Published online Nov 15, 2021. doi: 10.4239/wjd.v12.i11.1917
Peer-review started: February 5, 2021
First decision: March 30, 2021
Revised: April 18, 2021
Accepted: October 11, 2021
Article in press: October 11, 2021
Published online: November 15, 2021
Processing time: 283 Days and 0 Hours
Anaemia is common in patients with chronic kidney disease (CKD) and is a major risk factor that contributes to mortality in such patients. Type 2 diabetes mellitus (T2DM) is one of the leading causes of CKD. The association between admission hemoglobin levels and renal damage in patients with T2DM remains unclear.
To evaluate the relationship between admission hemoglobin levels and prognosis in patients with T2DM.
We performed a retrospective analysis of 265 consecutive patients presenting with T2DM between 2011 and 2015. The composite endpoint was end-stage renal disease or a 50% reduction in the estimated glomerular filtration rate.
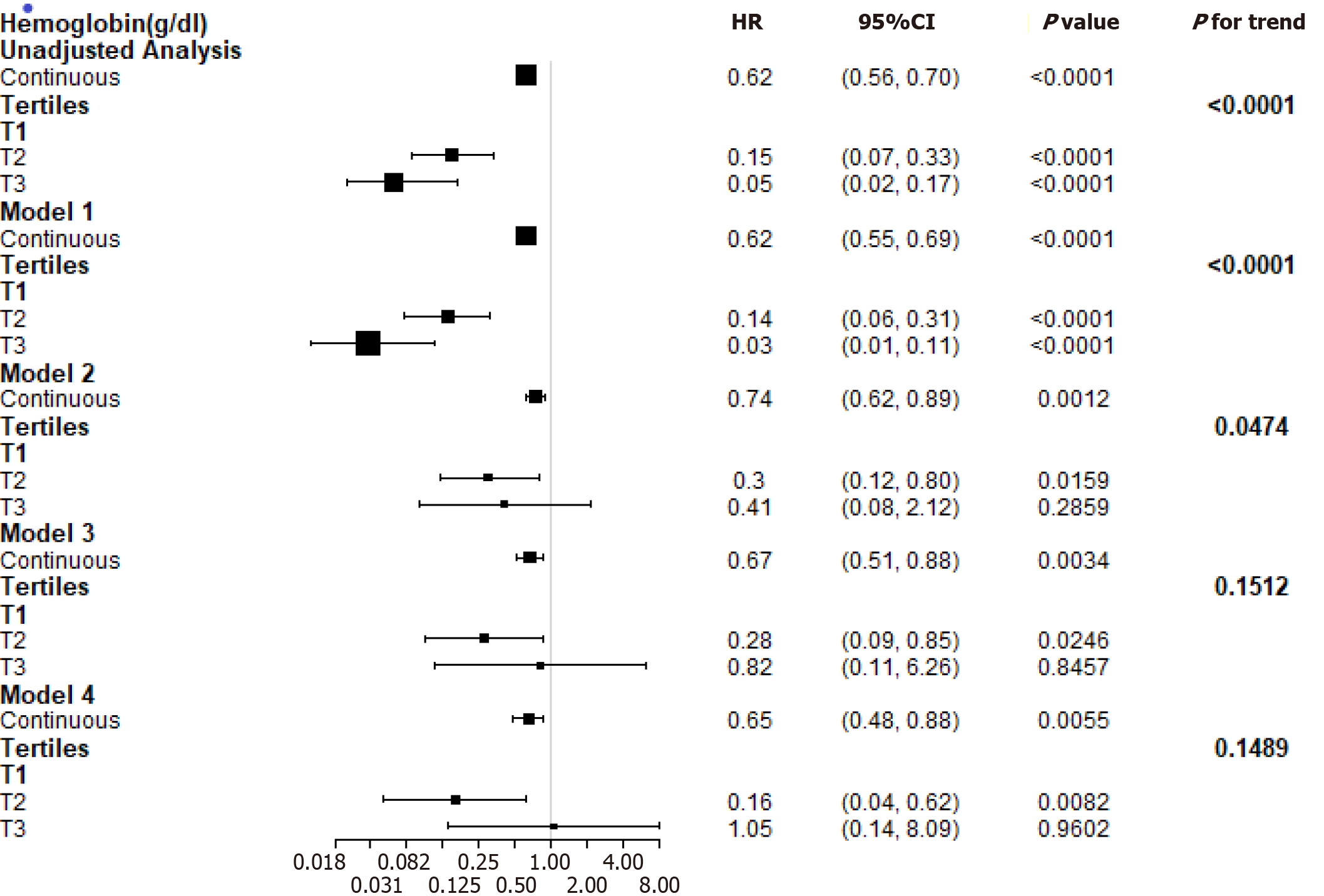
In multivariable-adjusted Cox proportional hazards models (adjusting for demographic factors, traditional risk factors, lipids), the adjusted hazard ratios (HRs) for the highest and middle tertiles compared to the lowest tertile of hemoglobin were 0.82 (95%CI: 0.11-6.26, P = 0.8457) and 0.28 (95%CI: 0.09-0.85, P = 0.0246), respectively. However, after further adjustment for glycaemia control, hemoglobin was positively related to the risk of the composite endpoint (HR: 1.05, 95%CI: 0.14-8.09, P = 0.9602) when the highest tertile was compared to the lowest tertile of hemoglobin. We found a U-shaped relationship between hemoglobin levels and the composite endpoint. The curve tended to reach the lowest level at an optimal hemoglobin level.
Among patients with T2DM, a U-shaped relationship was observed between hemoglobin levels and renal damage. A lower admission hemoglobin level (hemoglobin < 13.3 g/dL) is an independent predictor of renal damage.
Core Tip: A U-shaped exposure-response relationship exists between admission hemoglobin levels and the composite endpoint among patients with type 2 diabetes mellitus. A lower admission hemoglobin level (hemoglobin < 13.3 g/dL) is an independent predictor of renal damage. Hemoglobin is a convenient and feasible way to identify those patients who are at high risk of having a poor prognosis.
- Citation: Song HY, Wei CM, Zhou WX, Hu HF, Wan QJ. Association between admission hemoglobin level and prognosis in patients with type 2 diabetes mellitus. World J Diabetes 2021; 12(11): 1917-1927
- URL: https://www.wjgnet.com/1948-9358/full/v12/i11/1917.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i11.1917
Anaemia is a common complication of chronic kidney disease (CKD). Low hemoglobin level is one of the major risk factors for cardiovascular disease (CVD) and mortality and poor prognosis in CKD patients[1-4]. However, some studies have shown that higher hemoglobin levels slightly increase the risk of death[5], and elevations in hemoglobin levels have been implicated in a higher risk of mortality and cardio
This was a retrospective cohort study. The details of this study were described previously[8]. We used the database from the Shenzhen Second People’s Hospital and reviewed the records. A total of 265 patients diagnosed with T2DM were enrolled between January 2011 and December 2015 and followed until June 2016 at the Department of Nephrology and Endocrinology of Shenzhen Second People’s Hospital. The patients were followed every 3 mo for at least 3 mo until study endpoint or deadline. The deadline for the study was June 30, 2016. Patients with moderate to severe valvular disease, atrial fibrillation, other severe arrhythmias, congenital heart disease, or primary myocardial disease were excluded. The patients who had missing data for the admission hemoglobin levels and the composite endpoint were also excluded.
By using the database from the Shenzhen Second People’s Hospital, general clinical data, including age, gender, duration of T2DM, history of hypertension, use of angiotensin-converting enzyme inhibitors (ACEI) and/or angiotensin receptor blockers (ARB), body mass index (BMI; kg/m2), systolic blood pressure (SBP), and diastolic blood pressure (DBP), were recorded. Laboratory evaluations included fasting glycaemia, glycosylated hemoglobin (HbA1c), serum creatinine (Scr), 24-h urinary protein, hemoglobin, serum albumin (ALB), serum uric acid, blood urea nitrogen, total cholesterol (TC), and triglycerides (TG). All these laboratory tests were performed and checked at the Central Laboratory of Shenzhen Second People’s Hospital. The blood and urine samples were collected when patients were on admission. And the blood samples were fasting venous blood. Urine samples were collected in one or more containers over a period of 24 h. Reagent based method and automated analyzer were used to measure these laboratory variables. The eGFR was calculated using the CKD epidemiology collaboration equation[9]. The stages of CKD were classified as follows[10]: Stage 1, kidney damage with normal or increased eGFR (> 90 mL/min/1.73 m2); stage 2, mild reduction in eGFR (60-89 mL/min/1.73 m2); stage 3, moderate reduction in eGFR (30-59 mL/min/1.73 m2); stage 4, severe reduction in eGFR (15-29 mL/min/1.73 m2); stage 5, kidney failure (eGFR < 15 mL/min/1.73 m2 or dialysis). Our focus was to understand the effect of hemoglobin on the composite endpoint.
The patients were followed every 3 mo for at least 3 mo until June 2016. The composite endpoint was end-stage renal disease or a 50% reduction in the eGFR.
The Shapiro–Wilk test was used to test the normality of the data. Continuous variables are expressed as the mean ± SD (normal distribution) or median (quartiles) (skewed distribution), and categorical variables are expressed as frequencies or percentages. One-way ANOVA (normal distribution), Kruskal-Wallis H (skewed distribution) test, and chi-square test (categorical variables) were used to determine any significant differences between the means and proportions of the groups. The effects of hemoglobin levels on the composite endpoint were evaluated using Cox proportional hazards regression without adjustment and with adjustment for confounding variables. Hazard ratios (HRs) were reported per g/dL increment of hemoglobin levels (g/dL). Incremental models were fitted adjusting for (model 1) demographic factors (age, gender, and BMI); (model 2) demographic and traditional renal function risk factors (baseline eGFR, history of hypertension, SBP, DBP, 24-h urinary protein, ACEI and/or ARB use, and ALB); (model 3) demographic factors, traditional risk factors, and lipids (TC and TG); and (model 4) demographic factors, traditional risk factors, lipids, and glycaemia control (duration of diabetes, fasting glycaemia, and HbA1c). Kaplan–Meier plots were generated using tertiles of hemoglobin data to illustrate findings. A smoothing spline curve was used to describe the adjusted relationship between hemoglobin levels and the composite endpoint. Two-tailed probability values of < 0.05 were considered to indicate statistical significance. All statistical analyses were performed using Empowerstats (www.empowerstats.com) and R software (http://www.R-project.org).
The proposal was approved by the Clinical Research Ethical Committee of the Shenzhen Second People’s Hospital, and all subjects provided informed consent before enrollment. We adhered to the principles of the Declaration of Helsinki. The procedures followed were in accordance with institutional guidelines.
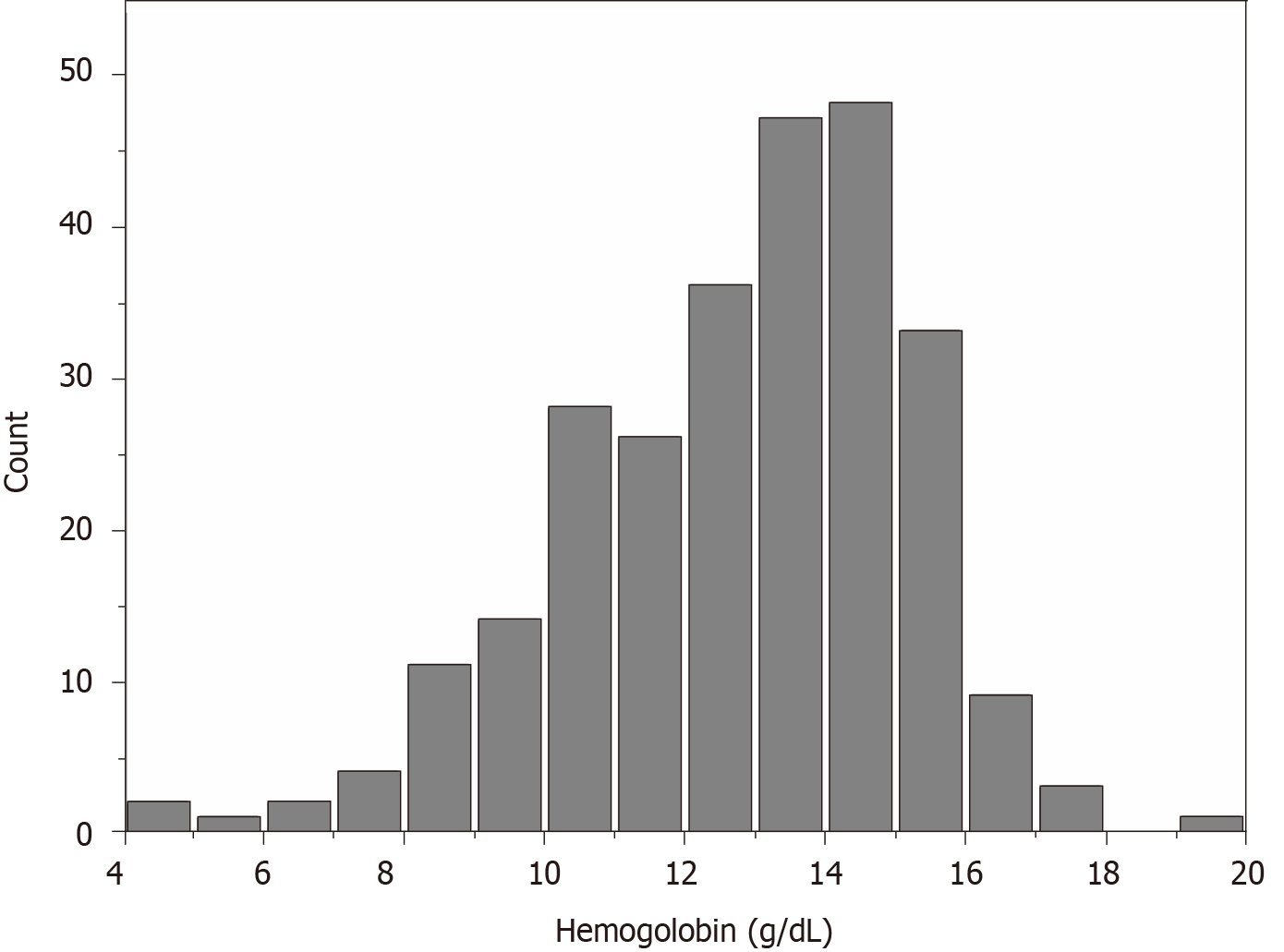
The average hemoglobin for the whole sample was 12.77 ± 2.42 g/dL. The distribution of hemoglobin levels in the full cohort is shown in Figure 1. We categorized the admission hemoglobin levels into tertiles to analyze. The cut-points for the tertile 1 (T1), tertile 2 (T2), and tertile 3 (T3) were < 11.97, 11.97-< 13.90, and 13.90-< 19.40, respectively. The baseline characteristics of study participants by hemoglobin tertiles are presented in Table 1. There were significant associations of higher hemoglobin with decreasing age, duration of diabetes, SBP, BUN, potassium, phosphate, HbA1c, 24-h urinary protein, and less history of hypertension. Male sex, ACEI/ARB use, and higher BMI, eGFR, DBP, ALB, and fasting blood glucose were also associated with higher hemoglobin.
| Variable | Hemoglobin tertile-Total | Hemoglobin tertile-Tertile 1 (< 11.97) | Hemoglobin tertile-Tertile 2 ( 11.97-< 13.90 ) | Hemoglobin tertile-Tertile 3 ( 13.90-< 19.40 ) | P value |
| Male, n (%) | 166 (62.64) | 34 (38.64) | 49 (59.04) | 83 (88.30) | < 0.001 |
| Female, n (%) | 99 (37.36) | 54 (61.36) | 34 (40.96) | 11(11.70) | < 0.001 |
| Age, years | 58.08 ± 12.37 | 61.67 ± 11.89 | 59.23 ± 10.35 | 53.69 ± 13.22 | < 0.001 |
| BMI, kg/m2 | 25.18 ± 3.22 | 24.55 ± 3.40 | 24.94 ± 2.97 | 25.98 ± 3.10 | 0.010 |
| Duration of diabetes, months | 33.54 ± 63.81 | 47.14 ± 79.51 | 37.60 ± 66.98 | 17.22 ± 35.07 | 0.005 |
| eGFR, mL/min per 1.73 m2 | 90.88 ± 46.71 | 60.63 ± 47.85 | 95.80 ± 41.82 | 114.85 ± 32.26 | < 0.001 |
| History of hypertension | 171 (64.53%) | 64 (72.73%) | 57 (68.67%) | 50 (53.19%) | 0.014 |
| SBP, mmHg | 141.78 ± 23.10 | 148.42 ± 26.85 | 141.14 ± 22.07 | 136.12 ± 18.33 | 0.001 |
| DBP, mmHg | 79.11 ± 12.51 | 75.66 ± 13.83 | 79.69 ± 10.50 | 81.84 ± 12.21 | 0.003 |
| ALB, g/L | 39.54 ± 5.59 | 35.98 ± 5.61 | 40.03 ± 5.20 | 42.46 ± 3.80 | < 0.001 |
| BUN, mmol/L | 7.47 ± 6.24 | 9.79 ± 5.99 | 7.61 ± 8.49 | 5.15 ± 1.73 | < 0.001 |
| SUA, μmol/L | 401.18 ± 250.95 | 449.22 ± 403.60 | 384.23 ± 106.65 | 370.82 ± 109.02 | 0.085 |
| Potassium, mmol/L | 4.30 ± 1.00 | 4.55 ± 1.26 | 4.31 ± 1.04 | 4.05 ± 0.50 | 0.003 |
| Total calcium, mmol/L | 2.31 ± 0.47 | 2.28 ± 0.56 | 2.35 ± 0.49 | 2.30 ± 0.34 | 0.619 |
| Phosphate, mmol/L | 1.25 ± 0.31 | 1.32 ± 0.34 | 1.28 ± 0.32 | 1.16 ± 0.24 | 0.002 |
| Fasting glycaemia, mmol/L | 7.95 ± 3.26 | 7.30 ± 3.09 | 7.65 ± 3.30 | 8.83 ± 3.22 | 0.004 |
| HbA1c, % | 11.40 ± 28.88 | 15.10 ± 49.59 | 10.22 ± 12.89 | 9.21 ± 2.13 | 0.015 |
| TG, mmol/L | 2.00 ± 1.71 | 1.83 ± 1.50 | 1.69 ± 1.24 | 2.41 ± 2.12 | 0.014 |
| TC, mmol/L | 4.52 ± 2.28 | 4.45 ± 1.53 | 4.70 ± 3.64 | 4.42 ± 0.96 | 0.703 |
| 24-h urinary protein, mg/d | 1179.17 ± 2346.72 | 2345.71 ± 3176.33 | 966.77 ± 2086.06 | 274.63 ± 450.60 | < 0.001 |
| ACEI/ARB use, n (%) | 228 (86.04) | 58 (65.91) | 77 (92.77) | 93 (98.94) | < 0.001 |
| CKD stage, n (%) | < 0.001 | ||||
| 1 | 148 (55.85) | 23 (26.14) | 51 (61.45) | 74 (78.72) | |
| 2 | 41 (15.47) | 10 (11.36) | 14 (16.87) | 17 (18.09) | |
| 3 | 38 (14.34) | 25 (28.41) | 12 (14.46) | 1 (1.06) | |
| 4 | 19 (7.17) | 13 (14.77) | 4 (4.82) | 2 (2.13) | |
| 5 | 19 (7.17) | 17 (19.32) | 2 (2.41) | 0 (0.00) |
During the follow-up period of 15.19 ± 9.33 mo, a total of 52 participants experienced the composite endpoint. Study entry hemoglobin levels independently and inversely correlated with the risk of the composite endpoint (HR: 0.74, P = 0.0012) in the model adjusted for demographic factors and known risk factors. The composite endpoint remained significant (HR: 0.65, P = 0.0055) after further adjustment for lipids and glycaemia control (Figure 2).
Figure 2 presents multivariable-adjusted HRs for the composite endpoint according to a 1 g/dL increase in the hemoglobin levels. The adjusted HR for the highest and middle tertiles compared to the lowest tertile of hemoglobin was 0.82 (P = 0.8457) and 0.28 (P = 0.0246), respectively, in the adjusted model (demographic factors, traditional risk factors, TC, and triglycerides). However, after further adjustment for the duration of diabetes, fasting glycaemia, and HbA1c, hemoglobin was positively related to the risk of the composite endpoint (HR: 1.05, P = 0.9602) when the highest tertile was compared to the lowest tertile of hemoglobin.
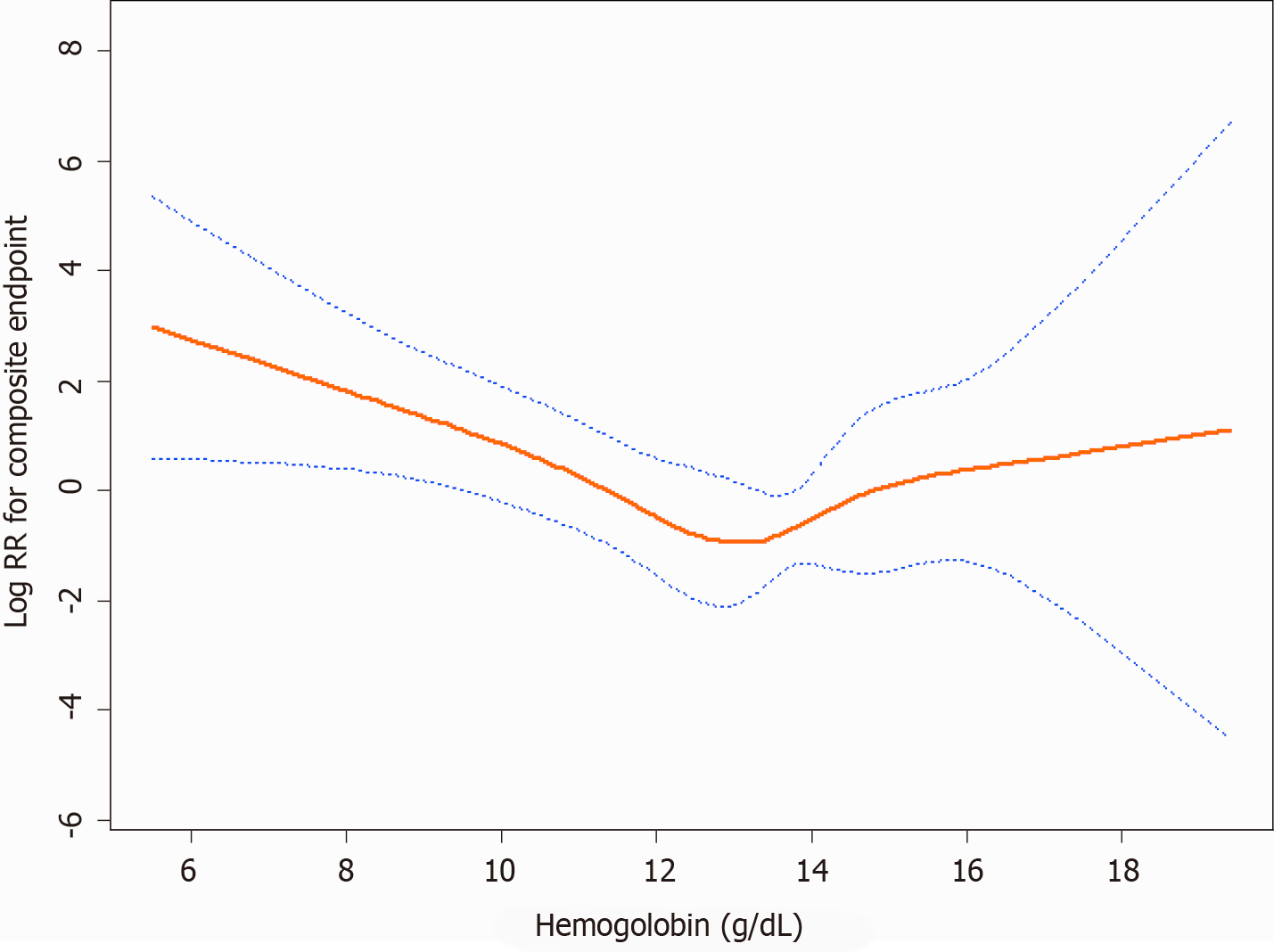
Figure 3 shows a U-shaped exposure-response relationship between admission hemoglobin levels and the composite endpoint after adjusting for age, gender, BMI, baseline eGFR, history of hypertension, SBP, DBP, 24-h urinary protein, ACEI and/or ARB use, ALB, TC, TG, duration of diabetes, fasting glycaemia, and HbA1c. The curve tended to reach the lowest level at an optimal hemoglobin level (approximately 13.3 g/dL). A higher admission hemoglobin level (hemoglobin < 13.3 g/dL) was associated with a lower risk of the composite endpoint (HR: 0.58, P = 0.0007). However, when hemoglobin was > 13.3 g/dL, the adjusted HR was 1.63 (P = 0.1585). Details are shown in Table 2.
| Cutoff point of hemoglobin level (K) | Hazard ratio (95%CI)1 | P value |
| < 13.3 g/dL | 0.58 (0.42-0.79) | 0.0007 |
| ≥ 13.3 g/dL | 1.63 (0.83-3.20) | 0.1585 |
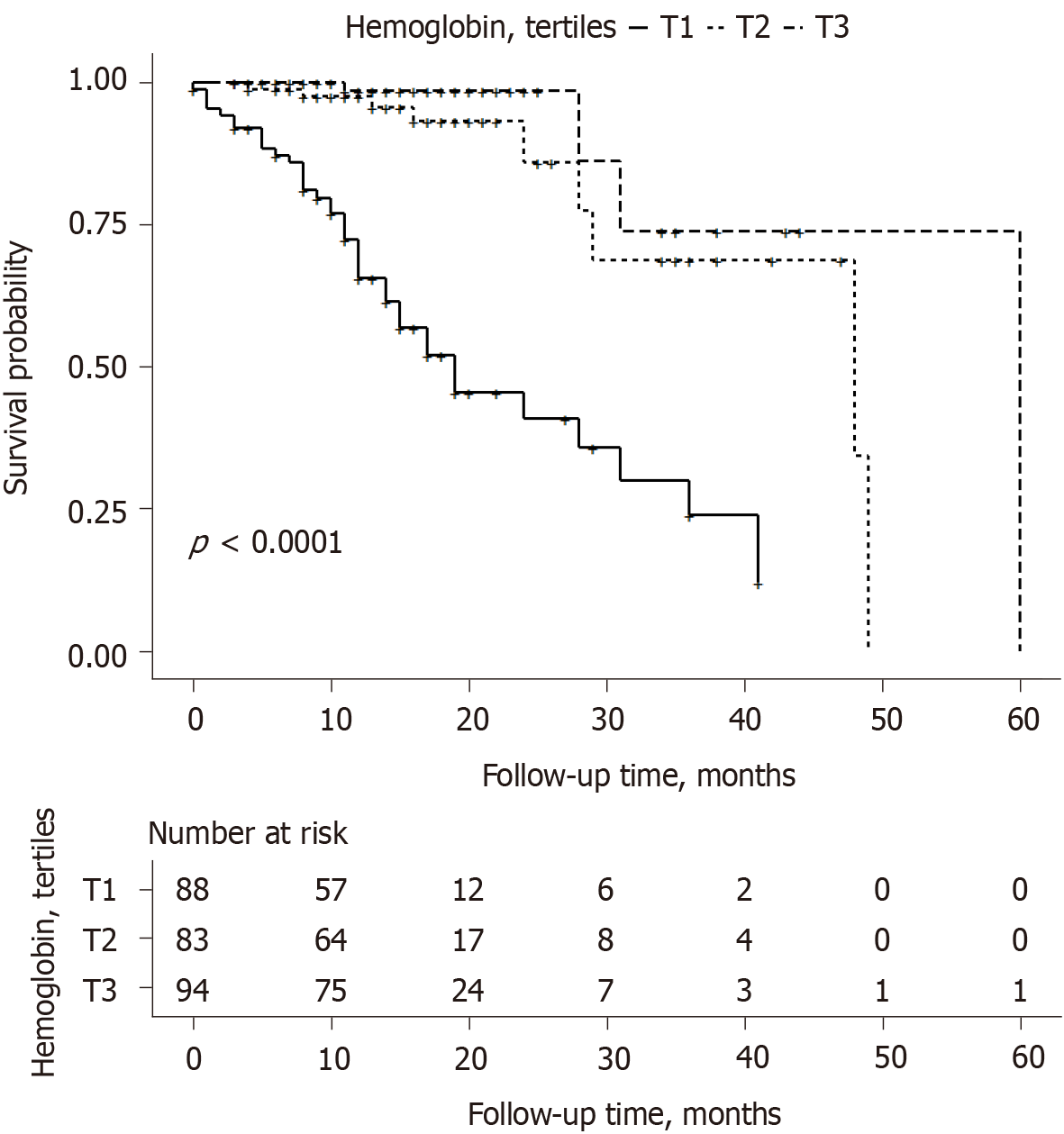
Kaplan–Meier curves show that patients within the upper tertiles of hemoglobin had a lower cumulative incidence of the composite endpoint (Figure 4).
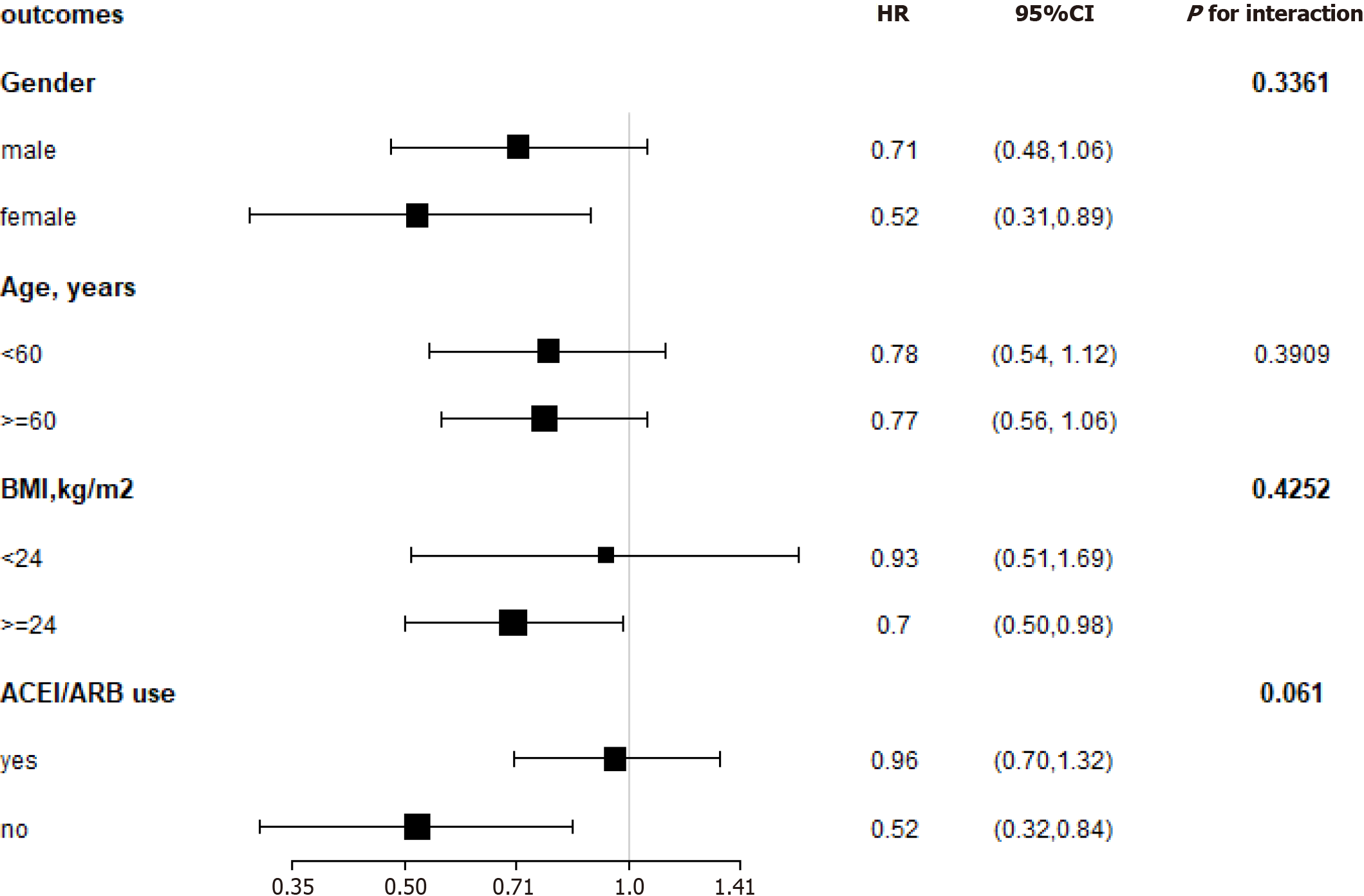
We performed stratified analyses by age (< 60 vs ≥ 60 years), gender, BMI (< 24 vs ≥ 24 kg/m2), and ACEI/ARB use (yes vs no). In our cohort, the impact of hemoglobin on the composite endpoint was not affected by age, gender, BMI, or ACEI/ARB use during the follow-up period (P for all interactions > 0.05) (Figure 5).
In this retrospective observational study involving patients with T2DM, we observed the relationship between the admission hemoglobin levels and the composite endpoint. In the Cox regression model, high levels of hemoglobin were associated with a decreased risk of the composite endpoint after adjusting for age, gender, BMI, traditional risk factors, TC, and TG.
By stratifying the patients into subgroups, the results showed that the impacts of hemoglobin on the composite endpoint were not affected by age, gender, BMI, or ACEI/ARB use. Many studies also showed that low hemoglobin was associated with adverse clinical outcomes and poor life quality[11-13]. Shacham et al[14] analyzed 1248 patients diagnosed with ST-segment elevation myocardial infarction and demon
Although higher hemoglobin targets were suggested to reduce the requirement for transfusions and benefit patients’ life quality[17-19], disadvantages were also been observed[7,20,21]. Several randomized controlled trials also showed that higher hemoglobin target levels were related to a higher risk of adverse outcomes[22-24]. In this study, after adjusting for the duration of diabetes, fasting glycaemia, and HbA1c, hemoglobin was positively related to the risk of the composite endpoint when the highest tertile was compared to the lowest tertile of hemoglobin. However, it is not statistically significant. We further performed a smooth spline curve and found a U-shaped exposure-response relationship between admission hemoglobin levels and the composite endpoint. The curve tended to reach the lowest level at an optimal hemoglobin level (approximately 13.3 g/dL). For the optimal hemoglobin level, lower and higher hemoglobin concentrations were related to higher rates of poor prognosis[25,26].
Furthermore, two large randomized controlled trials[24,27] showed no benefits of a higher hemoglobin target on cardiovascular events or death or found an increased rate of adverse events. Holst et al[28] found that patients assigned to blood transfusion at a higher hemoglobin threshold and at a lower threshold were similar on mortality at 90 d and risks of ischaemic events. Higher hemoglobin level patients had no benefit.
There are several explanations for the relationship between admission hemoglobin levels and the deterioration of renal function. Lower hemoglobin levels might decrease oxygen delivery and cause renal medullary hypoxia. The outer medullary region has high metabolic activity and low prevailing oxygen tension, so it is susceptible to ischaemic injury[29]. Angiotensin II is supposed to be a possible reason for tissue hypoxia during early CKD. The activated renin-angiotensin system induces tubular sodium reabsorption and vasoconstriction, so it results in higher oxygen consumption and relative tubular hypoxia[30-32]. However, an excessive hemoglobin level can result in increased blood viscosity and elevated blood pressure. Therefore, there is a U-shaped exposure-response relationship between hemoglobin levels and the composite endpoint. In our study, approximately 13.3 g/dL is an optimal hemoglobin level.
We acknowledge that there are several limitations to our study. First, this was a single-center, retrospective, nonrandomized trial. It was based on observational data, and many known or unknown confounding factors may exist, even though we attempted to adjust for confounding factors. Second, we used Scr to estimate GFR. As tubular secretion of creatinine and the variability in creatinine generation between individuals and for the same individual, the use of creatinine to estimate GFR has some limitations[33]. The eGFR can be validated by serum cystatin C or 51Cr-EDTA GFR, but these tools were not available in our study. Third, there were indeed fewer outcomes among patients with a higher Hb. Thus, the CIs around the estimates for the composite outcome in Figure 3 are very wide above an Hb >13 g/dL. The reason may be that this is a small-sized study. Fourth, this was only a single-center study, and whether these observations can be extended to whole Chinese and non-Chinese T2DM patients remains to be determined.
In brief, the results of this study demonstrated that among patients with T2DM, a U-shaped exposure-response relationship exists between admission hemoglobin levels and the composite endpoint. In our study, approximately 13.3 g/dL is an optimal hemoglobin level. A lower admission hemoglobin level (hemoglobin < 13.3 g/dL) is an independent predictor of the composite endpoint. These findings have important clinical and public health implications. As hemoglobin is a common and easily available measurement in clinical activity, it is a convenient and feasible way to identify those patients who are at high risk of developing the composite endpoint and have a poor prognosis.
Information on the association of hemoglobin with renal damage is uncertain, and the optimal hemoglobin target remains controversial.
The admission hemoglobin levels would influence the prognosis in patients with type 2 diabetes mellitus (T2DM).
To evaluate the relationships between admission hemoglobin levels and prognosis in patients with T2DM
A total of 265 patients with T2DM were included to perform a retrospective analysis. The general information and biochemical indices of these patients were statistically analyzed.
We found a U-shaped relationship between hemoglobin levels and the composite endpoint. The curve tended to reach the lowest level at an optimal hemoglobin level.
There is a U-shaped relationship between hemoglobin levels and renal damage in these patients.
We used a retrospective study to evaluate the relationships between admission hemoglobin levels and prognosis in patients with T2DM.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haile K S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | McFarlane SI, Chen SC, Whaley-Connell AT, Sowers JR, Vassalotti JA, Salifu MO, Li S, Wang C, Bakris G, McCullough PA, Collins AJ, Norris KC; Kidney Early Evaluation Program Investigators. Prevalence and associations of anemia of CKD: Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999-2004. Am J Kidney Dis. 2008;51:S46-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Covic A, Jackson J, Hadfield A, Pike J, Siriopol D. Real-World Impact of Cardiovascular Disease and Anemia on Quality of Life and Productivity in Patients with Non-Dialysis-Dependent Chronic Kidney Disease. Adv Ther. 2017;34:1662-1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Kleine CE, Soohoo M, Ranasinghe ON, Park C, Marroquin MV, Obi Y, Rhee CM, Moradi H, Kovesdy CP, Kalantar-Zadeh K, Streja E. Association of Pre-End-Stage Renal Disease Hemoglobin with Early Dialysis Outcomes. Am J Nephrol. 2018;47:333-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Wetmore JB, Li S, Yan H, Xu H, Peng Y, Sinsakul MV, Liu J, Gilbertson DT. Predialysis anemia management and outcomes following dialysis initiation: A retrospective cohort analysis. PLoS One. 2018;13:e0203767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Akizawa T, Saito A, Gejyo F, Suzuki M, Nishizawa Y, Tomino Y, Tsubakihara Y, Akiba T, Hirakata H, Watanabe Y, Kawanishi H, Bessho M, Udagawa Y, Aoki K, Uemura Y, Ohashi Y; JET Study Group. Low hemoglobin levels and hypo-responsiveness to erythropoiesis-stimulating agent associated with poor survival in incident Japanese hemodialysis patients. Ther Apher Dial. 2014;18:404-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol. 2005;16:2180-2189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 269] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 7. | Coyne DW. The health-related quality of life was not improved by targeting higher hemoglobin in the Normal Hematocrit Trial. Kidney Int. 2012;82:235-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Song H, Hu H, Liao D, Wei J, Wei C, Liao F, Zhou W, Mo Z, Jiang S, Ruan X, He Y. Left ventricular hypertrophy predicts the decline of glomerular filtration rate in patients with type 2 diabetes mellitus. Int Urol Nephrol. 2018;50:2049-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11183] [Cited by in RCA: 11821] [Article Influence: 454.7] [Reference Citation Analysis (0)] |
| 10. | Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63:713-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 1232] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 11. | Barrett BJ, Fenton SS, Ferguson B, Halligan P, Langlois S, Mccready WG, Muirhead N, Weir RV. Clinical practice guidelines for the management of anemia coexistent with chronic renal failure. Canadian Society of Nephrology. J Am Soc Nephrol. 1999;10 Suppl 13:S292-S296. [PubMed] |
| 12. | Yotsueda R, Tanaka S, Taniguchi M, Fujisaki K, Torisu K, Masutani K, Hirakata H, Kitazono T, Tsuruya K. Hemoglobin concentration and the risk of hemorrhagic and ischemic stroke in patients undergoing hemodialysis: the Q-cohort study. Nephrol Dial Transplant. 2018;33:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | He J, Shlipak M, Anderson A, Roy JA, Feldman HI, Kallem RR, Kanthety R, Kusek JW, Ojo A, Rahman M, Ricardo AC, Soliman EZ, Wolf M, Zhang X, Raj D, Hamm L; CRIC (Chronic Renal Insufficiency Cohort) Investigators. Risk Factors for Heart Failure in Patients With Chronic Kidney Disease: The CRIC (Chronic Renal Insufficiency Cohort) Study. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Shacham Y, Gal-Oz A, Leshem-Rubinow E, Arbel Y, Flint N, Keren G, Roth A, Steinvil A. Association of admission hemoglobin levels and acute kidney injury among myocardial infarction patients treated with primary percutaneous intervention. Can J Cardiol. 2015;31:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Dangas G, Iakovou I, Nikolsky E, Aymong ED, Mintz GS, Kipshidze NN, Lansky AJ, Moussa I, Stone GW, Moses JW, Leon MB, Mehran R. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 426] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 16. | Shavit L, Hitti S, Silberman S, Tauber R, Merin O, Lifschitz M, Slotki I, Bitran D, Fink D. Preoperative hemoglobin and outcomes in patients with CKD undergoing cardiac surgery. Clin J Am Soc Nephrol. 2014;9:1536-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, Pellegrini F, Ravani P, Jardine M, Perkovic V, Graziano G, McGee R, Nicolucci A, Tognoni G, Strippoli GF. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010;153:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 18. | Foley RN, Curtis BM, Parfrey PS. Hemoglobin targets and blood transfusions in hemodialysis patients without symptomatic cardiac disease receiving erythropoietin therapy. Clin J Am Soc Nephrol. 2008;3:1669-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Foley RN, Curtis BM, Parfrey PS. Erythropoietin therapy, hemoglobin targets, and quality of life in healthy hemodialysis patients: a randomized trial. Clin J Am Soc Nephrol. 2009;4:726-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Corrigendum to: "Surgery for Degenerative Cervical Myelopathy: A Nationwide Registry-Based Observational Study With Patient-Reported Outcomes" by Sasha Gulati, MD, PhD, Vetle Vangen-Lønne, MS, Øystein P Nygaard, MD, PhD, Agnete M Gulati, MD, PhD, Tommy A Hammer, MD, Tonje O Johansen, MD, Wilco C Peul, MD, PhD, Øyvind O Salvesen, MSc, PhD, Tore K Solberg, MD, PhD. Neurosurgery, nyab259, https://doi.org/10.1093/neuros/nyab259. Neurosurgery. 2021;89:943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Clement FM, Klarenbach S, Tonelli M, Johnson JA, Manns BJ. The impact of selecting a high hemoglobin target level on health-related quality of life for patients with chronic kidney disease: a systematic review and meta-analysis. Arch Intern Med. 2009;169:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071-2084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1462] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 23. | Lee YK, Kim SG, Seo JW, Oh JE, Yoon JW, Koo JR, Kim HJ, Noh JW. A comparison between once-weekly and twice- or thrice-weekly subcutaneous injection of epoetin alfa: results from a randomized controlled multicentre study. Nephrol Dial Transplant. 2008;23:3240-3246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019-2032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1506] [Cited by in RCA: 1493] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 25. | Panwar B, Judd SE, Warnock DG, McClellan WM, Booth JN 3rd, Muntner P, Gutiérrez OM. Hemoglobin Concentration and Risk of Incident Stroke in Community-Living Adults. Stroke. 2016;47:2017-2024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Jung MY, Hwang SY, Hong YA, Oh SY, Seo JH, Lee YM, Park SW, Kim JS, Wang JK, Kim JY, Lee JE, Ko GJ, Pyo HJ, Kwon YJ. Optimal hemoglobin level for anemia treatment in a cohort of hemodialysis patients. Kidney Res Clin Pract. 2015;34:20-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Levin A. Understanding recent haemoglobin trials in CKD: methods and lesson learned from CREATE and CHOIR. Nephrol Dial Transplant. 2007;22:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, Johansson PI, Aneman A, Vang ML, Winding R, Nebrich L, Nibro HL, Rasmussen BS, Lauridsen JR, Nielsen JS, Oldner A, Pettilä V, Cronhjort MB, Andersen LH, Pedersen UG, Reiter N, Wiis J, White JO, Russell L, Thornberg KJ, Hjortrup PB, Müller RG, Møller MH, Steensen M, Tjäder I, Kilsand K, Odeberg-Wernerman S, Sjøbø B, Bundgaard H, Thyø MA, Lodahl D, Mærkedahl R, Albeck C, Illum D, Kruse M, Winkel P, Perner A; TRISS Trial Group; Scandinavian Critical Care Trials Group. Lower vs higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 594] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 29. | Feldkamp T, Kribben A. Contrast media induced nephropathy: definition, incidence, outcome, pathophysiology, risk factors and prevention. Minerva Med. 2008;99:177-196. [PubMed] |
| 30. | Peti-Peterdi J, Harris RC. Macula densa sensing and signaling mechanisms of renin release. J Am Soc Nephrol. 2010;21:1093-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Emans TW, Patinha D, Joles JA, Koeners MP, Janssen BJ, Krediet CTP. Angiotensin II-induced hypertension in rats is only transiently accompanied by lower renal oxygenation. Sci Rep. 2018;8:16342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Vlahakos DV, Marathias KP, Madias NE. The role of the renin-angiotensin system in the regulation of erythropoiesis. Am J Kidney Dis. 2010;56:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Ferguson MA, Waikar SS. Established and emerging markers of kidney function. Clin Chem. 2012;58:680-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |