Published online Nov 15, 2021. doi: 10.4239/wjd.v12.i11.1875
Peer-review started: May 26, 2021
First decision: June 16, 2021
Revised: June 29, 2021
Accepted: August 20, 2021
Article in press: August 20, 2021
Published online: November 15, 2021
Processing time: 173 Days and 6.6 Hours
The accumulation of advanced glycation end products (AGEs) have been implicated in the development and progression of diabetic vasculopathy. However, the role of profilin-1 as a multifunctional actin-binding protein in AGEs-induced atherosclerosis (AS) is largely unknown.
To explore the potential role of profilin-1 in the pathogenesis of AS induced by AGEs, particularly in relation to the Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3) signaling pathway.
Eighty-nine individuals undergoing coronary angiography were enrolled in the study. Plasma cytokine levels were detected using ELISA kits. Rat aortic vascular smooth muscle cells (RASMCs) were incubated with different compounds for different times. Cell proliferation was determined by performing the MTT assay and EdU staining. An AGEs-induced vascular remodeling model was established in rats and histological and immunohistochemical analyses were performed. The mRNA and protein levels were detected using real-time PCR and Western blot analysis, respectively. In vivo, shRNA transfection was performed to verify the role of profilin-1 in AGEs-induced proatherogenic mediator release and aortic remodeling. Statistical analyses were performed using SPSS 22.0 software.
Compared with the control group, plasma levels of profilin-1 and receptor for AGEs (RAGE) were significantly increased in patients with coronary artery disease, especially in those complicated with diabetes mellitus (P < 0.01). The levels of profilin-1 were positively correlated with the levels of RAGE (P < 0.01); additionally, the levels of both molecules were positively associated with the degree of coronary artery stenosis (P < 0.01). In vivo, tail vein injections of AGEs induced the release of proatherogenic mediators, such as asymmetric dimethylarginine, intercellular adhesion molecule-1, and the N-terminus of procollagen III peptide, concomitant with apparent aortic morphological changes and significantly upregulated expression of the profilin-1 mRNA and protein in the thoracic aorta (P < 0.05 or P < 0.01). Downregulation of profilin-1 expression with an shRNA significantly attenuated AGEs-induced proatherogenic mediator release (P < 0.05) and aortic remodeling. In vitro, incubation of vascular smooth muscle cells (VSMCs) with AGEs significantly promoted cell proliferation and upregulated the expression of the profilin-1 mRNA and protein (P < 0.05). AGEs (200 μg/mL, 24 h) significantly upregulated the expression of the STAT3 mRNA and protein and JAK2 protein, which was blocked by a JAK2 inhibitor (T3042-1) and/or STAT3 inhibitor (T6308-1) (P < 0.05). In addition, pretreatment with T3042-1 or T6308-1 significantly inhibited AGEs-induced RASMC proliferation (P < 0.05).
AGEs induce proatherogenic events such as VSMC proliferation, proatherogenic mediator release, and vascular remodeling, changes that can be attenuated by silencing profilin-1 expression. These results suggest a crucial role for profilin-1 in AGEs-induced vasculopathy.
Core Tip: The formation and accumulation of advanced glycation end products (AGEs) contribute to accelerated macrovascular complications in individuals with diabetes. Currently, few studies have examined the role of profilin-1 in vasculopathy induced by AGEs. The present study proved that AGEs induced proatherogenic events such as vascular smooth muscle cell proliferation, proatherogenic mediator expression, and vascular remodeling, changes that were attenuated by silencing profilin-1 gene expression. These data suggested for the first time that profilin-1 was involved in AGEs-induced aortic atheroma formation in rats, indicating that drugs targeting profilin-1 may become a potential therapeutic strategy for ameliorating atherosclerosis secondary to diabetes.
- Citation: Xiao ZL, Ma LP, Yang DF, Yang M, Li ZY, Chen MF. Profilin-1 is involved in macroangiopathy induced by advanced glycation end products via vascular remodeling and inflammation. World J Diabetes 2021; 12(11): 1875-1893
- URL: https://www.wjgnet.com/1948-9358/full/v12/i11/1875.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i11.1875
Diabetic macroangiopathy is the leading cause of morbidity and mortality in patients with diabetes. The pathophysiological basis of diabetic macroangiopathy is atherosclerosis (AS), a complicated biological process including endothelial dysfunction, macrophage accumulation, inflammatory factor infiltration, vascular smooth muscle cell (VSMC) proliferation, and intima-media remodeling[1], which ultimately leads to vessel wall thickening and lumen stenosis. A large number of clinical studies have shown that the incidence of diabetic vascular complications has not decreased in patients with chronic long-term hyperglycemia, despite strict blood glucose and risk factor control[2,3]. Thus, the identification of effective new targets for the prevention and treatment of diabetic macroangiopathy is an urgent unsolved problem.
The production of advanced glycation end products (AGEs) is due to chronic hyperglycemia, aging, and chronic degenerative diseases[4]. The formation and accumulation of AGEs contribute to accelerated macrovascular complications in individuals with diabetes, and high serum levels of AGEs and receptor for AGEs (RAGE) are associated with an increased incidence and severity of coronary artery disease (CAD)[5-7]. According to previous studies, AGEs are involved in the development of AS through two major pathways: Binding to RAGE or cross-linking of proteins[8]. On the one hand, AGEs in the vascular wall cross-link extracellular matrix proteins, leading to arterial stiffness and atherosclerotic plaque formation[9]. On the other hand, the binding of AGEs to RAGE elicits the generation of reactive oxidative species (ROS), subsequently activating mitogen-activated protein kinase and nuclear factor kappa-B signaling and activating the pathways related to diabetic complications[10].
Profilin-1 is a well-known highly conserved and ubiquitously expressed multifunctional actin-binding protein (12 to 15 KD) that plays an essential role in regulating cytoskeletal rearrangement and redistribution by promoting actin polymerization and remodeling[11,12]. Based on accumulating evidence, profilin-1 levels are markedly increased in serum, the diabetic endothelium, and atherosclerotic lesions of patients with pathological conditions such as hypertension, AS, and diabetes[13-15]. Cardio
As shown in our previous study, AGEs induce endothelial dysfunction and cardiomyocyte hypertrophy in vitro, changes that are attenuated by the knockdown of profilin-1 expression[12,23]. However, to date, few studies have examined the role of profilin-1 in vascular remodeling induced by AGEs. Thus, the present study aimed to explore the role of profilin-1 in AGEs-induced macroangiopathy and identify vascular remodeling- and inflammation-dependent mechanisms that might account for the atheroprotective effects of profilin-1 silencing on AS both in vivo and in vitro and to provide a theoretical basis for targeting profilin-1 in the therapy of the diabetes complication of AS.
AGEs were purchased from Merck (Darmstadt, Germany). Bovine serum albumin (BSA) was supplied by Every Green Co. Ltd. (Hangzhou, Zhejiang Province, China). Asymmetric dimethylarginine (ADMA) and the anti-β-actin antibody were purchased from Sigma (St. Louis, MO, United States). Intercellular adhesion molecule-1 (ICAM-1) ELISA kits and Griess reagents were purchased from Jiancheng Biological Medical Engineering Institute (Nanjing, Jiangsu Province, China). N-terminal propeptide of type III procollagen (PIIINP) ELISA kits were supplied by Vscn Life Sciences, Inc. (Wuhan, Hubei Province, China). Phenylmethylsulfonyl fluoride (PMSF) and BCA protein kits were purchased from Beyotime Company (Jiangsu Province, China). The anti-profilin-1 antibody was obtained from Abcam (Shanghai, China). Western blotting kits and secondary IgG antibodies were purchased from KPL (Gaithersburg, MD, United States). The primers were synthesized by Shanghai Sangong Co. (Shanghai, China). RT reagent kits were obtained from Thermo Scientific (Waltham, MA, United States). Profilin-1 shRNA adenovirus vectors (Ad-profilin-1 shRNA) and blank control adenovirus vectors (Ad-GFP) were constructed by Hanbio Co. (Shanghai, China).
Eighty-nine patients undergoing coronary angiography (CAG) at the Department of Cardiology of XiangYa Hospital of Central South University from September 2017 to March 2018 were enrolled. Groups were divided as follows: Control group (27 patients) with stenosis < 50% in all coronary vessels; CAD group (31 patients) with stenosis ≥ 50% in at least one vessel but without diabetes; and CAD + diabetes mellitus (DM) group (31 patients) with stenosis ≥ 50% in at least one vessel with diabetes. The diagnosis of type 2 diabetes was made according to the criteria of the American Diabetes Association reported in 2017[24]. Informed consent was obtained from enrolled individuals prior to participation in the study, and privacy rights were always observed. The present study was approved by the ethical review committee of Xiangya Hospital of Central South University.
Peripheral blood was collected from all patients, and the levels of biochemical factors, such as fasting plasma glucose, glycosylated serum protein, glycosylated hemoglobin, total cholesterol, low-density lipoprotein, high-density lipoprotein, triglyceride, serum creatinine, and uric acid, were measured using an automatic biochemical analyzer. Clinical data, including age, sex, weight, systolic blood pressure, diastolic blood pressure, and CAD course, were collected. Each patient underwent angiography of the left and right coronary arteries using a GE Innova 3000 angiography CAG examination through a radial artery puncture with a selective angiography catheter. Coronary artery disease was defined as ≥ 50% stenosis in major vessels (the left anterior descending artery, circumflex artery, and right coronary artery and their major branches). The severity of coronary artery stenosis was quantitatively assessed according to CAG using the Gensini scoring system.
Rat aortic vascular smooth muscle cells (RASMCs) were purchased from ATCC (Manassas, VA, United States) and placed in an incubator at 37 °C with 90% humidity and an atmosphere containing 5% CO2. RASMCs were cultured in DMEM medium supplemented with 10% FBS (Gibco BRL, Grand Island, NY, United States), 100 U/mL penicillin, and 100 mg/mL streptomycin (Beyotime, China). After reaching 90% confluence, the cells were subcultured and incubated with different concentrations (0, 50, 100, 150, 200, or 400 μg/mL) of AGEs for different times (0, 24, 48, and 72 h) at passages 2-3 or incubated with the JAK2 inhibitor (T3042-1, 15 μmol/L) or STAT3 inhibitor (T6308-1, 5 μmol/L).
The viability and proliferation of VSMCs were determined using a MTT (5 mg/mL, Beyotime Company, Shanghai, China) assay according to the manufacturer’s instructions. Briefly, RASMCs were seeded on 96-well plates at a density of 7 × 106 cells/mL in DMEM supplemented with 10% FBS. The medium was changed to DMEM containing 1% FBS to make them quiescent for 24 h, and then different concentrations of AGEs were added and cocultured with the cells. Finally, 20 μL of 5 mg/mL MTT was added to the culture medium and incubated for 4 h. The formazan crystals in each well were dissolved in 150 μL of DMSO, and the absorbance was evaluated at 595 nm using a microplate reader (PerkinElmer, United States) to assess cell proliferation.
RASMCs were seeded on 6-well plates at a density of 1 × 104 cells/mL. After reaching 60% confluence, proliferation was assessed according to the instructions of the EdU Staining Proliferation Kit (iFluor 647). Briefly, the EdU solution was added to RASMCs and incubated for 2-4 h. Then, fixative solution and permeabilization buffer were added successively. After incubation with the reaction mix to fluorescently label cells with EdU for 30 min, cell proliferation was detected and quantified using fluorescence microscopy and flow cytometry.
AGEs were prepared using a previously reported method[16]. Briefly, BSA (10 g/L) was incubated in 0.2 M phosphate buffer (PBS, pH 7.4) with D-glucose (90 g/L) containing 100 U/mL penicillin and 100 mg/mL streptomycin in the dark at 37 °C. After 12 wk of incubation, the preparations were dialyzed against PBS three times at 4 °C to remove free glucose for 24 h and then separated into aliquots and stored at -20 °C until use.
Sprague-Dawley (SD) rats were fed under conditions of a controlled temperature (22 ± 1 °C) and humidity (50%-60%). AGEs-BSA was injected into the tail veins at a dose of 25 mg/kg/d per rat for 0, 20, 40, and 60 d. Ad-profilin-1 shRNA or Ad-GFP was injected into the tail vein twice at a dose of 3 × 109 PFU per injection with an interval of 4 wk between injections. The experimental protocols used in this study were approved by the Medicine Animal Welfare Committee of Xiangya Hospital, Central South University (Changsha, Hunan Province, China).
One-month-old male SD rats were purchased from Slac Laboratory Co. (Shanghai, China) and randomly divided into different groups (n = 6) after 1 wk of acclimation: (1) Control group: SD rats were intravenously injected with normal saline; (2) AGEs group: SD rats were intravenously injected with AGEs-BSA for 20, 40, or 60 d; (3) AGEs-S group: SD rats were intravenously injected with AGEs-BSA for 60 d and Ad-profilin-1 shRNA; and (4) AGEs-V group: SD rats were intravenously injected with AGEs-BSA for 60 d and Ad-GFP.
All rats were anesthetized with pentobarbital sodium (1%), and blood samples were collected after decapitation and centrifuged for 10 min at 4 °C at a rate of 3000 g. Supernatants were stored at -80 °C until analysis. The thoracic aorta was separated and divided into three parts: (1) One part was fixed with 10% formalin for 24 h, sequentially dehydrated in 70%, 80%, 90%, and 100% ethanol, rendered transparent with xylene, embedded in paraffin wax blocks, and stored at 4 °C until hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC); (2) The second part was homogenized in TRIzol reagent for real-time PCR analysis; and (3) The third part was homogenized in RIPA lysis buffer and 0.1 mmol/L PMSF for Western blot analysis. All procedures were performed in compliance with guidelines from the Ethics Committee for Animal Care and Research at Xiangya Hospital of Central South University (Changsha, Hunan Province, China).
H&E staining was performed on 3 mm sections from formalin-fixed and paraffin-embedded thoracic aorta tissue using standard protocols. After H&E staining, the morphological changes in blood vessels were observed under a light microscope. An Image-Pro Plus computer pathological image analysis system was used to calculate the media thickness (MT), lumen diameter (LD), medial area (MA), lumen area (LA), and smooth muscle layers (SMLs) of the thoracic aorta. The median MT/LD and MA/LA indicated the degree of hyperplasia.
IHC was performed as previously described[25] to detect the expression of profilin-1. Briefly, sections of aortic tissues with a thickness of 3 mm were incubated overnight at 4 °C with the primary antibody against profilin-1 (1:50) and then incubated with a horseradish peroxidase-labeled goat anti-rabbit immunoglobulin-G secondary antibody (1:1000) at 37 °C for 20 min. After three washes with PBS, the samples were incubated with diaminobenzidine for coloration and counterstained with hematoxylin for 2 min to stain the nuclei. Photographs were captured under a high-power microscope (200 ×); positively stained cells were brown in color, and integral optical density values were measured using an NIS-Elements AR 3.0 pathological image analysis system.
Total RNA was extracted from cells grown in a 6-well plate using TRIzol reagent, and cDNA was synthesized from 1 μg of RNA using the First-Strand Synthesis System for PCR according to the manufacturer’s instructions. The following primer pair was used to amplify profilin-1: Forward primer, 5’-TGACCTCATCTGTCCCTTCC-3’; reverse primer, 5’-ACAGGAGGGGGTATGGGTAG-3’. The following primer pair was used to amplify GAPDH: Forward primer, 5’-ACCCAGAAGACTGTGGATGG-3’; reverse primer, 5’-CACATTGGGGGTAGGAACAC-3’. The primer pair used for JAK2 amplification was as follows: Forward primer, 5’-CGGAACACCTTGTCCTGAAT-3’; reverse primer, 5’-GAGTCAGCTGGGAAAAGCAC-3’. The primer pair used for STAT3 amplification was as follows: Forward primer, 5’-TGATGCGCTCTTATGTGAGG-3’; reverse primer, 5’-GGCGGACAGAACATAGGTGT-3’. Amplification was performed with an initial incubation at 95 °C for 5 min, and 40 cycles of denaturation at 95 °C for 10 s and annealing and extension at 60 °C for 30 s to detect profilin-1, GAPDH, STAT3, and JAK2 expression. All amplification reactions for each sample were performed in triplicate, and data were analyzed using the 2-ΔΔCT method. The mRNA levels of profilin-1, STAT3, and JAK2 were normalized to the level of the GAPDH mRNA.
VSMCs were lysed in RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China) and centrifuged at 12000 g at 4 °C for 15 min. The concentration of the total protein extract was measured using BCA protein assay kits. Approximately 60 μg of protein was separated on 15% SDS-PAGE gels and electrotransferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, United States). The membranes were blocked with 5% fat-free milk in PBST buffer (0.1% Tween 20 in PBS) for 90 min and subsequently probed with primary antibodies against JAK2 (1:1000), STAT3 (1:2000), profilin-1 (1:1500), or β-actin (1:5000) at 4 °C overnight, followed by incubation with an HRP-conjugated IgG secondary antibody at room temperature for 1 h. The bands on the membranes were visualized by adding chemiluminescence reagent, and the gray value was then analyzed using ImageJ software v1.37c (National Institutes of Health, Bethesda, MD, United States).
The adenovirus interference vector Ad-profilin-1 shRNA was generated from pHBAd-U6-GFP using Gateway recombination technology. Briefly, Ad-profilin-1 shRNA was transfected into DH5a cells for adenovirus packaging and titer. The recombinant adenoviruses were measured using the immunization method. Primers for the profilin-1 shRNA were forward, 5’-TGCTGATTTCTTGTTGATCAAACCACGTTTTGGCCACTGACTGACGTGGTTTGCAACAAGAAAT-3’ and reverse, 5’-CCTGATTTCTTGTTGCAAACCACGTCAGTCAGTGGCCAAAACGTGGTTTGATCAACAAGAAATC-3’. The Ad-profilin-1 shRNA adenovirus vector was injected into the tail vein of SD rats at a dose of 3 × 109 PFU with an interval of 4 wk to silence the expression of profilin-1. The transfection efficiency in each experiment was determined by measuring profilin-1 protein expression using Western blot analysis.
The contents of ICAM-1 and PIIINP were detected using ELISA kits, and the concentration of ADMA was measured using high-performance liquid chromatography as previously described[26]. The standard curve for each assay was constructed in accordance with the manufacturer’s provided sample. Each sample was assayed in duplicate.
Test data of each sample accorded with the normal distribution and are expressed as the mean ± SD. Statistical analyses were performed using SPSS 22.0 software (IBM, Chicago, IL, United States). The comparisons among multiple groups were performed using one-way analysis of variance (ANOVA), followed by the test of pairwise comparisons. Associations between profilin-1, RAGE, and Gensini scores were assessed by Pearson correlation analysis. A value of P < 0.05 was considered to be of statistical difference.
Enrolled individuals with CAD and DM regularly received cardiovascular drug therapy, including antihypertensive and lipid-lowering drugs. The baseline characteristics of the subjects are presented in Table 1. None of the groups showed significant differences in age, percentage of males, SBP, DBP, LDL-cholesterol levels, high-density lipoprotein cholesterol levels, or serum uric acid levels (P > 0.05). Compared with the control group, the individuals in the CAD group and CAD + DM group had a higher weight and higher plasma levels of glycosylated hemoglobin (HbA1c), triglyceride (TG), total cholesterol (TC), high sensitivity C-reactive protein (hs-CRP), and blood urea nitrogen (BUN) (P < 0.05). Compared with the CAD group, those who developed CAD combined with DM showed higher plasma fasting plasma glucose (FPG), HbA1c, TG, and serum creatinine levels (P < 0.05).
| Control group (n = 27) | CAD group (n = 31) | CAD+DM group (n = 31) | |
| Age (yr) | 60 ± 8 | 61 ± 9 | 64 ± 8 |
| Sex (n) | |||
| Male | 14 | 15 | 17 |
| Female | 13 | 16 | 14 |
| Weight (kg) | 60.87 ± 7.67 | 67.15 ± 12.00a | 65.55 ± 10.30a |
| SBP (mmHg) | 123.54 ± 14.28 | 126.41 ± 16.56 | 127.35 ± 19.49 |
| DBP (mmHg) | 74.46 ± 6.66 | 75.71 ± 10.45 | 78.74 ± 9.57 |
| FPG (mmol/L) | 4.86 ± 0.56 | 5.47 ± 0.87 | 9.92 ± 3.26a,c |
| HbA1c (%) | 4.90 ± 0.58 | 6.07 ± 0.93a | 7.43 ± 1.70a,c |
| GSP (mmol/L) | 1.81 ± 0.19 | 2.27 ± 0.39 | 2.78 ± 1.19a,c |
| TG (mmol/L) | 1.21 ± 0.35 | 1.79 ± 1.25a | 2.44 ± 2.15a,c |
| TC (mmol/L) | 2.10 ± 1.61 | 4.40 ± 1.08a | 4.51 ± 1.26a |
| LDL-C (mmol/L) | 2.32 ± 0.53 | 2.63 ± 0.67 | 2.59 ± 1.22 |
| HDL-C (mmol/L) | 1.35 ± 0.31 | 2.48 ± 1.43 | 1.50 ± 1.14 |
| hs-CRP (mg/L) | 1.34 ± 0.61 | 3.84 ± 3.15a | 4.65 ± 2.70a |
| BUN (mmol/L) | 5.29 ± 0.73 | 5.53 ± 1.44a | 5.85 ± 1.65a |
| SCr (μmol/L) | 79.3 ± 15.2 | 82.3 ± 23.3 | 93.2 ± 21.4a,c |
| SUA (mmol/L) | 309.2 ± 92.3 | 316.3 ± 81.7 | 327.6 ± 69.8 |
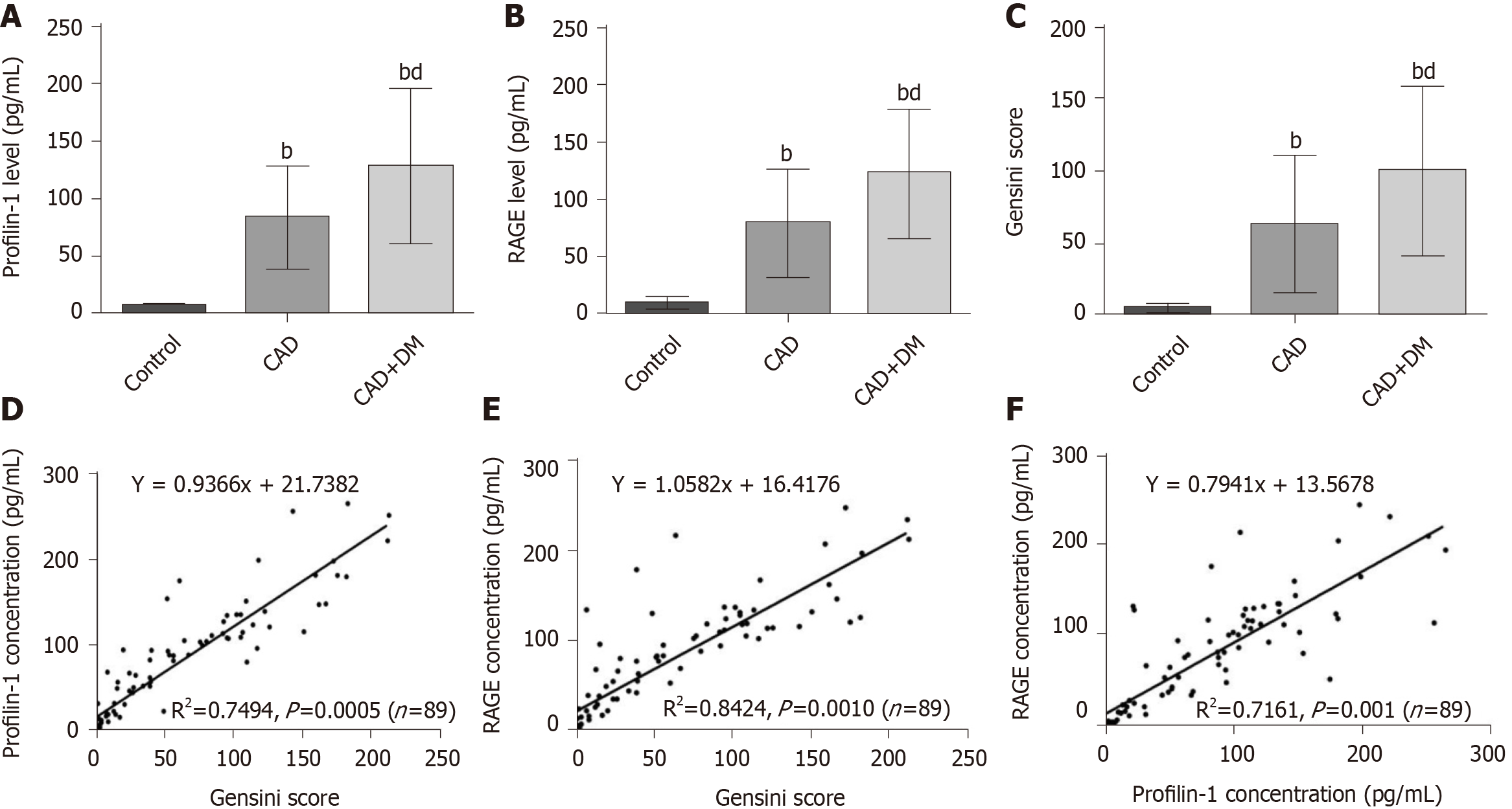
The plasma concentrations of profilin-1 and RAGE in enrolled individuals were detected using ELISA kits. The results of one-way ANOVA showed significantly increased concentrations of profilin-1 and RAGE in the CAD and CAD + DM groups compared with the control group (P < 0.01) (Figure 1A and B). As expected, plasma profilin-1 and RAGE levels were significantly higher in patients with CAD and DM than in those with CAD alone (P < 0.01). A similar significant difference was also observed in Gensini scores (Figure 1C).
| Control (n = 6) | AGEs-20 (n = 6) | AGEs-40 (n = 6) | AGEs-60 (n = 6) | |
| MT (mm) | 45.83 ± 3.54 | 57.17 ± 3.25a | 74.83 ± 4.62a | 79.33 ± 3.08a |
| LD (mm) | 737.5 ± 9.89 | 769.83 ± 15.73a | 862.33 ± 21.99a | 857.67 ± 15.06a |
| MT/LD (%) | 6.21 ± 0.41 | 7.42 ± 0.35a | 8.67 ± 0.37a | 9.25 ± 0.34a |
| MA (mm2) | 0.53 ± 0.01 | 0.54 ± 0.01a | 0.55 ± 0.01a | 0.56 ± 0.01a |
| LA (mm2) | 1.97 ± 0.04 | 1.85 ± 0.04a | 1.67 ± 0.04a | 1.64 ± 0.04a |
| MA/LA (%) | 26.97 ± 0.38 | 29.16 ± 0.73a | 32.99 ± 0.79a | 34.13 ± 0.62a |
| SML (layer) | 6.17 ± 0.75 | 7.17 ± 0.73 | 7.67 ± 0.82b | 8.17 ± 0.75b |
In the Pearson correlation analysis, both baseline profilin-1 and RAGE levels showed a strong significant positive correlation with Gensini scores (R2= 0.7494, P = 0.0005 and R2= 0.8424, P = 0.0010, respectively) (Figure 1D and E), and the plasma RAGE and profilin-1 levels were also positively correlated (R2= 0.7161, P = 0.0010) (Figure 1F).
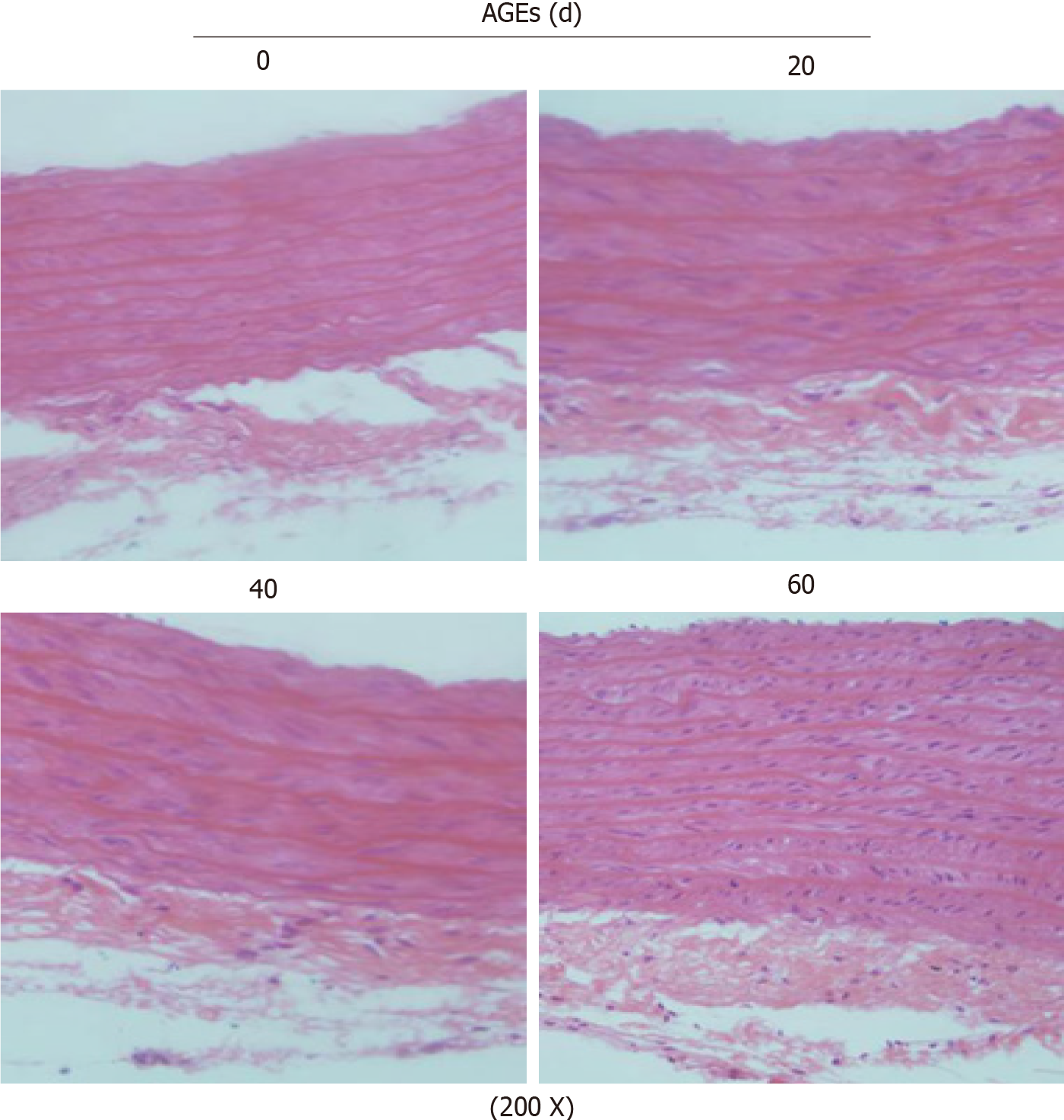
After tail vein injections of AGEs (25 mg/kg/d) for different times, the histological morphology of the thoracic aorta in rats was assessed to investigate the effect of AGEs on the aortic structure. H&E staining (200 ×) showed significant vascular remodeling of the thoracic aorta in AGEs-injected rats, including thickened aortic walls, looser or even broken elastic fibers, and broadened endothelial gaps. Significant proliferation and a disordered arrangement of SMCs were also observed in the AGEs group, leading to lumen stenosis (Figure 2). We measured the MT, LD, ratio of MT/LD, MA, LA, ratio of MA/LA, and SMLs of the thoracic aorta. Compared with the control group, the injection of AGEs significantly increased the MT/LD and MA/LA ratios, which are considered signs of vascular remodeling (P < 0.01, Table 2). The number of SMLs also increased 40 d after the AGEs injection (P < 0.05, Table 2).
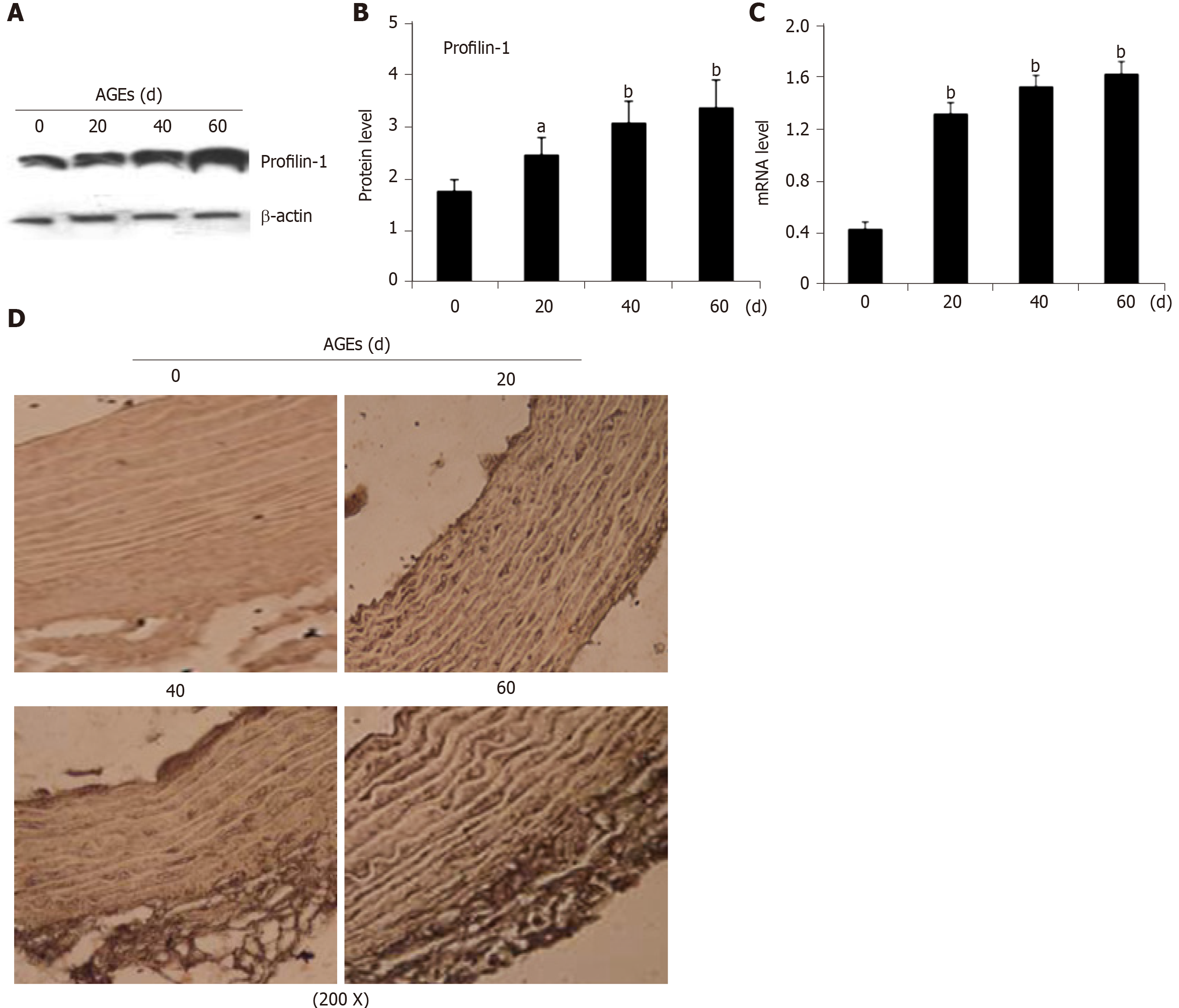
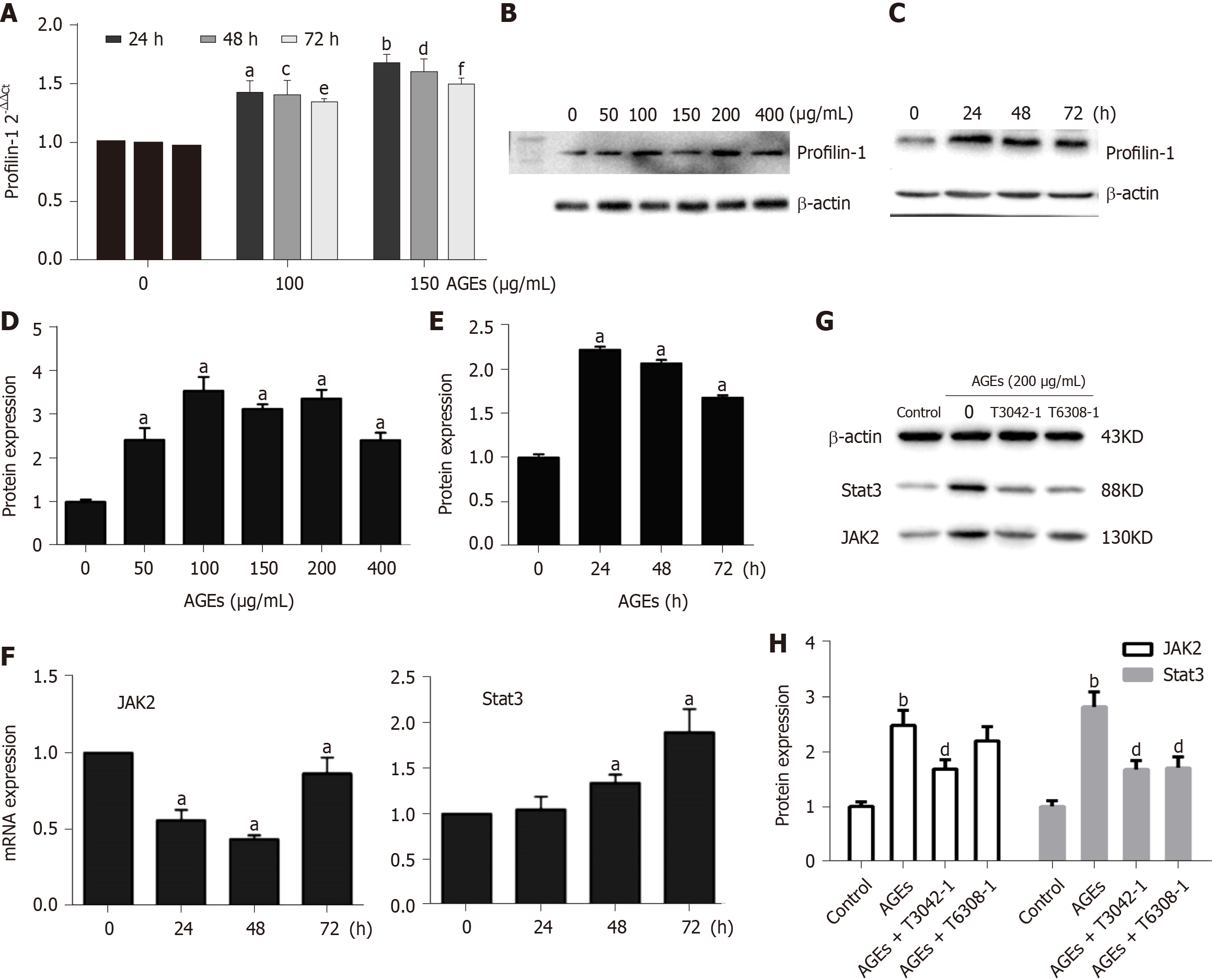
Real-time PCR and Western blot analysis of mRNA and protein extracts from thoracic aortae were performed, respectively, to confirm the effect of AGEs on profilin-1 expression in vivo. Consistent with the results from our in vitro study, the profilin-1 mRNA and protein were expressed at higher levels in the thoracic aorta wall of the AGEs-injection group compared to those of the control group (P < 0.05 or P < 0.01) (Figure 3A-C). Interestingly, the results of IHC staining showed that the positively stained cells were mainly located in the intima, along with a certain amount in the media, suggesting that profilin-1 was mainly expressed in endothelial cells and SMCs in local aortic AS plaques. Additionally, the expression of profilin-1 in the thoracic aorta of AGEs-injected rats was significantly higher than that of normal rats (Figure 3D). The average optical density, expression-positive area, and integrated optical density of profilin-1 in the thoracic aorta were also significantly increased in AGEs-injected rats compared with the control group (P < 0.001) (Table 3).
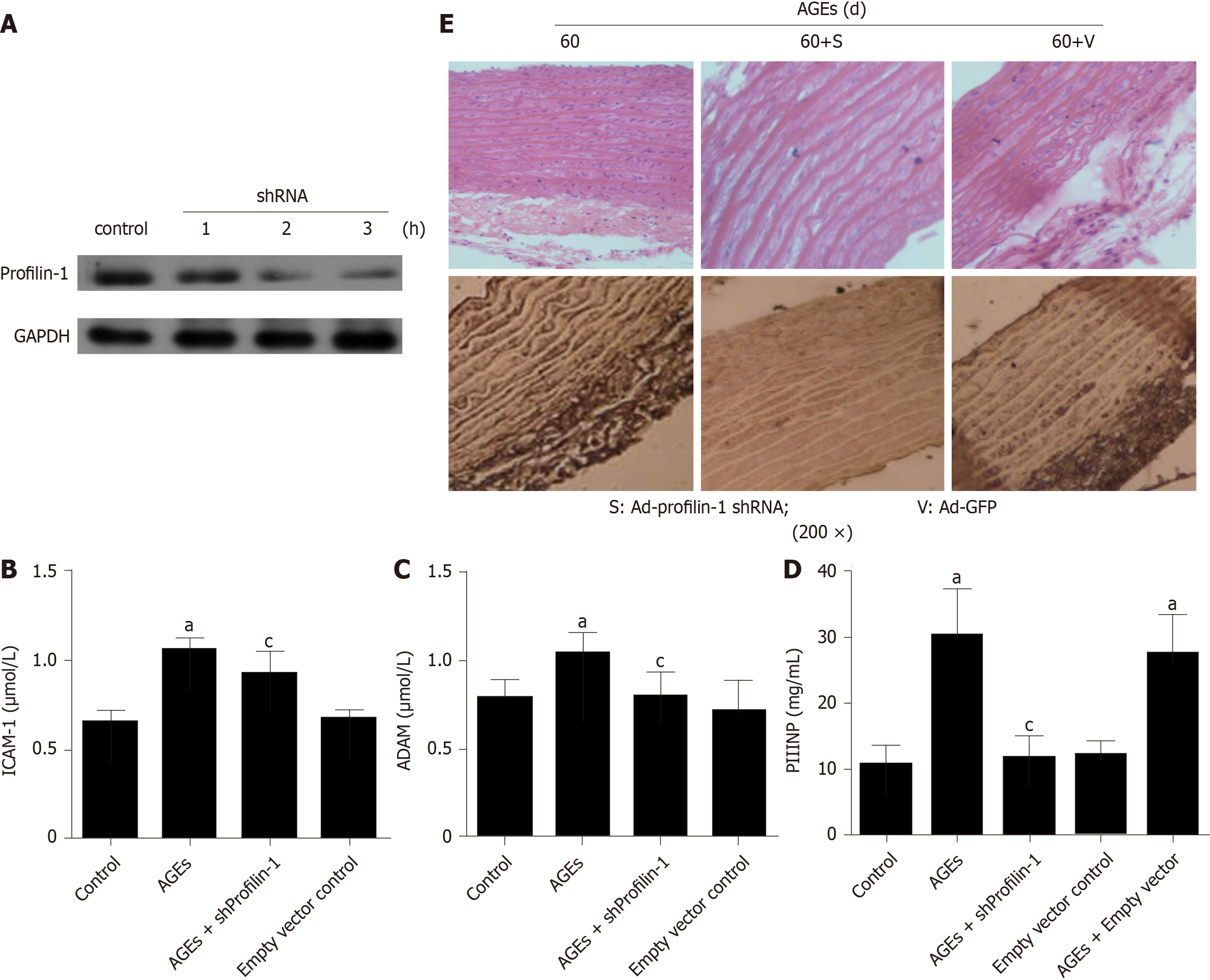
AGEs evoke inflammation in various cell types and organs, including endothelial cells, blood vessels, and the heart[27]. However, the role of AGEs-induced inflammation in vascular remodeling remains unclear. Here, after a chronic tail vein injection of AGEs, the serum levels of ADMA, ICAM-1, and PIIINP were significantly increased compared with the control group (Figure 4B-D) (P < 0.05); these changes were accompanied by increased expression of the profilin-1 protein and vascular remodeling in the thoracic aorta (Figure 4E).
We developed a profilin-1-specific shRNA to confirm the role of profilin-1 in the AGEs-mediated production of inflammatory mediators, and the transfection efficiency was confirmed by detecting the expression of the profilin-1 protein. As shown in Figure 4A and E, the profilin-1 shRNA successfully reduced the profilin-1 protein levels in thoracic aortae from AGEs-injected rats. Importantly, silencing of profilin-1 gene expression inhibited AGEs-induced ADMA, ICAM-1, and PIIINP release (Figure 4B-D) (P < 0.05) and attenuated AGEs-induced vascular remodeling of the thoracic aorta (Figure 4E). In contrast, the negative control shRNA had no such effect.
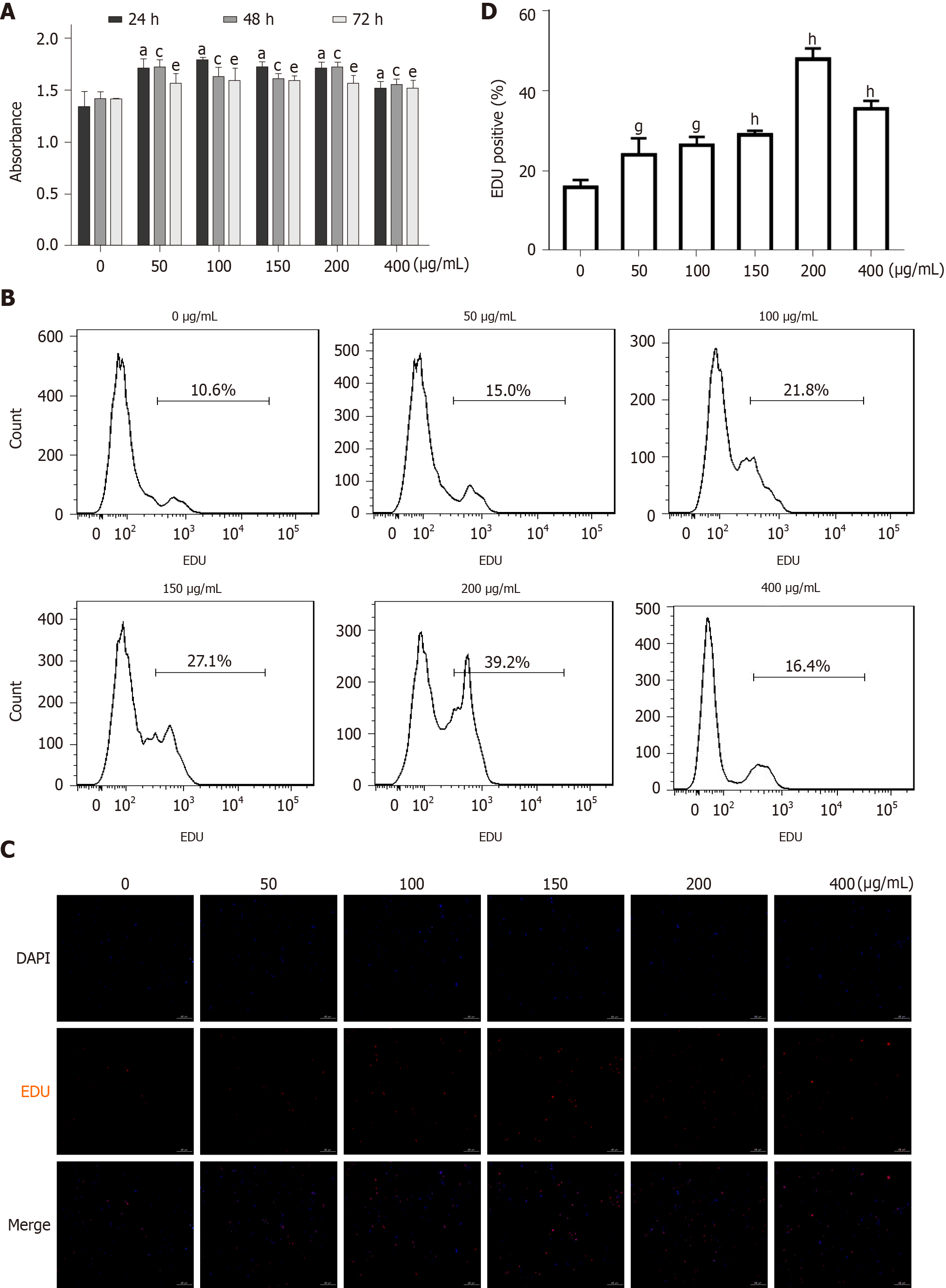
As mentioned above, vascular remodeling is an important pathological feature of diabetic macroangiopathy, in which endothelial dysfunction and VSMC proliferation plays a crucial role[28]. As the role of AGEs in endothelial cell injury has been proven[23], we focused on the effects of AGEs on VSMC proliferation in vitro. As shown in Figure 5A, after treatment with different concentrations of AGEs (0, 50, 100, 150, 200, or 400 μg/mL) for different times (0, 24, 48, or 72 h), the proliferation of RASMCs was significantly increased compared with that of the control group (Figure 5A, P < 0.05), and AGEs concentrations ranging from 100-200 μg/mL exerted the most robust effect on RASMC proliferation. EdU staining and flow cytometry produced similar results (Figure 5B-D).
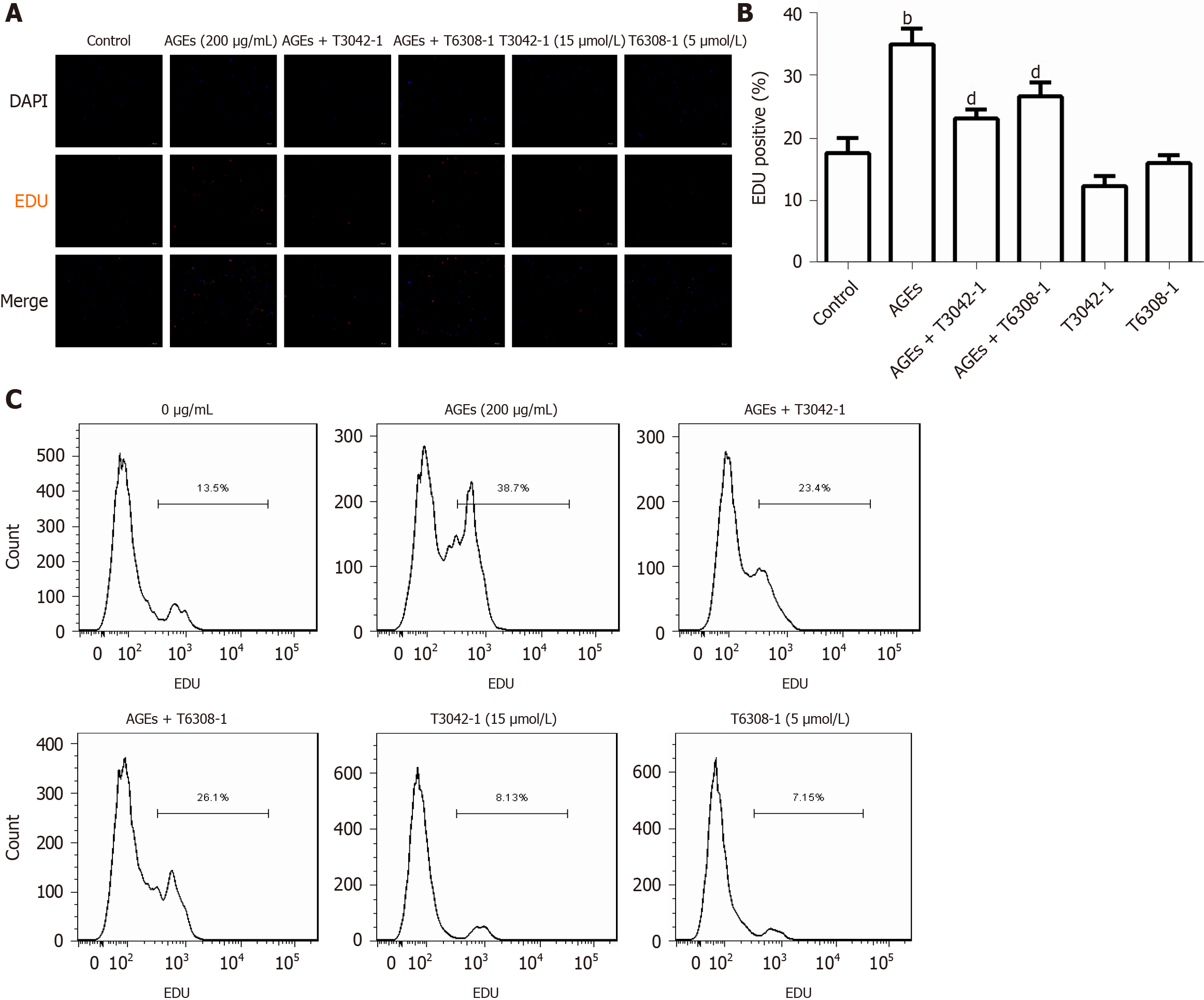
Previous studies have shown that the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway mediates the migration and proliferation of VSMCs and that AGEs induce apoptosis and inflammation by activating the JAK2/STAT3 pathway[29,30]. The expression of profilin-1, JAK2, and STAT3 in RASMCs exposed to medium alone or AGEs was examined to explore whether profilin-1 and the JAK2/STAT3 pathway are involved in AGEs-induced RASMC proliferation. Compared with the control group, treatment with AGEs at a dose of 100 or 150 μg/mL significantly upregulated the expression of both the profilin-1 mRNA and protein at different time points (P < 0.05, Figure 6A-E), which paralleled the increase in the proliferation of RASMCs induced by AGEs. AGEs (200 μg/mL) significantly upregulated the expression of STAT3 at both the mRNA and protein levels, but only upregulated the expression of the JAK2 protein (P < 0.05, Figure 6F and G). In addition, the AGEs-induced expression of the JAK2 and STAT3 proteins was effectively blocked by a JAK2 inhibitor (T3042-1) and/or STAT3 inhibitor (T6308-1) (P < 0.05, Figure 6H). Pretreatment with T3042-1 (15 μmol/L) or T6308-1 (5 μmol/L) significantly inhibited AGEs-induced RASMC proliferation, as detected by EdU staining and flow cytometry (P < 0.05). However, T3042-1 and T6308-1 alone had no effect on RASMC proliferation (Figure 7).
Epidemiological and clinical data suggest that diabetic macroangiopathy is the main cause of death in individuals with DM, and major biochemical pathways are involved in the development of diabetic macroangiopathy[27]. Among them, AGEs accumulation in tissues and the pivotal role of the AGEs-RAGE signaling pathway are most important for the central pathological features of DM, which rapidly accelerate AS[31,32]. High serum AGEs levels are associated with an increased incidence of CAD and a worse prognosis[7]. The present study also found significantly higher levels of RAGE in patients with CAD or CAD + DM than in healthy people, especially in patients with CAD + DM, and the levels of RAGE were positively correlated with Gensini scores. Thus, we focused on the relationship between AGEs and diabetic macroangiopathy characterized by abnormal hyperplasia of vascular smooth muscle, inflammation, and vascular remodeling.
Based on accumulating evidence, the increased proliferation and migration of VSMCs are hallmarks of vascular pathology in individuals with diabetic macroangiopathy[33]. A homogenous population of VSMCs is present within atherosclerotic plaques and responsible for early atherogenesis or preventing fibrous caps from rupturing in advanced plaques[34]. According to recent reports, dietary AGEs in patients with diabetes or an intraperitoneal injection of AGEs in rats causes an impairment in the vascular endothelium[35,36]. In an effort to understand the toxicity of AGEs in vasculopathy in vivo, a vascular injury model in rats was established by chronic tail vein injections of AGEs. In the AGEs injection group, thickened aortic walls, looser elastic fibers, broadened endothelial gaps, broken inner elastic tissue, and significant proliferation and disarrangement of VSMCs and luminal stenosis were observed. In addition, the injection of AGEs significantly increased the MT/LD and MA/LA ratios and the thickness of SMLs, which are considered indices of vascular remodeling[37], suggesting that exogenous AGEs directly induce vasculopathy and vascular remodeling in vivo. In vitro, incubation of VSMCs with AGEs significantly increased the proliferation of VSMCs and activated the proliferative JAK2/STAT3 signaling pathway. JAK2/STAT3 is an important signal transduction pathway that mediates VSMC proliferation and is involved in the process of AS in individuals with diabetes complicated with cardiovascular disease[38]. A previous study reported that AGEs exert proapoptotic and proinflammatory effects on mouse podocytes through the JAK2/STAT3 pathway[28]. Accordingly, we also found that exposure of VSMCs to AGEs induced the activation of JAK2/STAT3 signaling and that both a JAK2 inhibitor and STAT3 inhibitor attenuated the proliferation of VSMCs induced by AGEs. Therefore, AGEs induce VSMC proliferation that subsequently contributes to vascular remodeling under atherosclerotic conditions via the activation of the JAK2/STAT3 pathway. However, the precise mechanism of AGEs-induced vascular remodeling deserves further investigation.
Vascular remodeling is proven to be an adaptive process in response to chronic changes in hemodynamic conditions, and increased actin polymerization and stress fiber formation may play pivotal roles in the modulation of cellular morphology and function. Profilin-1, a multifunctional actin-binding protein, plays an essential role in regulating cytoskeletal rearrangement and redistribution by promoting vascular remodeling and inflammation. The most direct evidence is that overexpression of profilin-1 directly induces aortic remodeling in spontaneously hypertensive rats, manifesting as an increase in vessel size, wall thickness, and collagen content[19,39]. In fact, recent studies have shown that the biological effects of profilin-1 are far greater than those on regulating actin depolymerization and reorganization[40]. Thus, researchers have speculated that profilin-1 is involved in the vascular remodeling induced by AGEs. As shown in our study, the elevated levels of profilin-1 in patients with CAD or CAD + DM were positively correlated with coronary artery stenosis, and AGEs markedly upregulated the expression of profilin-1 in the aorta or cultured VSMCs concomitant with vascular remodeling and the hyperproliferation of VSMCs. Furthermore, profilin-1 silencing also downregulated AGEs-induced profilin-1 expression in aortic tissue and reversed AGEs-induced aortic vascular remodeling, suggesting that profilin-1 may participate in AGEs-induced vascular remodeling.
AS is a chronic inflammatory disorder that is involved in all stages of diabetic macroangiopathy, such as the formation, progression, and rupture of atherosclerotic plaques[41,42]. AGEs/RAGE activation may lead to increased production of proinflammatory and proatherogenic mediators that accelerate diabetes and its complication, AS[30,43]. Recently, the proinflammatory factors ADMA and ICAM-1 have been recognized as early markers of endothelial dysfunction with a high risk of cardiovascular events[44,45]. In patients with myocardial infarction, the levels of PIIINP potentially reflect the extent of extracellular matrix remodeling, and the increase in extracellular matrix contributes to intimal thickening and sclerosis[46,47]. Several studies have reported that AGEs markedly increase ADMA levels in endothelial cells by inducing local ROS production[48,49], and overexpression of profilin-1 upregulates the expression of ICAM-1[23]. In AGEs-injected rats, the production of ADMA, ICAM-1, and PIIINP was markedly increased and aortic vascular remodeling was induced in the present study, changes that were reversed by the inhibition of profilin-1 through shRNA transfection. Based on these results, profilin-1 may participate in AGEs-induced inflammation and vascular remodeling by activating the JAK2/STAT3 pathway, and profilin-1 is expected to become a promising therapeutic target for preventing diabetes-related vascular injury. However, the exact molecular mechanism of profilin-1 in AGEs-induced vasculopathy is worthy of further study.
Taken together, our results provide evidence that AGEs induce proatherogenic events such as VSMC proliferation, elevated expression of proatherogenic mediators, and vascular remodeling, which are attenuated by silencing profilin-1 gene expression. These data suggested for the first time that profilin-1 is involved in AGEs-induced aortic atheroma formation, indicating that increased profilin-1 expression in the aorta of patients with diabetes might trigger atherosclerosis-related events. Therefore, drugs targeting profilin-1 may become a potential therapeutic strategy for ameliorating AS secondary to diabetes.
The formation and accumulation of advanced glycation end products (AGEs) contribute to accelerated macrovascular complications, which are the leading cause of morbidity and mortality in patients with diabetes. Profilin-1 plays an important role in vascular remodeling and vascular inflammation.
Currently, few studies have investigated the role of profilin-1 in vasculopathy induced by AGEs, and the mechanism of profilin-1 in diabetic macroangiopathy remains unclear.
The aim of this study was to explore the potential role of profilin-1 in the pathogenesis of atherosclerosis induced by AGEs and to elucidate its probable mechanism.
Eighty-nine individuals undergoing coronary angiography were enrolled in the study. Plasma cytokine levels were detected using ELISA kits. Rat aortic vascular smooth muscle cells were incubated with different compounds for different times. Cell proliferation was determined by performing the MTT assay and EdU staining. An AGEs-induced vascular remodeling model was established in rats and histological and immunohistochemical analyses were performed. The mRNA and protein levels were detected using real-time PCR and Western blot analysis, respectively. In vivo, shRNA transfection was performed to verify the role of profilin-1 in AGEs-induced proatherogenic mediator release and aortic remodeling. Statistical analyses were performed using SPSS 22.0 software.
Compared with the control group, plasma levels of profilin-1 and receptor for AGEs (RAGE) were significantly increased in patients with coronary artery disease, especially in those complicated with diabetes mellitus (P < 0.01). The levels of profilin-1 were positively correlated with the levels of RAGE (P < 0.01); additionally, the levels of both molecules were positively associated with the degree of coronary artery stenosis (P < 0.01). In vivo, tail vein injections of AGEs induced the release of proatherogenic mediators, such as asymmetric dimethylarginine, intercellular adhesion molecule-1, and the N-terminus of procollagen III peptide, concomitant with apparent aortic morphological changes and significantly upregulated expression of the profilin-1 mRNA and protein in the thoracic aorta (P < 0.05 and P < 0.01). Downregulation of profilin-1 expression with an shRNA significantly attenuated AGEs-induced proatherogenic mediator release (P < 0.05) and aortic remodeling. In vitro, incubation of vascular smooth muscle cells (VSMCs) with AGEs significantly promoted cell proliferation and upregulated the expression of the profilin-1 mRNA and protein (P < 0.05). AGEs (200 μg/mL, 24 h) significantly upregulated the expression of the signal transducer and activator of transcription 3 (STAT3) mRNA and protein and the Janus kinase 2 (JAK2) protein, which were blocked by a JAK2 inhibitor (T3042-1) and/or STAT3 inhibitor (T6308-1) (P < 0.05). In addition, pretreatment with T3042-1 or T6308-1 significantly inhibited AGEs-induced rat aortic vascular smooth muscle cell proliferation (P < 0.05).
The present study proved that AGEs induce proatherogenic events such as VSMC proliferation, proatherogenic mediator expression, and vascular remodeling, which are attenuated by silencing profilin-1 gene expression.
Profilin-1 is expected to be a promising therapeutic target for the prevention of diabetes mellitus associated with vascular damage. Further studies are needed to develop a targeted drug against profilin-1 and elucidate the exact molecular mechanism of profilin-1 in AGEs-induced vasculopathy.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhong G S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | Zhang S, Xu W, Gao P, Chen W, Zhou Q. Construction of dual nanomedicines for the imaging and alleviation of atherosclerosis. Artif Cells Nanomed Biotechnol. 2020;48:169-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3867] [Cited by in RCA: 3436] [Article Influence: 171.8] [Reference Citation Analysis (0)] |
| 3. | Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5314] [Cited by in RCA: 5281] [Article Influence: 310.6] [Reference Citation Analysis (0)] |
| 4. | Hajizadeh-Sharafabad F, Sahebkar A, Zabetian-Targhi F, Maleki V. The impact of resveratrol on toxicity and related complications of advanced glycation end products: A systematic review. Biofactors. 2019;45:651-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Kizer JR, Benkeser D, Arnold AM, Ix JH, Mukamal KJ, Djousse L, Tracy RP, Siscovick DS, Psaty BM, Zieman SJ. Advanced glycation/glycoxidation endproduct carboxymethyl-lysine and incidence of coronary heart disease and stroke in older adults. Atherosclerosis. 2014;235:116-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Koska J, Saremi A, Howell S, Bahn G, De Courten B, Ginsberg H, Beisswenger PJ, Reaven PD; VADT Investigators. Advanced Glycation End Products, Oxidation Products, and Incident Cardiovascular Events in Patients With Type 2 Diabetes. Diabetes Care. 2018;41:570-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 7. | Wang X, Xu T, Mungun D, Zhou C, Zha Z, Lu M, Fen C, Guo Y. The Relationship between Plasma Soluble Receptor for Advanced Glycation End Products and Coronary Artery Disease. Dis Markers. 2019;2019:4528382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Deluyker D, Evens L, Bito V. Advanced glycation end products (AGEs) and cardiovascular dysfunction: focus on high molecular weight AGEs. Amino Acids. 2017;49:1535-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 9. | Lee TW, Kao YH, Chen YJ, Chao TF, Lee TI. Therapeutic potential of vitamin D in AGE/RAGE-related cardiovascular diseases. Cell Mol Life Sci. 2019;76:4103-4115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Fukami K, Yamagishi S, Okuda S. Role of AGEs-RAGE system in cardiovascular disease. Curr Pharm Des. 2014;20:2395-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Alkam D, Feldman EZ, Singh A, Kiaei M. Profilin1 biology and its mutation, actin(g) in disease. Cell Mol Life Sci. 2017;74:967-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Yang D, Wang Y, Jiang M, Deng X, Pei Z, Li F, Xia K, Zhu L, Yang T, Chen M. Downregulation of Profilin-1 Expression Attenuates Cardiomyocytes Hypertrophy and Apoptosis Induced by Advanced Glycation End Products in H9c2 Cells. Biomed Res Int. 2017;2017:9716087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Yang D, Liu W, Ma L, Wang Y, Ma J, Jiang M, Deng X, Huang F, Yang T, Chen M. Profilin1 contributes to cardiac injury induced by advanced glycation endproducts in rats. Mol Med Rep. 2017;16:6634-6641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Li X, Liu J, Chen B, Fan L. A Positive Feedback Loop of Profilin-1 and RhoA/ROCK1 Promotes Endothelial Dysfunction and Oxidative Stress. Oxid Med Cell Longev. 2018;2018:4169575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Romeo GR, Kazlauskas A. Oxysterol and diabetes activate STAT3 and control endothelial expression of profilin-1 via OSBP1. J Biol Chem. 2008;283:9595-9605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Song B, Jin H, Yu X, Zhang Z, Yu H, Ye J, Xu Y, Zhou T, Oudit GY, Ye JY, Chen C, Gao P, Zhu D, Penninger JM, Zhong JC. Angiotensin-converting enzyme 2 attenuates oxidative stress and VSMC proliferation via the JAK2/STAT3/SOCS3 and profilin-1/MAPK signaling pathways. Regul Pept. 2013;185:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Cheng JF, Ni GH, Chen MF, Li YJ, Wang YJ, Wang CL, Yuan Q, Shi RZ, Hu CP, Yang TL. Involvement of profilin-1 in angiotensin II-induced vascular smooth muscle cell proliferation. Vascul Pharmacol. 2011;55:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Katakami N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J Atheroscler Thromb. 2018;25:27-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 306] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 19. | Wang Y, Zhang J, Gao H, Zhao S, Ji X, Liu X, You B, Li X, Qiu J. Profilin-1 promotes the development of hypertension-induced artery remodeling. J Histochem Cytochem. 2014;62:298-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Horrevoets AJ. Profilin-1: an unexpected molecule linking vascular inflammation to the actin cytoskeleton. Circ Res. 2007;101:328-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Romeo GR, Moulton KS, Kazlauskas A. Attenuated expression of profilin-1 confers protection from atherosclerosis in the LDL receptor null mouse. Circ Res. 2007;101:357-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Romeo GR, Pae M, Eberlé D, Lee J, Shoelson SE. Profilin-1 haploinsufficiency protects against obesity-associated glucose intolerance and preserves adipose tissue immune homeostasis. Diabetes. 2013;62:3718-3726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Li Z, Zhong Q, Yang T, Xie X, Chen M. The role of profilin-1 in endothelial cell injury induced by advanced glycation end products (AGEs). Cardiovasc Diabetol. 2013;12:141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Introduction: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42:S1-S2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 25. | Yao W, Ji S, Qin Y, Yang J, Xu J, Zhang B, Xu W, Liu J, Shi S, Liu L, Liu C, Long J, Ni Q, Li M, Yu X. Profilin-1 suppresses tumorigenicity in pancreatic cancer through regulation of the SIRT3-HIF1α axis. Mol Cancer. 2014;13:187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Blackwell S, O'Reilly DS, Talwar DK. HPLC analysis of asymmetric dimethylarginine (ADMA) and related arginine metabolites in human plasma using a novel non-endogenous internal standard. Clin Chim Acta. 2009;401:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Yuan T, Yang T, Chen H, Fu D, Hu Y, Wang J, Yuan Q, Yu H, Xu W, Xie X. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 2019;20:247-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 468] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 28. | Li M, Qian M, Kyler K, Xu J. Endothelial-vascular smooth muscle cells interactions in atherosclerosis. Front Cardiovasc Med. 2018;5:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 29. | Yu J, Wu H, Liu ZY, Zhu Q, Shan C, Zhang KQ. Advanced glycation end products induce the apoptosis of and inflammation in mouse podocytes through CXCL9-mediated JAK2/STAT3 pathway activation. Int J Mol Med. 2017;40:1185-1193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Yu L, Zhang Y, Zhang H, Li Y. SOCS3 overexpression inhibits advanced glycation end product-induced EMT in proximal tubule epithelial cells. Exp Ther Med. 2017;13:3109-3115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Fukushima Y, Daida H, Morimoto T, Kasai T, Miyauchi K, Yamagishi S, Takeuchi M, Hiro T, Kimura T, Nakagawa Y, Yamagishi M, Ozaki Y, Matsuzaki M; JAPAN-ACS Investigators. Relationship between advanced glycation end products and plaque progression in patients with acute coronary syndrome: the JAPAN-ACS sub-study. Cardiovasc Diabetol. 2013;12:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Jensen LJ, Flyvbjerg A, Bjerre M. Soluble Receptor for Advanced Glycation End Product: A Biomarker for Acute Coronary Syndrome. Biomed Res Int. 2015;2015:815942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Silvério de Barros R, Dias GS, Paula do Rosario A, Paladino FV, Lopes GH, Campos AH. Gremlin-1 potentiates the dedifferentiation of VSMC in early stages of atherosclerosis. Differentiation. 2019;109:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Bennett MR, Sinha S, Owens GK. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res. 2016;118:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1041] [Cited by in RCA: 1593] [Article Influence: 177.0] [Reference Citation Analysis (0)] |
| 35. | Negrean M, Stirban A, Stratmann B, Gawlowski T, Horstmann T, Götting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri J, Vlassara H, Tschoepe D. Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 36. | Soro-Paavonen A, Zhang WZ, Venardos K, Coughlan MT, Harris E, Tong DC, Brasacchio D, Paavonen K, Chin-Dusting J, Cooper ME, Kaye D, Thomas MC, Forbes JM. Advanced glycation end-products induce vascular dysfunction via resistance to nitric oxide and suppression of endothelial nitric oxide synthase. J Hypertens. 2010;28:780-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 37. | Li Q, Wo D, Huang Y, Yu N, Zeng J, Chen H, Wang H, Bao L, Lin S, Chu J, Peng J. Alkaloids from Nelumbinis Plumula (AFNP) ameliorate aortic remodeling via RhoA/ROCK pathway. Biomed Pharmacother. 2019;112:108651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Zhou W, Ye SD, Wang W. Elevated retinol binding protein 4 levels are associated with atherosclerosis in diabetic rats via JAK2/STAT3 signaling pathway. World J Diabetes. 2021;12:466-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Elnakish MT, Hassanain HH, Janssen PM. Vascular remodeling-associated hypertension leads to left ventricular hypertrophy and contractile dysfunction in profilin-1 transgenic mice. J Cardiovasc Pharmacol. 2012;60:544-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Söderberg E, Hessle V, von Euler A, Visa N. Profilin is associated with transcriptionally active genes. Nucleus. 2012;3:290-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Nguyen MT, Fernando S, Schwarz N, Tan JT, Bursill CA, Psaltis PJ. Inflammation as a Therapeutic Target in Atherosclerosis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 42. | Marchio P, Guerra-Ojeda S, Vila JM, Aldasoro M, Victor VM, Mauricio MD. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid Med Cell Longev. 2019;2019:8563845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 419] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 43. | Prasad K, Tiwari S. Therapeutic Interventions for Advanced Glycation-End Products and its Receptor- Mediated Cardiovascular Disease. Curr Pharm Des. 2017;23:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 44. | Gursel O, Tapan S, Sertoglu E, Taşçılar E, Eker I, Ileri T, Uysal Z, Kurekci AE. Elevated plasma asymmetric dimethylarginine levels in children with beta-thalassemia major may be an early marker for endothelial dysfunction. Hematology. 2018;23:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 45. | Dzikowska-Diduch O, Domienik-Karłowicz J, Górska E, Demkow U, Pruszczyk P, Kostrubiec M. E-selectin and sICAM-1, biomarkers of endothelial function, predict recurrence of venous thromboembolism. Thromb Res. 2017;157:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Madahar P, Duprez DA, Podolanczuk AJ, Bernstein EJ, Kawut SM, Raghu G, Barr RG, Gross MD, Jacobs DR Jr, Lederer DJ. Collagen biomarkers and subclinical interstitial lung disease: The Multi-Ethnic Study of Atherosclerosis. Respir Med. 2018;140:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Stienen S, Rossignol P, Barros A, Girerd N, Pitt B, Zannad F, Ferreira JP. Determinants of anti-fibrotic response to mineralocorticoid receptor antagonist therapy: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) and Early Eplerenone Treatment in Patients with Acute ST-elevation Myocardial Infarction without Heart Failure (REMINDER) trials. Clin Res Cardiol. 2020;109:194-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Ojima A, Ishibashi Y, Matsui T, Maeda S, Nishino Y, Takeuchi M, Fukami K, Yamagishi S. Glucagon-like peptide-1 receptor agonist inhibits asymmetric dimethylarginine generation in the kidney of streptozotocin-induced diabetic rats by blocking advanced glycation end product-induced protein arginine methyltranferase-1 expression. Am J Pathol. 2013;182:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 49. | Ando R, Ueda S, Yamagishi S, Miyazaki H, Kaida Y, Kaifu K, Yokoro M, Nakayama Y, Obara N, Fukami K, Takeuchi M, Okuda S. Involvement of advanced glycation end product-induced asymmetric dimethylarginine generation in endothelial dysfunction. Diab Vasc Dis Res. 2013;10:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |