Published online Oct 15, 2021. doi: 10.4239/wjd.v12.i10.1789
Peer-review started: April 27, 2021
First decision: May 12, 2021
Revised: May 25, 2021
Accepted: September 14, 2021
Article in press: September 14, 2021
Published online: October 15, 2021
Processing time: 169 Days and 3.1 Hours
Previous studies have shown that diabetes mellitus is a common comorbidity of coronavirus disease 2019 (COVID-19), but the effects of diabetes or anti-diabetic medication on the mortality of COVID-19 have not been well described.
To investigate the outcome of different statuses (with or without comorbidity) and anti-diabetic medication use before admission of diabetic after COVID-19.
In this multicenter and retrospective study, we enrolled 1422 consecutive hospitalized patients from January 21, 2020, to March 25, 2020, at six hospitals in Hubei Province, China. The primary endpoint was in-hospital mortality. Epidemiological material, demographic information, clinical data, laboratory parameters, radiographic characteristics, treatment and outcome were extracted from electronic medical records using a standardized data collection form. Most of the laboratory data except fasting plasma glucose (FPG) were obtained in first hospitalization, and FPG was collected in the next day morning. Major clinical symptoms, vital signs at admission and comorbidities were collected. The treatment data included not only COVID-19 but also diabetes mellitus. The duration from the onset of symptoms to admission, illness severity, intensive care unit (ICU) admission, and length of hospital stay were also recorded. All data were checked by a team of sophisticated physicians.
Patients with diabetes were 10 years older than non-diabetic patients [(39 - 64) vs (56 - 70), P < 0.001] and had a higher prevalence of comorbidities such as hypertension (55.5% vs 21.4%, P < 0.001), coronary heart disease (CHD) (9.9% vs 3.5%, P < 0.001), cerebrovascular disease (CVD) (3% vs 2.2%, P < 0.001), and chronic kidney disease (CKD) (4.7% vs 1.5%, P = 0.007). Mortality (13.6% vs 7.2%, P = 0.003) was more prevalent among the diabetes group. Further analysis revealed that patients with diabetes who took acarbose had a lower mortality rate (2.2% vs 26.1, P < 0.01). Multivariable Cox regression showed that male sex [hazard ratio (HR) 2.59 (1.68 - 3.99), P < 0.001], hypertension [HR 1.75 (1.18 - 2.60), P = 0.006), CKD [HR 4.55 (2.52-8.20), P < 0.001], CVD [HR 2.35 (1.27 - 4.33), P = 0.006], and age were risk factors for the COVID-19 mortality. Higher HRs were noted in those aged ≥ 65 (HR 11.8 [4.6 - 30.2], P < 0.001) vs 50-64 years (HR 5.86 [2.27 - 15.12], P < 0.001). The survival curve revealed that, compared with the diabetes only group, the mortality was increased in the diabetes with comorbidities group (P = 0.009) but was not significantly different from the non-comorbidity group (P = 0.59).
Patients with diabetes had worse outcomes when suffering from COVID-19; however, the outcome was not associated with diabetes itself but with comorbidities. Furthermore, acarbose could reduce the mortality in diabetic.
Core Tip: Previous studies have shown that diabetes mellitus is a common comorbidity of coronavirus disease 2019 (COVID-19), but the effects of diabetes or antidiabetic medication on the mortality of COVID-19 have not been well described. This retrospective and multiple-center study investigate the outcome of different statuses (with or without comorbidity) and antidiabetic medication use before admission of diabetic after COVID-19.
- Citation: Luo SK, Hu WH, Lu ZJ, Li C, Fan YM, Chen QJ, Chen ZS, Ye JF, Chen SY, Tong JL, Wang LL, Mei J, Lu HY. Diabetes patients with comorbidities had unfavorable outcomes following COVID-19: A retrospective study. World J Diabetes 2021; 12(10): 1789-1808
- URL: https://www.wjgnet.com/1948-9358/full/v12/i10/1789.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i10.1789
Coronavirus disease 2019 (COVID-19) has become an ongoing pandemic and has caused considerable mortality worldwide[1]. Diabetes is a common comorbidity, especially in elderly patients, but the effects of diabetes or anti-diabetic medication on the severity and mortality of COVID-19 have not been well described. As of April 27, 2021, nearly 150 million COVID-19 cases had been confirmed around the world, and more than 3 million patients died of COVID-19 (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). Well-controlled blood glucose (3.9-10.0 mmol/L) in preexisting diabetes was associated with a significant reduction in the composite adverse outcomes and death of patients with COVID-19[2]. Patients with diabetes often have several comorbidities, and previous research has revealed that hypertension, chronic obstructive pulmonary disease (COPD), chronic kidney disease (CKD), cardiovascular disease and cerebrovascular disease (CVD) are also associated with worse outcomes in patients suffering from COVID-19[3-6]. However, few studies have described the outcome of different comorbidity statuses of patients with diabetes after infection with COVID-19. In addition, few studies have focused on whether anti-diabetic medication would influence the outcome of patients with preexisting diabetes who suffer from COVID-19. Considering this, we performed a multicenter study to investigate the outcome of different statuses (with or without comorbidity) and anti-diabetic medication before admission of patients with diabetes with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
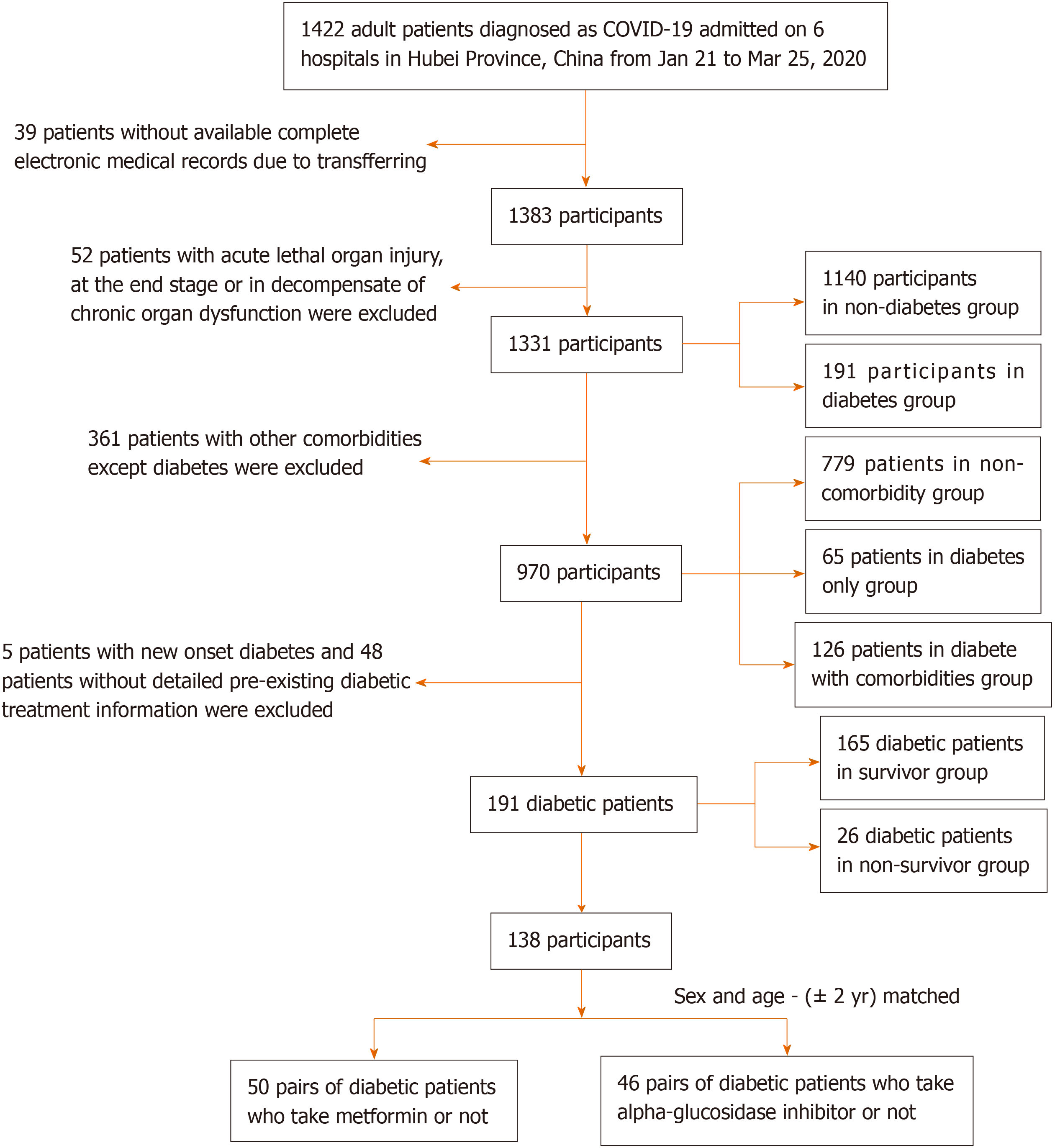
This is a multicenter, observational, retrospective, real-world study that included adult inpatients from six designated tertiary centers (Supplementary Table 1) between January 21 and March 25, 2020. A total of 1422 patients with COVID-19 were screened for this study (Figure 1). All patients were diagnosed with COVID-19 in accordance with WHO interim guidance.
Epidemiological material, demographic information, clinical data, laboratory parameters, radiographic characteristics, treatment and outcome were extracted from electronic medical records using a standardized data collection form. Most of the laboratory data except fasting plasma glucose (FPG) were obtained in first hospitalization, and FPG was collected in the next day morning. Major clinical symptoms, vital signs at admission and comorbidities were collected. The treatment data included not only COVID-19 but also diabetes mellitus. The duration from the onset of symptoms to admission, illness severity, intensive care unit (ICU) admission, and length of hospital stay were also recorded. All data were checked by a team of sophisticated physicians.
Diabetes was defined as a history record of diabetes and the use of anti-diabetic medication; otherwise, newly diagnosed diabetes was based on the level of fasting plasma glucose (FPG) (≥ 7.0 mmol/L), random plasma glucose (≥ 11.1 mmol/L), glycosylated hemoglobin (HbA1c) ≥ 6.5% and classic symptoms of hyperglycemia during hospital stay (as the oral glucose tolerance test may lead to hyperglycemia and then to worsening of a COVID-19 patient’s illness, it was not used for diagnosis of diabetes in our study[7]). Hypertension was defined by a history of hypertension, the use of anti-hypertensive drugs, or the National Heart Lung and Blood Institute criteria[8]. Coronary heart disease was defined by a history of coronary heart disease. CVD was defined by a history of CVD. ARDS was defined according to the Berlin definition[9]. Acute kidney injury (AKI) was diagnosed according to the KDIGO (Kidney Disease: Improving Global Outcomes) clinical practice guidelines[10]. Acute cardiac injury (ACI) was reported if serum levels of myocardial injury biomarkers were higher than the upper limit of normal[2]. The criteria for classification of COVID-19 severity were according to the Diagnosis and Treatment Protocol for COVID-19 (Trial Version 8)[11]. We divided the patients into two groups: the non-severe group (mild and general types) and the severe group (severe and critical types).
The primary outcome was all-cause mortality after admission. Secondary outcomes were ICU admission and incidence of SARS-CoV-2-related complications, including ARDS, AKI, ACI, secondary infection, shock and hypoproteinemia.
Continuous variables were described as the mean ± SD or median (IQR). Categorical variables were calculated as frequencies and percentages with available data. The differences in continuous variables among groups were assessed using the independent sample t-test or one-way ANOVA for normally distributed continuous variables or the Mann-Whitney U test or Kruskal-Wallis H test for skewed continuous variables. Pearson’s χ2 test and Fisher’s exact test were performed for unordered categorical variables. The Mann-Whitney U test or the Kruskal-Wallis H test was used for ordered categorical variables. To explore the risk factors associated with mortality, multivariable Cox regression models were performed. The Kaplan-Meier plot was performed to compare the survival probability for the diabetes and non-diabetes groups and among the patients with no comorbidities, only diabetes and diabetes with comorbidities by log-rank test. Additionally, we did not process the missing data. The statistical analyses were conducted with SPSS (version 25.0). A two-sided P value less than 0.05 was considered statistically significant. The statistical methods of this study were reviewed by Yameng Fan from Wuhan University.
The characteristics of this study population at baseline are given in Table 1. The median age was 54 years old (39-64) and 64 years old (56-70) in the non-diabetes and diabetes groups, respectively. Comorbidities such as hypertension (55.5% vs 21.4%), coronary heart disease (9.9% vs 3.5%), CVD (7.3% vs 2.2%), and CKD (4.7% vs 1.5%) were significantly more prevalent in the diabetes group. Mean systolic blood pressure (SBP) was higher in the diabetes group. Moreover, decreased blood oxygen saturation (lower than 93%) occurred more frequently in the diabetes group vs the non-diabetes group (19.8% vs 19.6%) on admission. Chest CT scan revealed that the incidence of bilateral lesions was higher (94% vs 80.1%) in the diabetes group than in the non-diabetes group.
| Total (n = 1331) | Non-diabetes (n = 1140) | Diabetes (n = 191) | P1 value | Non-comorbidity (n = 779) | Diabetes only (n = 65) | Diabetes with comorbidities (n = 126) | P2 value | |
| Demographic | ||||||||
| Male | 673 (50.6) | 565 (49.6) | 108 (56.5) | 0.074 | 369 (47.4) | 40 (61.5) | 68 (54.0) | 0.046 |
| Age, yr | 56.0 (42.0-65.0) | 54.0 (39.0-64.0) | 64.0 (56.0-70.0) | < 0.001 | 48.0c (36.0-60.0) | 57.0 (50.0-64.0) | 67.0c (59.0-72.0) | < 0.001 |
| 18-49 | 500(37.6) | 477 (41.8) | 23 (12.0) | <0.001 | 415 (53.3)c | 16 (24.6) | 7 (5.6)c | < 0.001d |
| 50-64 | 458 (34.4) | 382 (33.5) | 76 (39.8) | 253 (32.5) | 34 (52.3) | 42 (33.3) | ||
| ≥ 65 | 373 (28.0) | 281 (24.6) | 92 (48.2) | 111 (14.2) | 15 (23.1) | 77 (61.1) | ||
| Wuhan exposure | 1190 (89.4) | 1008 (88.4) | 182 (95.3) | 0.004 | 686 (88.3) | 61 (95.3) | 120 (95.2) | 0.018 |
| Current smoking | 107 (8.1) | 93 (8.2) | 14 (7.4) | 0.736 | 55 (7.2) | 3 (4.7) | 11 (8.9) | 0.149 |
| Onset of symptom, d | 8.0 (5.0-14.0) | 8.0 (5.0-14.0) | 10.0 (6.0-13.0) | 0.217 | 8.0 (4.8-14.0) | 10.0 (6.5-16.5) | 10.0 (5.8-12.0) | 0.109 |
| Symptoms | ||||||||
| Fever | 955 (71.8) | 823 (72.2) | 132 (69.1) | 0.381 | 570 (73.2) | 46 (70.8) | 86 (68.3) | 0.496 |
| Dyspnea | 270 (20.3) | 227 (19.9) | 43 (22.5) | 0.408 | 135 (17.3) | 9 (13.8) | 34 (27.0) | 0.021 |
| Cough | 777 (58.4) | 660 (57.9) | 117 (61.3) | 0.383 | 433 (55.6) | 46 (70.8) | 71 (56.7) | 0.060 |
| Sputum production | 138 (10.4) | 126 (11.1) | 12 (6.3) | 0.045 | 84 (10.8) | 4 (6.2) | 8 (6.3) | 0.175 |
| Hemoptysis | 3 (0.2) | 3 (0.3) | 0 (0.0) | 1.000 | 1 (0.1) | 0 (0.0) | 0 (0.0) | 0.885 |
| Fatigue | 362 (27.2) | 306 (26.8) | 56 (29.3) | 0.476 | 212 (27.2) | 16 (24.6) | 40 (31.7) | 0.489 |
| Headache | 47 (3.5) | 44 (3.9) | 3 (1.6) | 0.169 | 29 (3.7) | 3 (4.6) | 0 (0.0) | 0.010 |
| Nausea or vomiting | 44 (3.3) | 39 (3.4) | 5 (2.6) | 0.566 | 25 (3.2) | 2 (3.1) | 3 (2.4) | 0.939 |
| Diarrhea | 112 (8.4) | 97 (8.5) | 15 (7.9) | 0.763 | 63 (8.1) | 9 (13.8) | 6 (4.8) | 0.091 |
| Temperature, ℃ | 36.8 (36.5-37.5) | 36.8 (36.5-37.5) | 36.7 (36.4-37.4) | 0.018 | 36.8 (36.5-37.5) | 36.8 (36.5-37.6) | 36.6 (36.4-37.3) | 0.018 |
| ≥ 39 | 30 (2.4) | 25 (2.3) | 5 (2.7) | 0.980 | 16 (2.2) | 2 (3.1) | 3 (2.4) | 0.889 |
| Pulse ≥ 100 beats per min | 244 (18.5) | 209 (18.5) | 35 (18.3) | 0.955 | 125 (16.1) | 12 (18.5) | 23 (18.30) | 0.761 |
| Blood oxygen saturation < 93% | 124 (11.1) | 92 (9.6) | 32 (19.8) | < 0.001 | 43 (6.5) | 7 (12.5) | 25 (23.6) | < 0.001 |
| Respiratory rate > 24 breaths/min | 71 (5.4) | 56 (5.0) | 15 (7.9) | 0.105 | 27 (3.5) | 2 (3.1) | 13 (10.3) | 0.002 |
| Mean systolic blood pressure, mmHg | 125 (120-135) | 124 (119-135) | 128 (120-140) | 0.001 | 121 (118-131) | 127 (120-133) | 130 (120-140) | < 0.001 |
| Mean diastolic blood pressure, mmHg | 80.0 (74.0-85.0) | 80.0 (74.0-85.0) | 80.0 (74.0-85.0) | 0.777 | 80.0 (73.0-83.0) | 80.0 (72.5-85.0) | 80.0 (74.0-84.3) | 0.550 |
| Radiological findings | ||||||||
| Ground glass opacity | 294 (22.1) | 265 (23.2) | 29 (15.2) | 0.013 | 195 (25.0) | 9 (13.8) | 20 (15.9) | 0.014 |
| Bilateral patchy shadowing | 813 (61.1) | 687 (60.3) | 126 (66.0) | 0.134 | 62 (8.0) | 5 (7.7) | 4 (3.2) | 0.107 |
| Bilateral lesions | 962 (82.1) | 805 (80.1) | 157 (94.0) | < 0.001 | 524 (76.2)a | 53 (91.4) | 104 (95.4) | < 0.001 |
| Comorbidity | ||||||||
| Hypertension | 350 (26.3) | 244 (21.4) | 106 (55.5) | < 0.001 | - | - | - | - |
| CHD | 59 (4.4) | 40 (3.5) | 19 (9.9) | < 0.001 | - | - | - | - |
| Chronic liver disease | 20 (1.5) | 18 (1.6) | 2 (1.0) | 0.812 | - | - | - | - |
| CVD | 39 (2.9) | 25 (2.2) | 14 (7.3) | < 0.001 | - | - | - | - |
| CKD | 26 (2.0) | 17 (1.5) | 9 (4.7) | 0.007 | - | - | - | - |
| COPD | 10 (0.8) | 10 (0.9) | 0 (0.0) | 0.397 | - | - | - | - |
There were numerous differences in laboratory results between the diabetes group and the non-diabetes group with COVID-19 (Table 2). FPG levels were significantly higher in the diabetes group than in the non-diabetes group, as expected, with higher levels of HbA1c. Patients with diabetes had a higher white blood cell count (WBC), neutrophil count (NEU), neutrophil to lymphocyte ratio (NLR), and C-reactive protein (CRP) and a lower lymphocyte count (LY) than the non-diabetic group. These results revealed that diabetes represented more severe inflammation. The percentage of high levels of prothrombin time (PT) and D-dimer among the diabetes group was higher than that among the non-diabetes group. The serum level of albumin (ALB) was lower in the diabetes group than in the non-diabetes group. Meanwhile, urea nitrogen (BUN), a marker of kidney function, was higher in the diabetes group. Non-diabetes participants had significantly lower serum levels of lactate dehydrogenase. Compared with the non-diabetes group, the diabetes group had higher levels of total cholesterol (TCH) and lower high-density lipoprotein cholesterol (HDL-C).
| Total (n = 1331) | Non-diabetes (n = 1140) | Diabetes (n = 191) | P1 value | Non-comorbidity (n = 779) | Diabetes only (n = 65) | Diabetes with comorbidities (n = 126) | P2 value | |
| WBC, × 109/L | 5.42 (4.18-7.10) | 5.35 (4.10-6.95) | 5.93 (4.49-7.53) | 0.003 | 5.28 (4.00-6.77) | 6.11 (4.27-7.68) | 5.85 (4.57-7.32) | 0.001 |
| NEUT, × 109/L | 3.58 (2.53-5.12) | 3.45 (2.46-5.07) | 4.25 (3.13-5.37) | < 0.001 | 3.29 (2.33-4.64) | 4.16 (2.67-5.40) | 4.37 (3.20-5.29) | < 0.001 |
| LY, × 109/L | 1.15 (0.78-1.59) | 1.17 (0.80-1.61) | 1.04 (0.72-1.43) | 0.015 | 1.25 (0.86-1.65) | 1.27 (0.84-1.73) | 0.93 (0.68-1.33)b | < 0.001 |
| NLR | 2.95 (1.97-5.26) | 2.79 (1.88-4.93) | 3.84 (2.45-6.37) | < 0.001 | 2.54 (1.79-4.36) | 3.15 (2.08-5.06) | 4.29 (2.62-7.30)a | < 0.001 |
| Hb, g/L | 130 (118-140) | 130 (118-140) | 120 (117-140) | 0.195 | 131 ± 16.3 | 132 ± 14.4 | 125 ± 17.6b | < 0.001 |
| PLT, × 109/L | 196 (150-251) | 196 (151-251) | 196 (147-255) | 0.714 | 196 (152-242) | 197 (147-265) | 196 (146-255) | 0.974 |
| PCT, ng/mL | ||||||||
| < 0.5 | 981 (94.4) | 838 (94.4) | 143 (94.7) | 0.869 | 585 (97.3) | 53 (100) | 90 (91.8) | 0.006 |
| ≥ 0.5 | 58 (5.6) | 50 (5.6) | 8 (5.3) | 16 (2.7) | 0 (0.0) | 8 (8.2) | ||
| CRP | 10.9 (1.7-46.7) | 9.1 (1.4-39.0) | 29.8 (5.3-75.7) | < 0.001 | 6.11 (1.0-27.7)a | 13.2 (3.0-61.5) | 39.9 (6.6-77.7)a | < 0.001 |
| IL-6, pg/mL | 2.77 (1.5-14.09) | 2.73 (1.5-13.5) | 3.09 (1.5-20.4) | 0.471 | 1.80 (1.50-6.27) | 2.32 (1.50-5.06) | 4.01 (2.72-28.56) | 0.008 |
| PT, s | 13.0 (11.3-14.9) | 12.9 (11.3-14.6) | 14.3 (11.9-15.5) | < 0.001 | 12.80 (11.20-14.30) | 13.70 (10.80-15.05) | 14.50 (12.35-16.03) | < 0.001 |
| < 16 | 830 (85.7) | 712 (87.1) | 118 (78.1) | 0.004 | 493 (87.7) | 41 (83.7) | 75 (73.5) | 0.001 |
| ≥ 16 | 138 (14.3) | 105 (12.9) | 33 (21.9) | 69 (12.3) | 8 (16.3) | 27 (26.5) | ||
| D-dimer, mg/L | 0.49 (0.26-1.14) | 0.46 (0.25-1.10) | 0.69 (0.35-1.35) | < 0.001 | 0.38 (0.23-0.80) | 0.46 (0.26-0.91) | 0.83 (0.46-1.94)b | < 0.001 |
| ≤ 0.5 | 555 (52.5) | 497 (55.0) | 58 (37.9) | < 0.001 | 386 (63.4) | 27 (50.9) | 31 (31.0)b | < 0.0013 |
| > 0.5 to ≤ 1.0 | 209 (19.8) | 163 (18.0) | 46 (30.1) | 100 (16.4) | 18 (34.0) | 28 (28.0) | ||
| > 1.0 | 293 (27.7) | 244 (27.0) | 49 (32.0) | 123 (20.2) | 8 (15.1) | 41 (41.0) | ||
| ALB, g/L | 38.1 ± 5.8 | 38.5 ± 5.7 | 35.7 ± 5.5 | < 0.001 | 39.3 ± 5.9c | 36.5 ± 6.4 | 35.3 ± 5.0 | < 0.001 |
| ALT, U/L | 23.1 (14.2-39.0) | 23.3 (14.0-40.0) | 23.0 (16.0-34.0) | 0.844 | 22.0 (13.8-39.0) | 21.0 (16.3-33.5) | 24.0 (15.9-34.0) | 0.801 |
| AST, U/L | 28.8 (22.0-40.4) | 28.8 (22.0-40.0) | 29.0 (20.0-41.0) | 0.583 | 27.0 (21.0-38.0) | 26.0 (18.2-36.5) | 31.0 (22.0-43.0)a | 0.034 |
| ALP, U/L | 58.0 (46.0-73.0) | 58.0 (46.0-73.0) | 55.0 (43.5-74.0) | 0.171 | 58.0 (45.0-72.0) | 53.0 (38.0-68.5) | 58.0 (45.0-77.0) | 0.086 |
| TBIL, mmol/L | 10.9 (8.2-14.7) | 10.8 (8.2-14.5) | 11.4 (8.3-15.7) | 0.196 | 10.8 (8.2-14.7) | 11.4 (9.5-14.8) | 11.3 (8.0-15.8) | 0.429 |
| Potassium, mmol/L | 3.90 (3.59-4.20) | 3.90 (3.60-4.20) | 3.88 (3.52-4.21) | 0.325 | 3.94 ± 0.51 | 3.94 ± 0.49 | 3.86 ± 0.62 | 0.279 |
| Sodium, mmol/L | 139 (137-141) | 140 (137-141) | 138 (136-141) | 0.001 | 140 (138-141)a | 138 (136-141) | 139 (136-142) | 0.002 |
| Chlorine ion, mmol/L | 104 (102-107) | 105 (102-107) | 103 (100-106) | 0.002 | 104.2 ± 5.3 | 103.1 ± 4.5 | 103.7 ± 5.1 | 0.218 |
| Calcium, mmol/L | 2.11 (2.00-2.21) | 2.12 (2.01-2.21) | 2.09 (1.95-2.17) | 0.005 | 2.13 ± 0.22 | 2.11 ± 0.22 | 2.07 ± 0.18 | 0.011 |
| Phosphorus, mmol/L | 1.03 (0.89-1.19) | 1.03 (0.89-1.19) | 1.01 (0.73-1.18) | 0.359 | 1.04 (0.90-1.19) | 1.03 (0.92-1.17) | 1.00 (0.85-1.19) | 0.300 |
| BUN, mmol/L | 3.96 (3.10-5.25) | 3.90 (3.10-5.13) | 4.68 (3.60-6.20) | < 0.001 | 3.70 (2.96-4.66)a | 4.30 (3.51-5.07) | 4.93 (3.60-7.01) | < 0.001 |
| Creatinine, μmol/L | 63.6 (53.3-78.0) | 63.0 (53.0-77.4) | 66.3 (54.0-83.8) | 0.088 | 62.00 (52.70-73.00) | 60.00 (52.00-76.60) | 67.75 (55.25-90.23)a | < 0.001 |
| UA, μmol/L | 258 (204-336) | 257 (205-336) | 258 (193-332) | 0.725 | 253 (203-327) | 248 (194-306) | 264 (191-352) | 0.499 |
| CK, U/L | 65.0 (43.0-110) | 64.5 (44.0-109) | 66.5 (40.3-118) | 0.830 | 62.0 (44.0-98.0) | 58.5 (36.8-108) | 70.0 (43.8-122) | 0.233 |
| LDH, U/L | 205 (162-272) | 201 (160-261) | 229 (180-341) | < 0.001 | 186 (155-239) | 198 (164-282) | 251 (195-362)a | < 0.001 |
| Hs-cTnI > ULN, pg/mL | 130 (22.1) | 117 (23.4) | 13 (14.90) | 0.080 | 71 (23.5) | 3 (12.5) | 6 (9.5) | 0.027 |
| TG, mmol/L | 1.22 (0.92-1.78) | 1.20 (0.89-1.77) | 1.39 (1.04-1.83) | 0.002 | 1.18 (0.86-1.77) | 1.50 (1.05-2.08) | 1.36 (1.03-1.79)a | 0.004 |
| TCH, mmol/L | 4.00 (3.40-4.80) | 4.01 (3.42-4.80) | 4.00 (3.22-4.78) | 0.180 | 4.25 ± 1.09 | 4.34 ± 1.07 | 3.88 ± 1.08a | 0.004 |
| LDL-C, mmol/L | 2.50 (3.00-3.12) | 2.51 (2.02-3.10) | 2.48 (1.87-3.15) | 0.368 | 2.65 ± 0.89 | 2.76 ± 0.91 | 2.41 ± 0.87a | 0.020 |
| HDL-C, mmol/L | 1.01 (0.82-1.21) | 1.03 (0.84-1.24) | 0.91 (0.76-1.08) | < 0.001 | 1.11 ± 0.42a | 0.97 ± 0.26 | 0.92 ± 0.27 | < 0.001 |
| FPG, mmol/L | 5.80 (5.00-7.46) | 5.57 (4.92-6.89) | 9.10 (6.50-11.63) | < 0.001 | 5.37 (4.83-6.50)c | 9.40 (6.48-11.59) | 8.80 (6.50-12.03) | < 0.001 |
| 3.9-6.9 | 693 (69.0) | 650 (76.7) | 43 (27.6) | < 0.001 | 475 (80.2)c | 17 (29.8)) | 26 (26.3) | < 0.0013 |
| 7.0-11.1 | 241 (24.0) | 179 (21.1) | 62 (39.7) | 108 (18.2) | 22 (38.6) | 40 (40.4) | ||
| ≥ 11.1 | 70 (7.0) | 19 (2.2) | 51 (32.7) | 9 (1.5) | 18 (31.6) | 33 (33.3) | ||
| HbA1C | 6.20 (5.55-7.30) | 5.90 (5.40-6.30) | 7.87 (6.27-9.03) | < 0.001 | 5.9 (5.44-6.20)b | 7.60 (5.64-8.98) | 7.89 (6.75-9.21) | < 0.001 |
In addition, a between-group comparison with only the diabetes group was performed. The baseline characteristics and radiological findings are also summarized in Table 1. Patients with diabetes with comorbidities were the oldest among the three groups. There was a significant difference in blood oxygen saturation and respiratory rate among the three groups but no significant differences in the comparison of the non-comorbidity group and only diabetes group or the comparison of the diabetes only group and diabetes with comorbidities group. Chest CT scans indicated that the diabetes only group had more incidences of bilateral lesions than the non-comorbidity group.
Although there were numerous differences in laboratory findings among the non-comorbidity group, diabetes only group and diabetes with comorbidities group (Table 2), only ten items had statistical significance between the non-comorbidity group and diabetes only group, including ALB, sodium, BUN, CRP, and HDL-C, as well as FPG and HbA1c, as expected. These results combined with oxygen saturation indicated that there was no difference in cardiac, liver, lung and coagulation function between the groups.
FPG and HbA1c in the diabetes only group and diabetes with comorbidities group were almost at the same level. Compared with the diabetes only group, the diabetes with comorbidities group had a lower LY and a higher NLR and CRP, which represented a more severe inflammatory response.
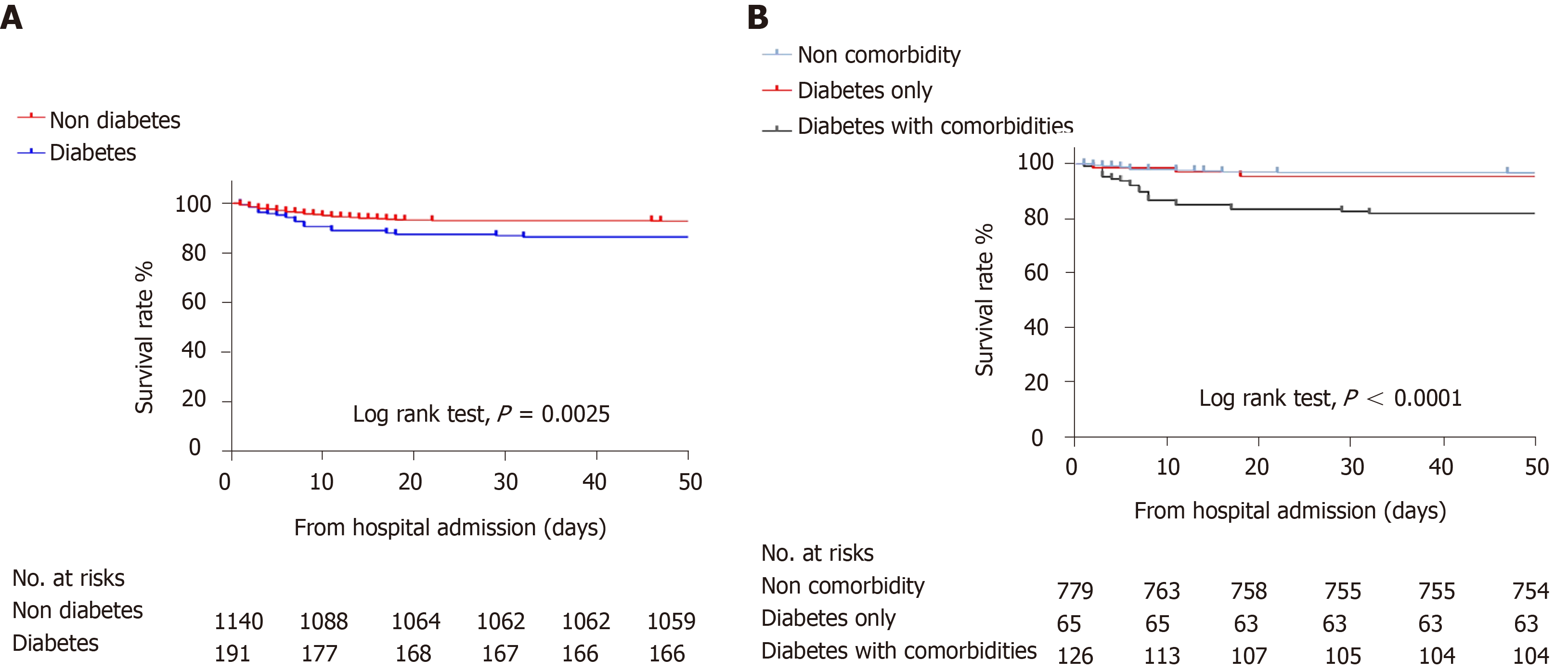
As shown in Table 3, 1223 of the 1331 patients (91.9%) were discharged from the hospital; the rate of mortality of the diabetes group was higher than that of the non-diabetes group (13.6% vs 7.2%). Kaplan-Meier survival analysis for all-cause mortality in patients with COVID-19 is shown in Figure 2. The overall survival rate was significantly lower in the diabetes group (log-rank P < 0.01, Figure 2A). Compared with non-diabetes patients, more patients with diabetes reported severe cases (34.6% vs 21.7%). The diabetes group had a higher rate of ARDS (11% vs 5.7%) and hypoproteinemia (15% vs 6.5%).
| Tota (n = 1331) | Non-diabetes (n = 1140) | Diabetes (n = 191) | P1 value | Non- comorbidity (n = 779) | Diabetes only (n = 65) | Diabetes with comorbidities (n = 126) | P2 value | |
| Treatments | ||||||||
| Antiviral therapy | 1227 (92.2) | 1057 (92.7) | 170 (89.0) | 0.077 | 725 (93.1) | 62 (95.4) | 108 (85.7) | 0.010 |
| Antibiotic therapy | 1142 (85.8) | 982 (86.1) | 160 (83.8) | 0.385 | 665 (85.4) | 57 (87.7) | 103 (81.7) | 0.472 |
| Systemic glucocorticoid | 533 (40.0) | 458 (40.2) | 75 (39.3) | 0.813 | 292 (37.5) | 23 (35.4) | 52 (41.3) | 0.657 |
| Intravenous immunoglobulin | 403 (30.3) | 342 (30.0) | 61 (31.9) | 0.590 | 210 (27.0) | 18 (27.7) | 43 (34.1) | 0.250 |
| Renal replacement therapy | 2 (0.2) | 1 (0.1) | 1 (0.5) | 0.267 | 0 (0.0) | 0 (0.0) | 1 (0.8) | 0.197 |
| Oxygen support | ||||||||
| Oxygenation | 786 (59.1) | 672 (58.9) | 114 (59.7) | 0.848 | 426 (54.7) | 38 (58.5) | 76 (60.3) | 0.446 |
| Mechanical ventilation | 154 (11.6) | 124 (10.9) | 30 (15.7) | 0.053 | 68 (8.7) | 7 (10.8) | 23 (18.3) | 0.004 |
| Illness severity | ||||||||
| Severe | 313 (23.5) | 247 (21.7) | 66 (34.6) | < 0.001 | 123 (15.8) | 14 (21.5) | 52 (41.3)a | < 0.001 |
| Complications | ||||||||
| ARDS | 86 (6.5) | 65 (5.7) | 21 (11.0) | 0.006 | 26 (3.3) | 2 (3.1) | 19 (15.1) | < 0.001 |
| ACI | 148 (11.1) | 132 (11.6) | 16 (8.4) | 0.193 | 77 (9.9) | 3 (4.6) | 12 (9.5) | 0.379 |
| AKI | 18 (1.4) | 14 (1.2) | 4 (2.1) | 0.535 | 6 (0.8) | 1 (1.5) | 3 (2.4) | 0.122 |
| Secondary infection | 161 (12.1) | 139 (12.2) | 22 (11.5) | 0.791 | 76 (9.8) | 4 (6.2) | 18 (14.3) | 0.162 |
| Shock | 25 (1.9) | 23 (2.0) | 2 (1.0) | 0.531 | 9 (1.2) | 0 (0.0) | 2 (1.6)a | 0.706 |
| Hypoproteinemia < 30g/l | 99 (7.7) | 71 (6.5) | 28 (15.0) | < 0.001 | 38 (5.0)c | 11 (16.9) | 17 (13.9) | < 0.001 |
| Length of hospital stay, d | 17.0 (10.0-24.0) | 17.0 (10.0-24.0) | 16.0 (10.0-25.0) | 0.655 | 17.0 (11.0-24.0) | 19.0 (11.5-27.0) | 16.0 (8.0-22.5) | 0.109 |
| ICU admission | 125 (9.4) | 103 (9.0) | 22 (11.5) | 0.276 | 57 (7.3) | 5 (7.7) | 17 (13.5) | 0.062 |
| Duration from admission to ICU, d | 4.00 (1.00-7.50) | 5.00 (1.00-8.00) | 3.50 (1.75-5.25) | 0.383 | 4.50 (1.00-8.00) | 5.00 (1.50-6.00) | 3 (1.50-4.50) | 0.733 |
| Prognosis | ||||||||
| Death, No | 108 (8.1) | 82 (7.2) | 26 (13.6) | 0.003 | 26 (3.3) | 3 (4.6) | 23 (18.3) | < 0.001 |
| Total (n = 191) | Survivors (n = 165) | Non-survivors (n = 26) | P value | |
| WBC, × 109per L | 5.94 (4.49-7.53) | 5.91 (4.42-7.29) | 7.26(5.19-13.07) | 0.016 |
| NEUT, × 109per L | 4.25 (3.13-5.37) | 4.09 (3.01-5.13) | 6.22 (3.69-11.33) | < 0.001 |
| LY, × 109per L | 1.04 (0.72-1.43) | 1.08 (0.78-1.48) | 0.65 (0.56-1.07) | < 0.001 |
| NLR | 3.85 (2.45-6.37) | 3.50 (2.33-5.53) | 10.43 (5.78-16.84) | < 0.001 |
| Hb, g/L | 127.3 ± 16.9 | 126.8 ± 16.7 | 131.6 ± 18.3 | 0.314 |
| PLT, × 109per L | 196 (147-255) | 201 (152-201) | 155 (110-230) | 0.033 |
| PCT, ng/mL | ||||
| < 0.5 | 143 (94.7) | 132 (98.5) | 11 (64.7) | < 0.001 |
| ≥ 0.5 | 8 (5.3) | 2 (1.5) | 6 (35.3) | |
| CRP | 29.8 (5.5-75.9) | 25.4 (4.4-63.0) | 115.3 (66.1-170.6) | < 0.001 |
| IL-6, pg/mL | 3.31 (1.64-17.49) | 3.09 (1.50-5.25) | 83.47 (35.75-243.60) | < 0.001 |
| PT, s | 14.30 (11.90-15.50) | 14.00 (11.60-15.40) | 16.20 (13.52-18.92) | 0.002 |
| < 16 | 116 (76.8) | 110 (81.5) | 6 (37.5) | < 0.001 |
| ≥ 16 | 35 (23.2) | 25 (18.5) | 10 (62.5) | |
| D-dimer, mg/L | 0.69 (0.35-1.35) | 0.62 (0.62-1.09) | 5.40 (1.50-21.00) | < 0.001 |
| ≤ 0.5 | 58 (37.9) | 57 (41.9) | 1 (5.9) | < 0.001 |
| > 0.5 to ≤ 1.0 | 46 (30.1) | 43 (31.6) | 3 (17.6) | |
| > 1.0 | 49 (32.0) | 36 (26.5) | 13 (76.5) | |
| ALB, g/L | 35.7 ± 5.5 | 36.0 ± 30.5 | 33.5 ± 23.4 | 0.031 |
| ALT, U/L | 23.0 (16.0-34.0) | 21.3 (15.3-32.3) | 31.0 (20.9-46.6) | 0.008 |
| AST, U/L | 29.0 (20.0-41.0) | 27.0 (19.0-38.7) | 43.0 (31.0-60.5) | < 0.001 |
| ALP, U/L | 55.0 (43.5-74.0) | 55.0 (41.5-73.0) | 57.0 (49.5-89.5) | 0.241 |
| TBIL, mmol/L | 11.3 (8.3-15.7) | 11.4 (9.0-15.1) | 11.2 (7.6-28.0) | 0.642 |
| Potassium, mmol/L | 3.88 (3.52-4.21) | 3.90 (3.54-4.21) | 3.65 (3.37-4.30) | 0.381 |
| Sodium, mmol/L | 138.4 ± 4.3 | 138.2 ± 3.9 | 139.3 ± 6.4 | 0.418 |
| Chlorine ion, mmol/L | 103.5 ± 4.9 | 103.2 ± 4.7 | 105.3 ± 6.0 | 0.052 |
| Calcium, mmol/L | 2.09 (1.95-2.17) | 2.10 (1.95-2.20) | 2.00 (1.89-2.11) | 0.042 |
| Phosphorus, mmol/L | 1.01 (0.86-1.18) | 1.02 (0.87-1.19) | 0.93 (0.76-1.18) | 0.268 |
| BUN, mmol/L | 4.70 (3.60-6.22) | 4.5 (3.59-5.82) | 6.51 (4.92-17.45) | < 0.001 |
| Creatinine, μmol/L | 66.3 (54.0-83.8) | 64.0 (44.6-81.0) | 73.0 (64.0-129.6) | 0.006 |
| UA, μmol/L | 258 (193-332) | 258 (147-321) | 293 (179-428) | 0.286 |
| CK, U/L | 66.5 (40.3-117.8) | 61.0 (36.5-111.0) | 85.0 (71.0-364.0) | 0.002 |
| LDH, U/L | 229 (180-341) | 216 (172-219) | 522 (420-611) | < 0.001 |
| Hs-cTnI > ULN, pg/mL | 10/88 (11.4) | 8/72 (11.1) | 2/16 (12.5) | 1.000 |
| TG, mmol/L | 1.39 (1.04-1.83) | 1.41 (1.05-1.98) | 1.31 (0.99-1.57) | 0.398 |
| TCH, mmol/L | 4.04 ± 1.10 | 4.12 ± 1.06 | 3.39 ± 1.16 | 0.009 |
| LDL-C, mmol/L | 2.54 ± 0.90 | 2.59 ± 0.88 | 2.10 ± 0.93 | 0.036 |
| HDL-C, mmol/L | 0.94 ± 0.26 | 0.94 ± 0.26 | 0.89 ± 0.33 | 0.450 |
| FPG, mmol/L | 9.10 (6.50-11.72) | 8.70 (6.50-11.36) | 12.00 (9.40-16.81) | 0.011 |
| 3.9-6.9 | 43 (27.7) | 40 (28.6) | 3 (20.0) | 0.0691 |
| 7.0-11.1 | 61 (39.4) | 58 (41.4) | 3 (20.0) | |
| ≥ 11.1 | 51 (32.9) | 42 (30.0) | 9 (60.0) | |
| HbA1C | 7.77 ± 1.97 | 7.61 ± 1.90 | 9.53 ± 2.02 | 0.021 |
| Total (n = 191) | Survivors (n = 165) | Non-survivors (n = 26) | P value | |
| Treatments | ||||
| Antiviral therapy | 170 (89.0) | 149 (90.3) | 21 (80.8) | 0.268 |
| Antibiotic therapy | 160 (83.8) | 139 (84.2) | 21 (80.8) | 0.873 |
| Systemic glucocorticoids | 74 (38.7) | 55 (33.3) | 19 (73.1) | < 0.001 |
| Intravenous immunoglobulin | 60 (31.4) | 50 (30.3) | 10 (38.5) | 0.405 |
| Renal replacement therapy | 1 (0.5) | 0 (0.0) | 1 (3.8) | 0.136 |
| Insulin | 88 (46.1) | 75 (45.5) | 13 (50.0) | 0.666 |
| Metformin | 54 (28.3) | 51 (30.9) | 3 (11.5) | 0.041 |
| Sulfonylurea | 37 (19.4) | 36 (21.8) | 1 (3.8) | 0.031 |
| DPP-4 inhibitor | 11 (5.8) | 10 (6.1) | 1 (3.8) | 1.000 |
| Acarbose | 77 (40.3) | 75 (45.5) | 2 (7.7) | < 0.001 |
| Thiazolidinedione | 7 (3.7) | 7 (4.2) | 0 (0.0) | 0.596 |
| Oxygen support | ||||
| Oxygenation | 115 (60.2) | 95 (57.6) | 20 (76.9) | 0.061 |
| Mechanical ventilation | 35 (18.3) | 15 (9.1) | 20 (76.9) | < 0.001 |
| Illness severity | ||||
| Severe | 63 (33.0) | 37 (33.7) | 26 (100) | < 0.001 |
| Complications | ||||
| ARDS | 21 (11.0) | 5 (3.0) | 16 (61.5) | < 0.001 |
| ACI | 16 (8.4) | 9 (5.5) | 7 (26.9) | 0.001 |
| AKI | 3 (1.6) | 1 (0.6) | 2 (7.7) | 0.049 |
| Secondary infection | 22 (11.5) | 10 (6.1) | 12 (46.2) | < 0.001 |
| Shock | 3 (1.6) | 0 (0.0) | 3 (11.5) | 0.002 |
| Hypoproteinemia < 30 g/L | 28 (14.7) | 22 (13.3) | 6 (23.1) | 0.314 |
| Coagulopathy | 36 (18.8) | 26 (15.8) | 10 (38.5) | 0.013 |
| Length of hospital stay, d | 16.0 (10.0-25.0) | 18.0 (11.5-26.0) | 7.0 (3.0-11.0) | < 0.001 |
| ICU admission | 22 (11.5) | 11 (6.7) | 11 (42.3) | < 0.001 |
| Duration from admission to ICU, d | 4.00 ± 3.51 | 3.91 ± 3.11 | 4.09 ± 4.01 | 0.907 |
| Metformin (n = 50) | Matched non-Metformin (n = 50) | Acarbose (n = 46) | Matched non-acarbose (n = 46) | |
| WBC, × 109/L | 6.33 ± 2.25 | 6.27 ± 2.62 | 4.83 (4.04-6.68) | 5.91 (4.42-9.35)c |
| NEUT, × 109/L | 4.20 (3.02-5.18) | 4.17 (3.21-5.87) | 3.50 (2.48-4.74) | 4.60 (3.14-8.13) |
| LY, × 109/L | 1.20 (0.69-1.74) | 1.14 (0.82-1.50) | 1.19 ± 0.55 | 1.04 ± 0.53 |
| NLR | 3.69 (2.11-6.05) | 3.74 (2.47-5.55) | 3.25 (2.05-4.41) | 4.88 (2.50-12.32)d |
| Hb, g/L | 126.0 ± 15.7 | 126.5 ± 14.9 | 126.0 ± 16.9 | 129.3 ± 17.3 |
| PLT, × 109/L | 229.5 ± 93.5 | 208.5 ± 103.8 | 233.0 ± 93.2 | 214.2 ± 99.7 |
| PCT, ng/mL | ||||
| < 0.5 | 43 (100.0) | 34 (91.9) | 37 (100.0) | 34 (87.2) |
| ≥ 0.5 | 0 (0.0) | 3 (8.1) | 0 (0.0) | 5 (12.8) |
| CRP | 50.7 (5.0-78.0) | 46.5 (6.3-106.8) | 26.2 (3.7-52.2) | 63.8 (10.8-83.4)c |
| IL-6, pg/mL | 2.07 (1.50-4.90) | 3.20 (1.68-67.28) | 2.58 (1.50-5.06) | 19.88 (1.95-67.28) |
| PT, s | 13.5 ± 2.7 | 14.2 ± 2.2 | 14.1 ± 2.6 | 14.2 ± 3.2 |
| < 16 | 37 (86.0) | 31 (79.5) | 30 (78.9) | 27 (73.0) |
| ≥ 16 | 6 (14.0) | 8 (20.5) | 8 (21.1) | 10 (27.0) |
| D-dimer, mg/L | 0.45 (0.26-1.19) | 0.83 (0.33-1.60) | 0.59 (0.33-0.98) | 0.96 (0.39-5.40) |
| ≤ 0.5 | 22 (52.4) | 13 (33.3) | 18 (42.9) | 12 (33.3) |
| > 0.5 to ≤ 1.0 | 6 (14.3) | 11 (28.2) | 15 (35.7) | 7 (19.4) |
| > 1.0 | 14 (33.3) | 15 (38.5) | 9 (21.4) | 17 (47.2) |
| ALB, g/L | 35.7 ± 5.9 | 35.8 ± 5.5 | 35.7 ± 6.5 | 35.1 ± 4.8 |
| ALT, U/L | 20.0 (13.5-27.5) | 22.0 (17.0-36.0) | 20.0 (14.0-31.0) | 23.00 (14.00-33.25) |
| AST, U/L | 25.5 (18.5-33.7) | 29.0 (20.0-42.0) | 23.0 (17.5-36.4) | 31.0 (21.5-39.6) |
| ALP, U/L | 51.0 (37.0-71.0) | 54.0 (44.0-68.0) | 59.5 24.6 | 61.5 25.5 |
| TBIL, mmol/L | 12.2 ± 5.4 | 13.4 ± 16.2 | 11.5 ± 4.8 | 13.5 ± 5.4 |
| Potassium, mmol/L | 3.81 ± 0.46 | 3.77 ± 0.54 | 3.90 ± 0.50 | 3.82 ± 0.63 |
| Sodium, mmol/L | 137.9 ± 3.9 | 138.2 ± 4.1 | 138. 5 ± 3.8 | 138.1 ± 4.7 |
| Chlorine ion, mmol/L | 103.3 ± 4.5 | 103.4 ± 5.0 | 103.2 ± 4.6 | 103.7 ± 5.3 |
| Calcium, mmol/L | 2.14 ± 0.22 | 2.05 ± 0.18a | 2.12 ± 0.22 | 2.08 ± 0.19 |
| Phosphorus, mmol/L | 1.02 (0.83-1.21) | 0.99 (0.87-1.17) | 1.07( 0.88-1.21) | 1.00 (0.77-1.21) |
| BUN,mmol/L | 4.40 (3.67-4.84) | 5.20 (3.50-5.75) | 4.16 (3.60-5.18) | 5.04 (3.80-6.64) |
| Creatinine, μmol/L | 54.0 (49.0-73.7) | 60.0 (52.5-90.3) | 59.5 (48.8-74.5) | 68.0 (55.0-89.3) |
| UA, μmol/L | 266.5 ± 96.6 | 260.9 ± 98.7 | 229 (168-263) | 258 (179-324) |
| CK, U/L | 64.0 (49.0-84. 0) | 82.0 (39.0-135.3) | 53.5 (35.5-73.8) | 71.0 (40.0-114.0) |
| LDH, U/L | 237 ± 115 | 304 ± 162a | 229 (185-263) | 267 (181-446) |
| Hs-cTnI > ULN , pg/mL | 0/24 (0.0) | 3/15 (20.0) | 1/21 (4.8) | 2/26 (7.7) |
| TG, mmol/L | 1.55 (1.15-1.82) | 1.32 (1.08-3.40) | 1.36 (1.05-1.83) | 1.15 (0.94-1.61) |
| TCH, mmol/L | 3.91 ± 0.87 | 3.80 ± 0.92 | 4.40 ± 1.14 | 4.04 ± 0.96 |
| LDL-C, mmol/L | 2.40 ± 0.73 | 2.43 ± 0.78 | 2.84 ± 0.87 | 2.57 ± 0.83 |
| HDL-C, mmol/L | 0.93 ± 0.22 | 0.88 ± 0.28 | 0.98 ± 0.27 | 0.93 ± 0.23 |
| FPG, mmol/L | 10.57 ± 4.92 | 8.32 ± 2.47b | 9.92 ± 4.90 | 10.00 ± 4.26 |
| 3.9-6.9 | 11 (44.0) | 13 (38.2) | 12 (30.8) | 8 (22.2) |
| 7.0-11.1 | 13 (52.0) | 21 (61.8) | 13 (33.3) | 16 (44.4) |
| ≥ 11.1 | 1 (4.0) | 0 (0.0) | 14 (35.9) | 12 (33.3) |
| HbA1C | 7.96 ± 1.85 | 6.71 ± 1.94 | 7.85 ± 1.78 | 8.25 ± 2.04 |
| Metformin (n = 50) | Matched non-Metformin (n = 50) | Acarbose (n = 46) | Matched non-acarbose (n = 46) | |
| Treatments | ||||
| Antiviral therapy | 49 (98.0) | 42 (84.0)a | 41 (89.1) | 43 (93.5) |
| Antibiotic therapy | 45 (90.0) | 37 (74.0)a | 40 (87.0) | 40 (87.0) |
| systemic glucocorticoids | 17 (34.0) | 16 (32.0) | 12 (26.1) | 22 (47.8)e |
| Intravenous immunoglobulin | 15 (30.0) | 11 (22.0) | 11 (23.9) | 22 (47.8)e |
| Renal replacement therapy | 0 (0.0) | 1 (2.0) | 0 (0.0) | 0 (0.0) |
| Insulin | 26 (52.0) | 10 (20.0)b | 23 (50.0) | 39 (84.8)g |
| Metformin | 50 | 0 | 21 (45.7) | 15 (32.6) |
| Sulfonylurea | 18 (36.0) | 1 (2.0)c | 17 (37.0) | 8 (17.4)e |
| DPP-4 inhibitor | 3 (6.0) | 0 (0.0) | 3 (6.5) | 3 (6.5) |
| Acarbose | 28 (56.0) | 3 (6.0)c | 46 (100.0) | 0 (0.0) |
| thiazolidinedione | 6 (12.0) | 0 (0.0)a | 4 (8.7) | 0 (0.0) |
| Oxygen support | ||||
| Oxygenation | 32 (64.0) | 21 (42.0) | 30 (65.2) | 30 (65.2) |
| Mechanical ventilation | 6 (12.0) | 11 (22.0) | 3 (6.5) | 10 (21.7)e |
| Illness severity | ||||
| Severe | 14 (28.0) | 21 (42.0) | 12 (26.1) | 18 (39.1) |
| Complications | ||||
| ARDS | 4 (8.0) | 8 (16.0) | 1 (2.2) | 8 (17.4)e |
| ACI | 1 (2.0) | 4 (8.0) | 2 (4.3) | 6 (13.0) |
| AKI | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.3) |
| Secondary infection | 8 (16.0) | 5 (10.0) | 0 (0.0) | 2 (4.3) |
| Shock | 1 (2.0) | 0 (0.0) | 1 (2.2) | 10 (21.7)e |
| Hypoproteinemia < 30 g/L | 9 (18.0) | 5 (10.0) | 10 (21.7) | 6 (13.0) |
| Coagulopathy | 6 (12.0) | 9 (18.0) | 8 (17.4) | 10 (21.7) |
| Length of hospital stay, d | 17.60 ± 8.74 | 16.80 ± 10.51 | 18.37 ± 8.15 | 16.52 ± 9.96 |
| ICU admission | 6 (12.0) | 6 (12.0) | 3 (6.5) | 8 (17.4) |
| Duration from admission to ICU, d | 3.83 ± 2.04 | 2.83 ± 2.14 | 6.00 (3.50-6.00) | 2.50 (2.00-5.00) |
| Prognosis | ||||
| Discharged | 47 (94) | 41 (82) | 45 (97.8) | 34 (73.9)f |
| Death | 3 (6.0) | 9 (18) | 1 (2.2) | 12 (26.1) |
| Factor | Hazard ratio | P value |
| Sex (male) | 2.59 (1.68-3.99) | < 0.001 |
| Age, yr | ||
| 18-49 | 1 (ref) | |
| 50-64 | 5.86 (2.27-15.12) | < 0.001 |
| ≥ 65 | 11.8 (4.6- 30.2) | < 0.001 |
| Hypertension | 1.75 (1.18-2.60) | 0.006 |
| CKD | 4.55 (2.52-8.20) | < 0.001 |
| CVD | 2.35 (1.27-4.33) | 0.006 |
| Diabetes | 0.98 (0.62-1.54) | 0.918 |
The treatment and primary outcome of the non-comorbidity group and diabetes only group were not different (Table 3), and the results for all-cause mortality were similar in both groups (log-rank P = 0.59) (Figure 2B). Regarding the secondary endpoint, there was no difference between the groups except for hypoproteinemia (5.0% vs 16.9%). Likewise, there was a similar frequency of COVID-19 pharmacological therapy in the diabetes only patients vs diabetes with comorbidities patients; however, the latter was more likely to receive mechanical ventilation (10.8% vs 18.3%), had a higher incidence of mortality (4.6% vs 18.3%), greater likelihood of shock (0 vs 1.6%) and more severe cases (21.5% vs 41.3%).
Diabetic survivors (n = 165) and non-survivors (n = 26) shared basic characteristics except for decreased blood oxygen saturation (10.9% vs 26.9%) and rapid breathing (18.2% vs 26.9%), which were more frequent in non-survivors (Supplementary Table 2), indicating that the latter had severe lung dysfunction. There were numerous differences in laboratory results between diabetic survivors and non-survivors with COVID-19 that reflected the functions of different organs and systems (Supplementary Table 2). Diabetic non-survivors had higher WBC, NEU, NLR, CRP, and IL-6 and lower LY, reflecting that mortality patients had severe inflammatory responses. Serum levels of PT, D-dimer, ALT, AST, BUN, creatinine, CK, and LDH were all significantly higher in non-survivors (Table 4), which reflected more severe coagulation, liver, kidney, and cardiac dysfunction. Diabetic non-survivors reported higher average FPG compared with survivors.
Undoubtedly, higher proportions of complications, including ARDS (3.0 vs 61.5%), ACI (5.5% vs 26.9%), shock (0 vs 11.5%), secondary infection (6.1% vs 46.2%), AKI (0.6% vs 7.7%) and coagulopathy (15.8% vs 38.5%), were found in non-survivors (Table 5). Likewise, the non-survivor group had a greater incidence of severe cases (33.7% vs 100%) and ICU admission (6.7% vs 42.3%) and was more likely to receive corticosteroids (33.3% vs 73.1%). There was a significantly lower frequency of hypoglycemic medication in diabetic non-survivors vs diabetic survivors, including metformin (30.9% vs 11.5%), sulfonylurea (21.8% vs 3.8%) and acarbose (45.5% vs 7.7%), which might be related to controlled blood glucose.
Of 191 patients with diabetes with COVID-19, 54 cases were using metformin, and after sex and age matching, there were 50 patients using metformin and 50 sex- and age-matched non-metformin users. The frequency of fever (54% vs 78%) and fatigue (38% vs 18%) showed significant differences in clinical characteristics between patients with diabetes with COVID-19 using metformin and matched non-metformin users (Supplementary Table 3). Laboratory findings (Table 6) revealed that metformin users had lower levels of LDH and FPG; however, the distribution of glucose was similar. The results that referred to liver, kidney, cardiac, coagulation and inflammatory response function were not statistically significant. The primary outcome and secondary outcome of patients who used metformin were comparable to matched non-metformin users (Table 7). The former group showed a higher need for antivirals (98% vs 84%) and antibiotics (90% vs 74%). Insulin (52.0% vs 20%), sulfonylurea (36.0% vs 2%), acarbose (56.0% vs 6%), and thiazolidinedione (12% vs 0) were also applied significantly more frequently to the individuals using metformin.
Of 191 patients with diabetes with COVID-19, 77 cases were treated with acarbose, and after sex and age matching, there were 46 patients treated with acarbose and 46 sex- and age-matched non-acarbose users. Supplementary Table 3 shows that the length of symptom onset to hospital admission was longer in the acarbose group than in the matched non-acarbose group, which indicated that the symptoms in the former patients might be relatively mild. Notably, some inflammatory response-related laboratory results, such as WBC, NLR, and CRP, were significantly lower in the acarbose group (Table 6). Furthermore, these differences were not related to glucose control, as the serum level of glucose in both groups was comparable.
The mortality rate (2.2% vs 26.1%) was lower in the acarbose group (Table 7), as were the rates of ARDS (2.2% vs 17.4%) and shock (2.2% vs 21.7%). At the same time, patients who were treated with acarbose indicated a lower need for treatment with corticosteroids (26.1% vs 47.8%), immunoglobin (23.9% vs 47.8%), mechanical ventilation (6.5% vs 21.7%), and insulin (50.0% vs 84.8%).
Among the 1131 included patients, multivariable Cox regression (Table 8) showed that male [hazard ratio (HR) 2.59, 95%CI 1.63-3.99], hypertension (HR 1.75, 95%CI 1.18-2.6), CKD (HR 4.55, 95%CI 2.52-8.20), and CVD (HR 2.35, 95%CI 1.27-4.33) were risk factors for COVID-19 mortality. Age was also a risk factor for COVID-19 mortality. However, diabetes alone was not an independent risk factor for mortality in patients with COVID-19.
A number of studies have demonstrated that patients with diabetes have a higher risk of mortality from COVID, as well as a greater risk of developing more severe cases[4,7,12,13]. Guo et al[13] reported that diabetes was a risk factor for the progression and prognosis of COVID-19. However, Shi et al[14] pointed out that diabetes was not independently associated with COVID mortality, while commonalities, such as hypertension and cardiovascular disease, played more important roles in contributing to the in-hospital death of patients with COVID-19, which was relatively limited in size. In this study, which had relatively rich clinical data, we found that diabetes alone was not an independent risk factor for in-hospital mortality from COVID-19, but comorbidities such as hypertension and CKD were risk factors; this result was consistent with a previous study[14]. Partially consistent with previous studies, our study found that compared with non-diabetic patients, patients with diabetes with COVID-19 were older, had worse outcomes, including a higher rate of mortality, severe cases and ARDS, and presented severe inflammatory response, lung and coagulation dysfunction[7,13,15]. In this study, up to 88% of diabetic patients were greater than or equal to 50 years of age, more over, older age was an independent risk factor of mortality in COVID-19, which was consistent with previous studies[3,14]. Additionally, patients with diabetes had increased levels of urea nitrogen and decreased levels of albumin. These abnormalities indicated that COVID-19 may be associated with progressive organ injury in patients with diabetes. Preexisting hypertension, CHD, CVD, and CKD had higher frequencies in the diabetic group. Recent studies reported that patients with cardiovascular hypertension, CKD, and CVD were more likely to develop severe cases[4,6,16], so we compared patients with diabetes and COVID-19 without comorbidity and patients with COVID-19 without any comorbidity to identify whether diabetes without comorbidity was a risk factor for COVID-19. In our study, there was no difference in the outcome between the non-comorbidity group and the diabetes only group. Shi et al[16] reported that even though patients with COVID-19 with diabetes had worse outcomes, it was not independently associated with in-hospital death, which was consistent with our results. In addition, most laboratory results were comparable between the non-comorbidity group and the diabetes only group, except for CRP, albumin, sodium, urea nitrogen, HDL-C and, of course, blood glucose. CRP is an inflammatory biomarker that is related to glucose homoeostasis, obesity and atherosclerosis[17] and was independently related to insulin sensitivity[18]. In addition, insulin resistance was a main characteristic of type 2 diabetes; since CRP was related to the chronic inflammatory situation, and the levels of WBC, NEU, and LY, which reflected the acute infection with the disease pathogen, were not statistically significant, we inferred that diabetes itself did not increase the degree of inflammation after SARS-CoV-2 infection.
Patients with diabetes with comorbidities were more seriously ill when compared with the diabetes only group and non-comorbidity group. The mortality was higher in the diabetes with comorbidities group, but the difference between both diabetes groups had no relation to FPG because the median FPG in both diabetes groups was comparable. Patients with diabetes with comorbidity were 10 years older than patients who had no comorbidity except diabetes; furthermore, age ≥ 65 years was associated with a greater risk of death[4]. As described above, patients with hypertension and CVD were more likely to develop severe cases[4]. Furthermore, our analysis indicated that age, hypertension, CKD, and CVD were risk factors for COVID-19 mortality. Since the diabetes with comorbidities group had a higher prevalence of hypertension, CKD and CVD, there was no doubt that patients with diabetes with comorbidities had worse outcomes.
Comparing to the survivor of diabetic patients with COVID-19, the diabetic patients who died of COVID-19 had more severe inflammatory response, progressive organ injury, and also, undoubtedly, higher proportions of complications and severe cases. Randomised Evaluation of COVID-19 Therapy (RECOVERY) Collaborative Group[19] and WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group[20] reported that systemic glucocorticoids was conducive to the reduction in mortality of COVID-19 severe cases. As the percentage of severe case in non-survivor group were 100%, while that rate in survivor group was just 33%, there was no doubt that non-survivor group had higher rate of using systemic glucocorticoids. Therefore, in such cases, higher rate of systemic glucocorticoids treatment in non-survivor group did not indicated higher rate of mortality in systemic glucocorticoids treatment.
One unanticipated result was that acarbose, not metformin, could improve prognosis through a decrease in the degree of inflammation, which was independent of the blood glucose level. In addition, acarbose accounted for 97% of the glycosidase inhibitors used. Feng et al[21] reported that acarbose could effectively block the metastasis of enterovirus 71 (EV71) from the intestine to the whole body. EV71 is one of the main causes of hand-foot-and-mouth disease (HFMD), and its infection relies on the interaction of the canyon region of its virion surface and the glycosylation of the SCARB2 protein, which is the cellular receptor of EV71 infection. Dang et al[22] found that acarbose not only inhibited cellular receptors of various glycosylated viruses but also competitively blocked the canyon region of the EV71 virion surface, blocking the metastasis of EV71 from the intestine. Angiotensin converting enzyme II (ACE2) is a SARS-CoV-2 cell entry receptor[23], and glycosylation sites play an important role in the combination of SARS-CoV-2 and its receptor[24,25]. Chloroquine was reported to block SARS-CoV-2 infection by interfering with the glycosylation of cellular receptors[26]. As previously stated, acarbose inhibited the glycosylation of EV71 receptors; additionally, patients with diabetes with COVID-19 who were treated with acarbose had better outcomes than patients who were not treated, suggesting that acarbose could improve the prognosis of COVID-19 infection by inhibiting the glycosylation of ACE2. In addition, compared to the non-acarbose group, the acarbose group had lower WBC, NLR, and CRP levels, indicating a decreased inflammatory response and further supporting the anti-SARS-CoV-2 function of acarbose. Furthermore, a previous study showed that acarbose could change the gut microbiota and then beneficially regulate the body’s immune function[27]. A recent study revealed that fetal microbiome changes occurred in patients with COVID-19, characterized by depletion of beneficial commensals and enrichment of opportunistic pathogens[28]. Therefore, we inferred that acarbose might increase the baseline abundance of microbiota that had inversely correlated with COVID-19.
As previous studies reported that metformin has multiple additional health benefits in patients with diabetes[29], we anticipated that metformin would improve prognosis after COVID-19 infection; however, the results were unexpected. Scanning the literature, we found that metformin improves ACE2 stability through AMPK[30], which means that metformin may increase ACE2 availability. In addition, the median level of FPG was higher in metformin users than in nonusers, as a previous study reported that improving glycemic control substantially reduced the risk of mortality from COVID-19.
The study has some limitations. First, due to the retrospective and multiple-center study design, some information, such as patients’ exposure history, the chronic disease severity and medication, diabetes medication, glycemic control and several laboratory items, was not available for all patients. There could be assay variability in different centers. Second, samples were only from Hubei Province, China; thus, more studies in other regions, even other countries, might obtain more comprehensive insight into COVID-19. However, this study is one of the largest retrospective and multicenter studies among patients with COVID-19. Additionally, this study is one of the first to investigate the influence of diabetes medications in patients with diabetes with COVID-19. The relatively abundant clinical data and numerous events also strengthen the results. The conclusion will help clinicians identify high-risk patients and choose suitable diabetes medication for patients with diabetes.
In conclusion, patients with diabetes had worse outcomes when suffering from COVID-19; however, the outcome was not related to diabetes itself but to comorbidities such as hypertension, CKD and CVD. Furthermore, the administration of acarbose could reduce the risk of death, ARDS, and shock in patients with diabetes.
Coronavirus disease 2019 (COVID-19) has become an ongoing pandemic and has caused considerable mortality worldwide. Previous studies have demonstrated that patients with diabetes have a higher risk of mortality from COVID-19, as well as a greater risk of developing more severe cases.
Diabetes was a risk factor for the progression and prognosis of COVID-19, however, the effects of diabetes or anti-diabetic medication on the mortality of COVID-19 have not been well described.
We aim to investigate the outcome of different statuses (with or without comorbidity) and anti-diabetic medication use before admission of diabetic after COVID-19.
The clinical characteristics of 1422 consecutive hospitalized patients were collected. The statistical analyses were conducted with SPSS (version 25.0).
The overall survival rate was significantly lower in the diabetes group (log-rank P < 0.01), but the results for all-cause mortality were similar in the non-comorbidity group and diabetes only group (log-rank P = 0.59). Male sex [hazard ratio (HR) 2.59, P < 0.001], hypertension (HR 1.75, P = 0.006), chronic kidney disease (CKD) (HR 4.55, P < 0.001), cerebrovascular disease (CVD) (HR 2.35, P = 0.006), and age were independent risk factors for the COVID-19 mortality in multivariable Cox regression. However, diabetes alone was not an independent risk factor for mortality in patients with COVID-19.
Although diabetes is associated with a higher risk of mortality in patients with COVID-19, the outcome was not related to diabetes itself. Age, hypertension, CKD and CVD were the independent risk factor of mortality.
The present study calls more attention to the impact of older age and comorbid chronic disease, such as hypertension, CKD and CVD on disease progression among diabetic patients with COVID-19.
We acknowledge all health-care workers involved in the diagnosis and treatment of patients in Hubei Province.
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alberca RW S-Editor: Gong ZM L-Editor: A P-Editor: Yu HG
| 1. | Du M, Lin YX, Yan WX, Tao LY, Liu M, Liu J. Prevalence and impact of diabetes in patients with COVID-19 in China. World J Diabetes. 2020;11:468-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 2. | Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen MM, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang BH. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31:1068-1077.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1198] [Cited by in RCA: 1098] [Article Influence: 219.6] [Reference Citation Analysis (0)] |
| 3. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18189] [Article Influence: 3637.8] [Reference Citation Analysis (0)] |
| 4. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5515] [Article Influence: 1103.0] [Reference Citation Analysis (1)] |
| 5. | Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020;8:433-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 6. | Ji HL, Zhao R, Matalon S, Matthay MA. Elevated Plasmin(ogen) as a Common Risk Factor for COVID-19 Susceptibility. Physiol Rev. 2020;100:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 273] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 7. | Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, Yu X, Dong K. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 360] [Cited by in RCA: 355] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 8. | Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8787] [Cited by in RCA: 8981] [Article Influence: 408.2] [Reference Citation Analysis (0)] |
| 9. | Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. N Engl J Med. 2017;377:562-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 921] [Cited by in RCA: 1178] [Article Influence: 147.3] [Reference Citation Analysis (0)] |
| 10. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3327] [Article Influence: 255.9] [Reference Citation Analysis (0)] |
| 11. | National Health Commission SAoTCM. Diagnosis and treatment of COVID-19 (trial version 8). Available from: http://www.gov.cn/zhengce/zhengceku/2020-08/19/content_5535757.htm. |
| 12. | Liu H, Chen S, Liu M, Nie H, Lu H. Comorbid Chronic Diseases are Strongly Correlated with Disease Severity among COVID-19 Patients: A Systematic Review and Meta-Analysis. Aging Dis. 2020;11:668-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 13. | Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;e3319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 884] [Cited by in RCA: 899] [Article Influence: 179.8] [Reference Citation Analysis (1)] |
| 14. | Shi Q, Zhang X, Jiang F, Hu N, Bimu C, Feng J, Yan S, Guan Y, Xu D, He G, Chen C, Xiong X, Liu L, Li H, Tao J, Peng Z, Wang W. Clinical Characteristics and Risk Factors for Mortality of COVID-19 Patients With Diabetes in Wuhan, China: A Two-Center, Retrospective Study. Diabetes Care. 2020;43:1382-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 285] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 15. | Bornstein SR, Rubino F, Khunti K, Mingrone G, Hopkins D, Birkenfeld AL, Boehm B, Amiel S, Holt RI, Skyler JS, DeVries JH, Renard E, Eckel RH, Zimmet P, Alberti KG, Vidal J, Geloneze B, Chan JC, Ji L, Ludwig B. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 535] [Cited by in RCA: 562] [Article Influence: 112.4] [Reference Citation Analysis (0)] |
| 16. | Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070-2079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 349] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 17. | Sjöholm A, Nyström T. Endothelial inflammation in insulin resistance. Lancet. 2005;365:610-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Festa A, D'Agostino R Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS). Circulation. 2000;102:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1606] [Cited by in RCA: 1590] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 19. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7376] [Article Influence: 1844.0] [Reference Citation Analysis (1)] |
| 20. | WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, Annane D, Azevedo LCP, Berwanger O, Cavalcanti AB, Dequin PF, Du B, Emberson J, Fisher D, Giraudeau B, Gordon AC, Granholm A, Green C, Haynes R, Heming N, Higgins JPT, Horby P, Jüni P, Landray MJ, Le Gouge A, Leclerc M, Lim WS, Machado FR, McArthur C, Meziani F, Møller MH, Perner A, Petersen MW, Savovic J, Tomazini B, Veiga VC, Webb S, Marshall JC. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324:1330-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1683] [Cited by in RCA: 1678] [Article Influence: 335.6] [Reference Citation Analysis (0)] |
| 21. | Feng Q, Zhou H, Zhang X, Liu X, Wang J, Zhang C, Ma X, Quan C, Zheng Z. Acarbose, as a potential drug, effectively blocked the dynamic metastasis of EV71 from the intestine to the whole body. Infect Genet Evol. 2020;81:104210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Dang M, Wang X, Wang Q, Wang Y, Lin J, Sun Y, Li X, Zhang L, Lou Z, Wang J, Rao Z. Molecular mechanism of SCARB2-mediated attachment and uncoating of EV71. Protein Cell. 2014;5:692-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 23. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14118] [Article Influence: 2823.6] [Reference Citation Analysis (1)] |
| 24. | Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444-1448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3274] [Cited by in RCA: 3673] [Article Influence: 734.6] [Reference Citation Analysis (0)] |
| 25. | Watanabe Y, Allen JD, Wrapp D, McLellan JS, Crispin M. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369:330-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1190] [Cited by in RCA: 1169] [Article Influence: 233.8] [Reference Citation Analysis (0)] |
| 26. | Alifano M, Alifano P, Forgez P, Iannelli A. Renin-angiotensin system at the heart of COVID-19 pandemic. Biochimie. 2020;174:30-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 27. | Gu Y, Wang X, Li J, Zhang Y, Zhong H, Liu R, Zhang D, Feng Q, Xie X, Hong J, Ren H, Liu W, Ma J, Su Q, Zhang H, Yang J, Zhao X, Gu W, Bi Y, Peng Y, Xu X, Xia H, Li F, Yang H, Xu G, Madsen L, Kristiansen K, Ning G, Wang W. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017;8:1785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 297] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 28. | Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159:944-955.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 739] [Cited by in RCA: 1062] [Article Influence: 212.4] [Reference Citation Analysis (0)] |
| 29. | Au Yeung SL, Luo S, Schooling CM. The impact of GDF-15, a biomarker for metformin, on the risk of coronary artery disease, breast and colorectal cancer, and type 2 diabetes and metabolic traits: a Mendelian randomisation study. Diabetologia. 2019;62:1638-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 30. | Zhang J, Dong J, Martin M, He M, Gongol B, Marin TL, Chen L, Shi X, Yin Y, Shang F, Wu Y, Huang HY, Zhang J, Zhang Y, Kang J, Moya EA, Huang HD, Powell FL, Chen Z, Thistlethwaite PA, Yuan ZY, Shyy JY. AMP-activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am J Respir Crit Care Med. 2018;198:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |