Published online Oct 15, 2021. doi: 10.4239/wjd.v12.i10.1731
Peer-review started: June 28, 2021
First decision: July 15, 2021
Revised: July 24, 2021
Accepted: August 6, 2021
Article in press: August 6, 2021
Published online: October 15, 2021
Processing time: 106 Days and 14.8 Hours
Recently, specific immunometabolic profiles have been postulated in patients with schizophrenia, even before full-blown disease and independent of antipsychotic treatment. Proteomic profiling studies offer a promising potential for elucidating the cellular and molecular pathways that may be involved in the onset and progression of schizophrenia symptoms, and co-occurrent metabolic changes. In view of all this, we were intrigued to explore galectin-3 (Gal-3) as a glycan, and in our previous study, we measured its elevated levels in remission of schizophrenia. The finding may be a consequence of antipsychotic treatment and may have an impact on the onset of inflammation, the development of obesity, and the presumed cognitive changes in schizophrenia. In the animal study, it was shown that downregulation of Gal-3 was beneficial in insulin regulation of obesity and cognitive preservation. Strategies involving plasma exchange are discussed in this review, particularly in the context of Gal-3 elimination.
Core Tip: Atypical antipsychotic use can be associated with undesired metabolic effects. In that context, glycosylation has become a new target in the investigation of schizophrenia pathophysiology. As a glycan, galectin-3 (Gal-3) might be involved in the inflammation-insulin resistance-obesity cascade in schizophrenia, leading to cognitive changes. Eliminating Gal-3 influence may be beneficial in preserving cognition and reestablishing metabolic balance.
- Citation: Borovcanin MM, Vesic K, Jovanovic M, Mijailovic NR. Galectin-3 possible involvement in antipsychotic-induced metabolic changes of schizophrenia: A minireview. World J Diabetes 2021; 12(10): 1731-1739
- URL: https://www.wjgnet.com/1948-9358/full/v12/i10/1731.htm
- DOI: https://dx.doi.org/10.4239/wjd.v12.i10.1731
Clinical practice raises many questions regarding somatic states that accompany or are a consequence of mental illnesses. As schizophrenia is an extremely complex and debilitating mental disorder, overall treatment must take into account the somatic comorbidity of the patients. Although schizophrenia requires special attention and care in terms of lifestyle and antipsychotic treatment, a particular immunometabolic profile has recently been postulated, even before the disease onset[1]. The use of atypical antipsychotics is often associated with undesired metabolic and endocrine side effects including obesity, dyslipidemia, hyperglycemia, and insulin resistance[2]. To summarize, patients with schizophrenia most probably could have other comorbidities, regardless of their specific immunometabolic profile and antipsychotic therapy, and the somatic states may also lead to metabolic changes.
The identification of defects in cell biology and molecular phenotype underlying schizophrenia represents a challenging new approach to the study of this complex neurodegenerative disorder. Proteomic profiling studies, in which many proteins are tested for their relevance to the disease, are still in their infancy but the potential for elucidating the cellular and molecular pathways that may be involved in the onset and progression of schizophrenia is promising[3].
Altered protein post translational modifications such as glycosylation have become a new target of investigation in the pathophysiology of schizophrenia[4]. Glycosy
Aberrant glycosylation has been identified in the serum, cerebrospinal fluid, urine, and postmortem brain tissue of schizophrenia patients[7]. Early evidence of glycosylation abnormalities in schizophrenia reported reduced glycoprotein expression in urine samples from male schizophrenia patients, and was consistent with abnormal glycan composition[8]. Altered monosaccharide composition of attached glycans was also found in the blood serum of the patients[9]. An increased serum glycoprotein level was also confirmed in young schizophrenia patients 13-17 years of age[10].
Abnormalities of N-linked glycosylation in schizophrenia have been observed in neurotransmitter receptor and transporter subunits, subunits from α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid, kainate, and gamma-aminobutyric acid (GABA)A receptor families in various brain regions, including the dorsolateral prefrontal cortex, anterior cingulate cortex, and superior temporal gyrus[11-14]. Receptors containing abnormally N-glycosylated subunits have also been shown to exhibit abnormal subcellular distribution in schizophrenia, suggesting cellular consequences of abnormal protein glycosylation[15]. Widespread glycosylation abnormalities due to abnormal glycosylation enzyme expression have also been reported in schizophrenia[16-18].
We have recently elaborated on the contrasting roles of the galectin-3 (Gal-3) through the schizophrenia continuance[19]. We also discussed the various somatic states co-occurring in schizophrenia that could be related to Gal-3. In this review, our interdisciplinary team seeks to further elucidate the mechanisms underlying the impact of glycans on early development, and how Gal-3 may further influence subsequent metabolic changes. However, our focus will be on the interplay of Gal-3 with antipsychotics during the course of the disease in an attempt to elucidate specific non-CNS systemic changes. Overall, that may lead to conclusions that allow more selective therapy of schizophrenia in the future.
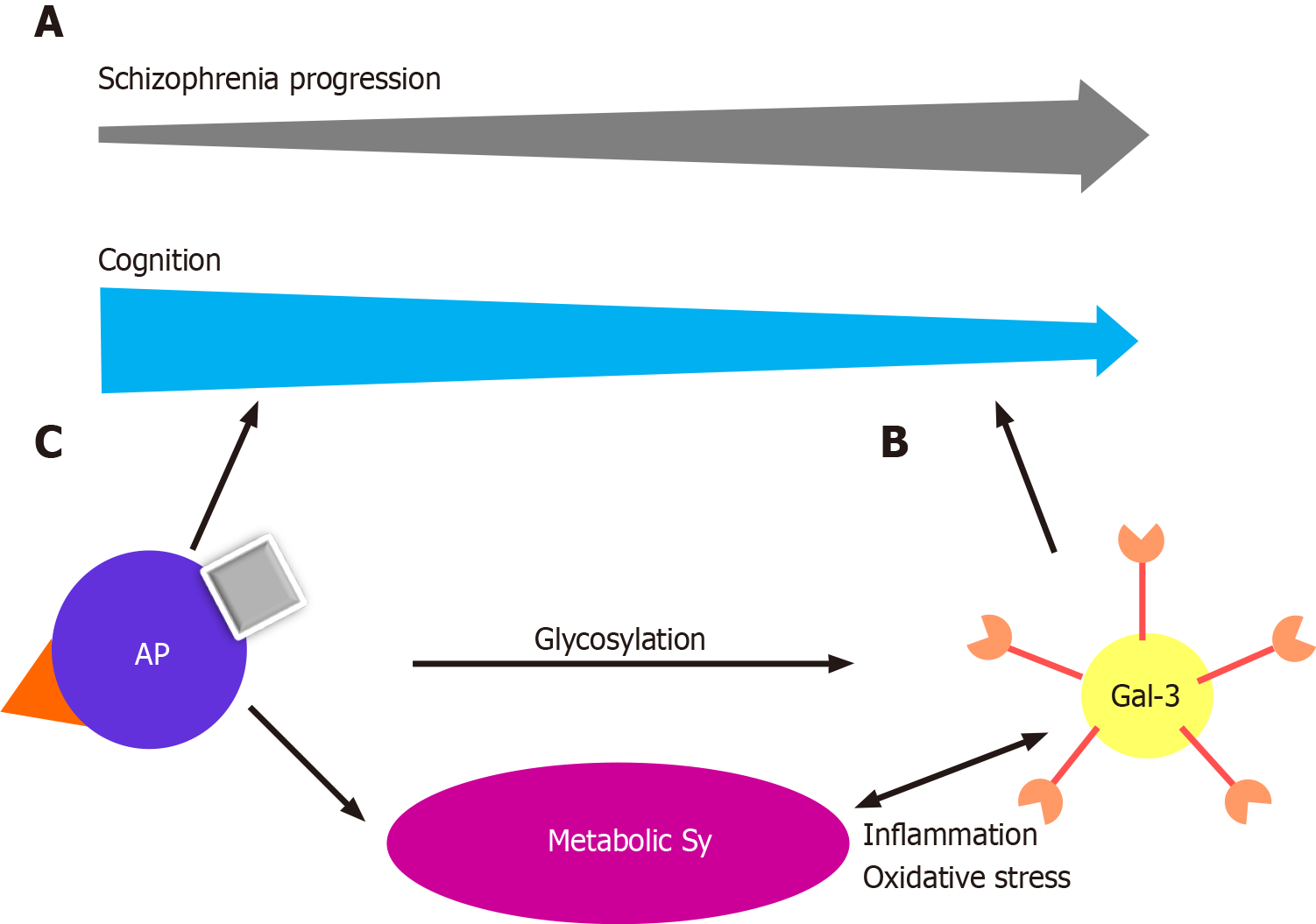
In recent years, an increasing body of evidence has highlighted the involvement of Gal-3 in neurodevelopment and neurodegenerative diseases[20]. Scientific advances during the last decade have led to the discovery that Gal-3 plays a significant role in normal murine brain development, neuroblast migration, oligodendrocyte differentiation, and basal gliogenesis[21-24]. Chronic inflammation, mitochondrial damage and oxidative stress are factors common to neurodegenerative and metabolic diseases, in which sustained responses to inflammation contribute to neurodegeneration and progression of the disease[24,25]. Glial cell dysregulation is the main characteristic of chronic inflammation in neurodegenerative diseases, leading to changes in glycan expression in brain cells[26,27]. Previous studies have shown that inflammatory stimuli upregulate Gal-3 expression in activated microglia, and conversely, Gal-3 has been proposed as a modulator of the inflammatory response through microglial activation, cell adhesion, and cytokine release[28-32]. Recently, Gal-3 was shown to regulate microglial response to promote remyelination[23]. All this leads to the conclusion that Gal-3 is a key player in control of the switch between protective and disruptive microglial effects. In multiple sclerosis, Gal-3 expression is increased in periventricular inflammatory lesions[33]. Nishihara et al[34] investigated whether anti-Gal-3 antibodies might be a novel diagnostic marker and a possible therapeutic target in patients with secondary, progressive multiple sclerosis. Gal-3 deficiency reduces inflammation and disease severity in experimental autoimmune encephalomyelitis, Alzheimer’s, and Parkinson’s disease[35-37]. We reported elevated levels of Gal-3 in the stable phase of schizophrenia, with the suggestion that this glycan has a proinflammatory effect in the later phase[19] (Figure 1A). All the data indicate that Gal-3 might be a potential biomarker and therapeutic agent in this cohort of neurodegenerative disorders. Gal-3 is not only found in the cells themselves but is also secreted into the extracellular space in kidneys and heart, suggesting its multiple functions[38]. In addition to cell proliferation and differentiation, it promotes oxidative stress and proinflammatory processes and plays an important role in angiotensin II and aldosterone-induced myocardial and kidney fibrosis[39,40]. Studies have shown that elevated levels of Gal-3 are predictors of coronary disease in diabetes mellitus type 2[41]. Gal-3 levels are elevated in maintenance hemodialysis patients, and can be used as a biomarker of vascular calcification, left ventricular hypertrophy, and left ventricular diastolic dysfunction[42-44].
Gal-3 has recently been recognized as an important modulator of biological functions and an emerging participant in the pathogenesis of immune/inflammatory and metabolic disorders[45-47] (Figure 1B). Gal-3 serum levels are elevated in women with polycystic ovary syndrome, especially those with insulin resistance, and those with increased insulin and glucose levels in the glucose tolerance test and it is considered a potential biomarker in prediabetes and diabetes[48-50]. The role of Gal-3 in metabolic disorders and the mechanism by which this lectin modulates excess fat mass, adipose tissue, systemic inflammation, and the associated impairment of glucose regulation, remains to be elucidated. Gal-3 is produced by many cell types, including adipocytes, and increased levels have been confirmed in obese patients[51,52]. Gal-3 is upregulated in growing adipose tissue and during inflammation[53,54]. Gal-3 is an important chemotactic factor for tissue macrophages in adipose tissue[55]. However, the role of Gal-3 in adipose tissue remains disputable because it exerts both deleterious and protective effects. In the general population, levels of circulating Gal-3 correlate positively with age, the prevalence of obesity, diabetes, hypercholesterolemia, and hypertension, markers of inflammation, and target organ damage, indicating a clear association of Gal-3 with metabolic disorders and associated risk factors and complications[50,52,56,57]. Seemingly contradictory results were reported by Ohkura et al[58], who demonstrated that Gal-3 affected the concentration of insulin more than that of glucose, and that the increase of Gal-3 activity in diabetic patients had a protective effect on insulin resistance.
Obesity may influence not only behavior, cognition, and mood, but also adipose tissue dysfunction and inflammation, trigger impairment of insulin signaling, compromise the storage of triglycerides, and contribute to insulin resistance with high levels of free fatty acids[59]. Moreover, all the processes associated with insulin resistance and chronic hyperglycemia induce oxidative stress and inflammatory responses that lead to neuronal death, cognitive impairment, and neurodegeneration.
Hippocampal insulin resistance is the key factor in cognitive deficits. In an animal model study, insulin signaling in the hippocampus was shown to be affected by a cascade in which obesity induced chronic inflammation and chronic inflammation had role in obesity-related insulin resistance[60]. Moreover, chronic inflammation is suppressed by Gal-3, so Gal-3 directly impacts insulin signaling and might be a targetable link between inflammation and insulin sensitivity. Qin et al[60] suggested that the development of cognitive deficits in obese people could be inhibited through Gal-3 decrement.
Obesity is reported in approximately 50% of patients, metabolic syndrome in up to 40%, glucose intolerance in up to 25%, and diabetes in up to 15% of patients with schizophrenia[61]. The increased prevalence of these conditions is multifactorial. Antipsychotics can cause weight gain, glucose intolerance, and other metabolic complications[62] (Figure 1C). A recent meta-analysis of metabolic parameters in patients with first-episode psychosis, which can be described as early schizophrenia, showed increased insulin resistance and impaired glucose tolerance in the patients compared with healthy, matched controls, implying that schizophrenia might share intrinsic inflammatory disease pathways with type 2 diabetes[63]. We have previously discussed our findings of the possibly protective properties of Gal-3 in type-2 diabetes, but triggering metabolic changes and myocardial fibrosis[19].
Relatively few studies have investigated the effects of antipsychotic treatment on the serum glycosylation profiles in schizophrenia patients. Reports examining glycan expression in schizophrenia patients showed that the glycan profile in serum and cerebrospinal fluid of first onset, unmedicated schizophrenia patients differs from the profile of healthy controls[64]. The results showed that some types of sialylated N-glycans derived from low-abundance serum proteins are significantly increased in patients with schizophrenia compared with controls. The study found a two-fold increase in serum glycan levels in male schizophrenia patients, with gender-specific differences also apparent[65]. Glycemic differences have also been reported in patients with acute paranoid schizophrenia before and after 6 wk of treatment with olanzapine, an atypical antipsychotic medication[65]. Olanzapine administration increased galactosylation and sialylation of serum N-glycans, suggesting increased activity of specific galactosyltransferases and increased availability of galactose residues for sialylation. The results indicate that the glycosylation profile of serum proteins can be used to monitor patients with schizophrenia after treatment. Given the confirmed effects of olanzapine on hepatic enzymes, it is possible that the reported changes in glycosylation induced by olanzapine treatment may occur because of the altered activity of hepatic glycosylation-processing enzymes[66].
As schizophrenia may have an evolving, progressive pathology, Narayan et al[67] focused on changes in gene expression and molecular pathways throughout illness progression. They assessed the alterations in patients treated with the typical antipsychotic medication, chlorpromazine, at early (≤ 4 years), intermediate (7-18 years), and late (≥ 28 years) stages of schizophrenia. The results showed that biopolymer glycosylation, protein amino acid glycosylation, and glycoprotein biosynthesis were increased in intermediate-stage patients. Analysis of differences in gene expression revealed that carbohydrate metabolism was dominant in short-term illness, whereas lipid metabolism prevailed in intermediate-term illness. Overall, short-term illness was particularly associated with disruptions in gene expression, metal ion binding, ribonucleic acid processing, and vesicle-mediated transport. Considerably different from short-term illness, long-term illness was associated with inflammation, glycosylation, apoptosis, and immune dysfunction.
A postmortem study compared the effects of atypical (olanzapine and risperidone) vs typical antipsychotics (chlorpromazine and haloperidol) on the livers, various genes, and molecular functions of patients[68]. The results demonstrated that typical antipsychotics affected genes associated with nuclear protein, stress responses, and phosphorylation, whereas atypical antipsychotics increased gene expression associated with Golgi/endoplasmic reticulum, and cytoplasmic transport, suggesting that atypical antipsychotics affect post translational modifications. The study showed that olanzapine treatment increased the expression of the B4GALT1 gene in the liver of schizophrenia patients. That gene encodes β1,4-galactosyltransferase I (Gal-T1). Increased expression and activity of the enzyme lead to increased galactosylation of GlcNAc residues in glycans, which is consistent with the results of a study performed by Telford et al[65]. Genes associated with lipid metabolism were consistently downregulated in the typical compared with the atypical antipsychotic group.
However, dysregulation of adipose tissue homeostasis appears to be a critical factor[69]. An untargeted proteomic analysis of the effect of antipsychotics on adipose tissue was performed in a rat schizophrenia-like methylazoxymethanol acetate model[70]. Chronic, 8-wk-long application of three antipsychotics was characterized by differences in the likelihood of inducing metabolic alterations. Olanzapine, risperidone, and haloperidol, caused alterations in protein N-linked glycosylation in adipose tissue, providing further evidence that dysregulated glycosylation in schizophrenia may also be caused to some extent by antipsychotic treatment. Drug-specific effects included upregulation of insulin resistance (olanzapine), upregulation of fatty acid metabolism (risperidone), and upregulation of nucleic acid metabolism (haloperidol). Individual metabolic characteristics might also predispose to a different likelihood of becoming obese after antipsychotic treatment. Gal-3 has been shown to be associated with the onset of schizophrenia, and its elevation could have consequent deleterious effects (Figure 1). In addition, it must be taken into account that our patients were treated with risperidone or paliperidone, which are antipsychotics that may upregulate fatty acid metabolism and have Gal-3-elevating properties[71].
In this context, it is necessary and urgent to develop more selective treatment strategies. The phase of the illness also needs to be considered, with a focus on early interventions. The possibility that schizophrenia is secondary to a circulating, large molecular-weight substance has been explored with variable success. However, a double-blind evaluation of plasmapheresis in ten patients with schizophrenia yielded negative results, and the procedure did not lead to a reduction in psychosis[72]. As hypercholesterolemia has been treated with plasmapheresis, and recently the therapeutic usefulness of Gal-3 depletion apheresis has been demonstrated in inflammation-mediated disease, targeting Gal-3 molecule may be a useful way to address immunometabolic problems and cognitive deterioration in schizophrenia in the future[73,74].
The question is whether extrapolations of preclinical and research data are applicable in clinical practice. Gal-3 relevance could be very interesting in further exploration of the genesis of schizophrenia in parallel with the metabolic alterations of the patients. It might be useful for clinicians to become familiar with this molecule and its precise roles in each phase of the disease in order to improve cognition and reestablishing metabolic balance in schizophrenia.
This review was enriched in valuable interactions by the Center for Molecular Medicine and Stem Cell Research, at the Faculty of Medical Sciences, University of Kragujevac, Kragujevac, Serbia. We would like to thank Bojana Mircetic for language editing.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gaman MA, Sorić T S-Editor: Yan JP L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Kucerova J, Babinska Z, Horska K, Kotolova H. The common pathophysiology underlying the metabolic syndrome, schizophrenia and depression. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Reynolds GP, McGowan OO. Mechanisms underlying metabolic disturbances associated with psychosis and antipsychotic drug treatment. J Psychopharmacol. 2017;31:1430-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Huang JT, Wang L, Prabakaran S, Wengenroth M, Lockstone HE, Koethe D, Gerth CW, Gross S, Schreiber D, Lilley K, Wayland M, Oxley D, Leweke FM, Bahn S. Independent protein-profiling studies show a decrease in apolipoprotein A1 levels in schizophrenia CSF, brain and peripheral tissues. Mol Psychiatry. 2008;13:1118-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Williams SE, Mealer RG, Scolnick EM, Smoller JW, Cummings RD. Aberrant glycosylation in schizophrenia: a review of 25 years of post-mortem brain studies. Mol Psychiatry. 2020;25:3198-3207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Rudd PM, Dwek RA. Glycosylation: heterogeneity and the 3D structure of proteins. Crit Rev Biochem Mol Biol. 1997;32:1-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 317] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Kleene R, Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci. 2004;5:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 406] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 7. | Mueller TM, Meador-Woodruff JH. Post-translational protein modifications in schizophrenia. NPJ Schizophr. 2020;6:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Varma RS, Varma R, Mesmer R. Urinary glycoproteins in schizophrenia. Biochem Med. 1976;15:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Varma R, Hoshino AY. Serum glycoproteins in schizophrenia. Carbohydr Res. 1980;82:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Varma R, Michos GA, Gordon BJ, Varma RS, Shirey RE. Serum glycoconjugates in children with schizophrenia and conduct and adjustment disorders. Biochem Med. 1983;30:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res. 2010;117:92-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Tucholski J, Simmons MS, Pinner AL, Haroutunian V, McCullumsmith RE, Meador-Woodruff JH. Abnormal N-linked glycosylation of cortical AMPA receptor subunits in schizophrenia. Schizophr Res. 2013;146:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Tucholski J, Simmons MS, Pinner AL, McMillan LD, Haroutunian V, Meador-Woodruff JH. N-linked glycosylation of cortical N-methyl-D-aspartate and kainate receptor subunits in schizophrenia. Neuroreport. 2013;24:688-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Mueller TM, Haroutunian V, Meador-Woodruff JH. N-Glycosylation of GABAA receptor subunits is altered in Schizophrenia. Neuropsychopharmacology. 2014;39:528-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Hammond JC, McCullumsmith RE, Funk AJ, Haroutunian V, Meador-Woodruff JH. Evidence for abnormal forward trafficking of AMPA receptors in frontal cortex of elderly patients with schizophrenia. Neuropsychopharmacology. 2010;35:2110-2119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Kippe JM, Mueller TM, Haroutunian V, Meador-Woodruff JH. Abnormal N-acetylglucosaminyltransferase expression in prefrontal cortex in schizophrenia. Schizophr Res. 2015;166:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Mueller TM, Yates SD, Haroutunian V, Meador-Woodruff JH. Altered fucosyltransferase expression in the superior temporal gyrus of elderly patients with schizophrenia. Schizophr Res. 2017;182:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Mueller T, Simmons MS, Helix AT, Haroutunian V, Meador-Woodruff JH. Glycosylation enzyme mRNA expression in dorsolateral prefrontal cortex of elderly patients with schizophrenia: Evidence for dysregulation of multiple glycosylation pathways. 2018 Preprint. Available from: bioRxiv:369314. [DOI] [Full Text] |
| 19. | Borovcanin MM, Radosavljevic GD, Pantic J, Milovanovic J, Mijailovic NR, Arsenijevic AN, Arsenijevic NN. Contrasting Roles of the Galectin-3 in the Schizophrenia Onset, Clinical Presentation and Somatic Comorbidity. Curr Top Med Chem. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Puigdellívol M, Allendorf DH, Brown GC. Sialylation and Galectin-3 in Microglia-Mediated Neuroinflammation and Neurodegeneration. Front Cell Neurosci. 2020;14:162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 21. | Al-Dalahmah O, Campos Soares L, Nicholson J, Draijer S, Mundim M, Lu VM, Sun B, Tyler T, Adorján I, O'Neill E, Szele FG. Galectin-3 modulates postnatal subventricular zone gliogenesis. Glia. 2020;68:435-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Comte I, Kim Y, Young CC, van der Harg JM, Hockberger P, Bolam PJ, Poirier F, Szele FG. Galectin-3 maintains cell motility from the subventricular zone to the olfactory bulb. J Cell Sci. 2011;124:2438-2447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 23. | Thomas L, Pasquini LA. Galectin-3-Mediated Glial Crosstalk Drives Oligodendrocyte Differentiation and (Re)myelination. Front Cell Neurosci. 2018;12:297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1109] [Cited by in RCA: 1007] [Article Influence: 67.1] [Reference Citation Analysis (0)] |
| 25. | Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 649] [Cited by in RCA: 768] [Article Influence: 76.8] [Reference Citation Analysis (10)] |
| 26. | Tang Y, Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol. 2016;53:1181-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1627] [Cited by in RCA: 1550] [Article Influence: 172.2] [Reference Citation Analysis (0)] |
| 27. | Ramos-Martinez I, Martínez-Loustalot P, Lozano L, Issad T, Limón D, Díaz A, Perez-Torres A, Guevara J, Zenteno E. Neuroinflammation induced by amyloid β25-35 modifies mucin-type O-glycosylation in the rat's hippocampus. Neuropeptides. 2018;67:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Srejovic I, Selakovic D, Jovicic N, Jakovljević V, Lukic ML, Rosic G. Galectin-3: Roles in Neurodevelopment, Neuroinflammation, and Behavior. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Ramírez Hernández E, Sánchez-Maldonado C, Mayoral Chávez MA, Hernández-Zimbrón LF, Patricio Martínez A, Zenteno E, Limón Pérez de León ID. The therapeutic potential of galectin-1 and galectin-3 in the treatment of neurodegenerative diseases. Expert Rev Neurother. 2020;20:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Starossom SC, Mascanfroni ID, Imitola J, Cao L, Raddassi K, Hernandez SF, Bassil R, Croci DO, Cerliani JP, Delacour D, Wang Y, Elyaman W, Khoury SJ, Rabinovich GA. Galectin-1 deactivates classically activated microglia and protects from inflammation-induced neurodegeneration. Immunity. 2012;37:249-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 31. | Burguillos MA, Svensson M, Schulte T, Boza-Serrano A, Garcia-Quintanilla A, Kavanagh E, Santiago M, Viceconte N, Oliva-Martin MJ, Osman AM, Salomonsson E, Amar L, Persson A, Blomgren K, Achour A, Englund E, Leffler H, Venero JL, Joseph B, Deierborg T. Microglia-Secreted Galectin-3 Acts as a Toll-like Receptor 4 Ligand and Contributes to Microglial Activation. Cell Rep. 2015;10:1626-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 273] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 32. | Dhirapong A, Lleo A, Leung P, Gershwin ME, Liu FT. The immunological potential of galectin-1 and -3. Autoimmun Rev. 2009;8:360-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 33. | James RE, Hillis J, Adorján I, Gration B, Mundim MV, Iqbal AJ, Majumdar MM, Yates RL, Richards MM, Goings GE, DeLuca GC, Greaves DR, Miller SD, Szele FG. Loss of galectin-3 decreases the number of immune cells in the subventricular zone and restores proliferation in a viral model of multiple sclerosis. Glia. 2016;64:105-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Nishihara H, Shimizu F, Kitagawa T, Yamanaka N, Akada J, Kuramitsu Y, Sano Y, Takeshita Y, Maeda T, Abe M, Koga M, Nakamura K, Kanda T. Identification of galectin-3 as a possible antibody target for secondary progressive multiple sclerosis. Mult Scler. 2017;23:382-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Jiang HR, Al Rasebi Z, Mensah-Brown E, Shahin A, Xu D, Goodyear CS, Fukada SY, Liu FT, Liew FY, Lukic ML. Galectin-3 deficiency reduces the severity of experimental autoimmune encephalomyelitis. J Immunol. 2009;182:1167-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 36. | Tao CC, Cheng KM, Ma YL, Hsu WL, Chen YC, Fuh JL, Lee WJ, Chao CC, Lee EHY. Galectin-3 promotes Aβ oligomerization and Aβ toxicity in a mouse model of Alzheimer's disease. Cell Death Differ. 2020;27:192-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 37. | Yazar HO, Yazar T, Cihan M. A preliminary data: Evaluation of serum Galectin-3 levels in patients with Idiopathic Parkinson's Disease. J Clin Neurosci. 2019;70:164-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 38. | Wang L, Guo XL. Molecular regulation of galectin-3 expression and therapeutic implication in cancer progression. Biomed Pharmacother. 2016;78:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 39. | Lin YH, Chou CH, Wu XM, Chang YY, Hung CS, Chen YH, Tzeng YL, Wu VC, Ho YL, Hsieh FJ, Wu KD; TAIPAI Study Group. Aldosterone induced galectin-3 secretion in vitro and in vivo: from cells to humans. PLoS One. 2014;9:e95254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Kumric M, Ticinovic Kurir T, Borovac JA, Bozic J. Role of novel biomarkers in diabetic cardiomyopathy. World J Diabetes. 2021;12:685-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 41. | Ozturk D, Celik O, Satilmis S, Aslan S, Erturk M, Cakmak HA, Kalkan AK, Ozyilmaz S, Diker V, Gul M. Association between serum galectin-3 levels and coronary atherosclerosis and plaque burden/structure in patients with type 2 diabetes mellitus. Coron Artery Dis. 2015;26:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Wang Z, Chen Z, Ma X, Yu H, Chen X. The predictive value of serum galectin 3 for abdominal aortic calcification in maintenance hemodialysis patients: A prospective cohort study. Hemodial Int. 2020;24:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Yilmaz H, Gurel OM, Celik HT, Bozkurt A, Yildirim ME, Bilgic I, Bilgic MA, Bavbek N, Akcay A. Relationship of galectin-3 to left ventricular geometry and hypertrophy in chronic hemodialysis patients. Herz. 2015;40:702-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Gurel OM, Yilmaz H, Celik TH, Cakmak M, Namuslu M, Bilgiç AM, Bavbek N, Akcay A, Eryonucu B. Galectin-3 as a new biomarker of diastolic dysfunction in hemodialysis patients. Herz. 2015;40:788-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Dumic J, Dabelic S, Flögel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 851] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 46. | Pugliese G, Iacobini C, Pesce CM, Menini S. Galectin-3: an emerging all-out player in metabolic disorders and their complications. Glycobiology. 2015;25:136-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 47. | Pugliese G, Iacobini C, Ricci C, Blasetti Fantauzzi C, Menini S. Galectin-3 in diabetic patients. Clin Chem Lab Med. 2014;52:1413-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | Alves MT, de Souza IDP, Ferreira CN, Cândido AL, Bizzi MF, Oliveira FR, Reis FM, Gomes KB. Galectin-3 is a potential biomarker to insulin resistance and obesity in women with polycystic ovary syndrome. Gynecol Endocrinol. 2020;36:760-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Yilmaz H, Celik HT, Ozdemir O, Kalkan D, Namuslu M, Abusoglu S, Atalay CR, Yigitoglu R. Serum galectin-3 levels in women with PCOS. J Endocrinol Invest. 2014;37:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Yilmaz H, Cakmak M, Inan O, Darcin T, Akcay A. Increased levels of galectin-3 were associated with prediabetes and diabetes: new risk factor? J Endocrinol Invest. 2015;38:527-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 51. | Rhodes DH, Pini M, Castellanos KJ, Montero-Melendez T, Cooper D, Perretti M, Fantuzzi G. Adipose tissue-specific modulation of galectin expression in lean and obese mice: evidence for regulatory function. Obesity (Silver Spring). 2013;21:310-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 52. | Weigert J, Neumeier M, Wanninger J, Bauer S, Farkas S, Scherer MN, Schnitzbauer A, Schäffler A, Aslanidis C, Schölmerich J, Buechler C. Serum galectin-3 is elevated in obesity and negatively correlates with glycosylated hemoglobin in type 2 diabetes. J Clin Endocrinol Metab. 2010;95:1404-1411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 53. | Baek JH, Kim SJ, Kang HG, Lee HW, Kim JH, Hwang KA, Song J, Chun KH. Galectin-3 activates PPARγ and supports white adipose tissue formation and high-fat diet-induced obesity. Endocrinology. 2015;156:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 54. | Flotte TJ, Springer TA, Thorbecke GJ. Dendritic cell and macrophage staining by monoclonal antibodies in tissue sections and epidermal sheets. Am J Pathol. 1983;111:112-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 55. | Li P, Liu S, Lu M, Bandyopadhyay G, Oh D, Imamura T, Johnson AMF, Sears D, Shen Z, Cui B, Kong L, Hou S, Liang X, Iovino S, Watkins SM, Ying W, Osborn O, Wollam J, Brenner M, Olefsky JM. Hematopoietic-Derived Galectin-3 Causes Cellular and Systemic Insulin Resistance. Cell. 2016;167:973-984.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 56. | de Boer RA, van Veldhuisen DJ, Gansevoort RT, Muller Kobold AC, van Gilst WH, Hillege HL, Bakker SJ, van der Harst P. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med. 2012;272:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 282] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 57. | Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 455] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 58. | Ohkura T, Fujioka Y, Nakanishi R, Shiochi H, Sumi K, Yamamoto N, Matsuzawa K, Izawa S, Ohkura H, Ueta E, Kato M, Miyoshi E, Taniguchi S, Yamamoto K. Low serum galectin-3 concentrations are associated with insulin resistance in patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2014;6:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Flores-Dorantes MT, Díaz-López YE, Gutiérrez-Aguilar R. Environment and Gene Association With Obesity and Their Impact on Neurodegenerative and Neurodevelopmental Diseases. Front Neurosci. 2020;14:863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 60. | Qin S, Sun D, Mu J, Ma D, Tang R, Zheng Y. Purple sweet potato color improves hippocampal insulin resistance via down-regulating SOCS3 and galectin-3 in high-fat diet mice. Behav Brain Res. 2019;359:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Annamalai A, Tek C. An overview of diabetes management in schizophrenia patients: office based strategies for primary care practitioners and endocrinologists. Int J Endocrinol. 2015;2015:969182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | De Hert M, Schreurs V, Sweers K, Van Eyck D, Hanssens L, Sinko S, Wampers M, Scheen A, Peuskens J, van Winkel R. Typical and atypical antipsychotics differentially affect long-term incidence rates of the metabolic syndrome in first-episode patients with schizophrenia: a retrospective chart review. Schizophr Res. 2008;101:295-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 63. | Perry BI, McIntosh G, Weich S, Singh S, Rees K. The association between first-episode psychosis and abnormal glycaemic control: systematic review and meta-analysis. Lancet Psychiatry. 2016;3:1049-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 64. | Stanta JL, Saldova R, Struwe WB, Byrne JC, Leweke FM, Rothermund M, Rahmoune H, Levin Y, Guest PC, Bahn S, Rudd PM. Identification of N-glycosylation changes in the CSF and serum in patients with schizophrenia. J Proteome Res. 2010;9:4476-4489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Telford JE, Bones J, McManus C, Saldova R, Manning G, Doherty M, Leweke FM, Rothermundt M, Guest PC, Rahmoune H, Bahn S, Rudd PM. Antipsychotic treatment of acute paranoid schizophrenia patients with olanzapine results in altered glycosylation of serum glycoproteins. J Proteome Res. 2012;11:3743-3752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Pae CU, Lim HK, Kim TS, Kim JJ, Lee CU, Lee SJ, Lee C, Paik IH. Naturalistic observation on the hepatic enzyme changes in patients treated with either risperidone or olanzapine alone. Int Clin Psychopharmacol. 2005;20:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 67. | Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B, Thomas EA. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res. 2008;1239:235-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 68. | Choi KH, Higgs BW, Weis S, Song J, Llenos IC, Dulay JR, Yolken RH, Webster MJ. Effects of typical and atypical antipsychotic drugs on gene expression profiles in the liver of schizophrenia subjects. BMC Psychiatry. 2009;9:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 69. | Gonçalves P, Araújo JR, Martel F. Antipsychotics-induced metabolic alterations: focus on adipose tissue and molecular mechanisms. Eur Neuropsychopharmacol. 2015;25:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 70. | Kucera J, Horska K, Hruska P, Kuruczova D, Micale V, Ruda-Kucerova J, Bienertova-Vasku J. Interacting effects of the MAM model of schizophrenia and antipsychotic treatment: Untargeted proteomics approach in adipose tissue. Prog Neuropsychopharmacol Biol Psychiatry. 2021;108:110165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Borovcanin MM, Janicijevic SM, Jovanovic IP, Gajovic N, Arsenijevic NN, Lukic ML. IL-33/ST2 Pathway and Galectin-3 as a New Analytes in Pathogenesis and Cardiometabolic Risk Evaluation in Psychosis. Front Psychiatry. 2018;9:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Schulz SC, van Kammen DP, Waters R, Klein HG, Balow JE, Bunney WE Jr. Double-blind evaluation of plasmapheresis in schizophrenic patients: a pilot study. Artif Organs. 1983;7:317-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Dann EJ, Shamir R, Mashiach T, Shaoul R, Badian A, Stravets T, Kerzman Y, Finkelbaum S, Gaitini D, Lorber A, Bonstein L. Early-onset plasmapheresis and LDL-apheresis provide better disease control for pediatric homozygous familial hypercholesterolemia than HMG-CoA reductase inhibitors and ameliorate atherosclerosis. Transfus Apher Sci. 2013;49:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 74. | Navarro-Alvarez N, Goncalves B, Andrews AR, Wang Z, Harrington E, Shah J, Sachs DH, Eliaz I, Huang CA. The effects of galectin-3 depletion apheresis on induced skin inflammation in a porcine model. J Clin Apher. 2018;33:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |