INTRODUCTION
Diabetes mellitus (DM) is not only a common chronic disease but also a global health problem that has expanded significantly over the past several decades. World Health Organization has reported that the number of people with DM has risen from 108 million in 1980 to 422 million and 8.5% of world adult population had DM in 2014[1]. The number of diabetic patients worldwide has been estimated to rise to 592 million in 2035 by the International Diabetes Federation[2]. DM is characterized by hyperglycemia, which is secondary to insulin deficiency, which may be relative or absolute. More than 50% of the patients with DM are not aware of their diagnosis and are at risk for developing complications. It causes microvascular and macrovascular complications. Microvascular complications are generally manifested as nephropathy and retinopathy. Macrovascular complications include coronary artery disease, cerebrovascular disease and peripheral arterial disease[3]. In addition to these complications, pulmonary complications which include reduction in lung function as well as pulmonary fibrosis have been reported in literature[4]. The pulmonary system is prone to undergo microvascular damage and non-enzymatic glycation because of its large vascular bed and presence of abundant connective tissue. Our review is mainly going to focus on lung as a target organ for DM and the association of DM and interstitial lung disease (ILD).
PATHOPHYSIOLOGY OF DM IN ILD
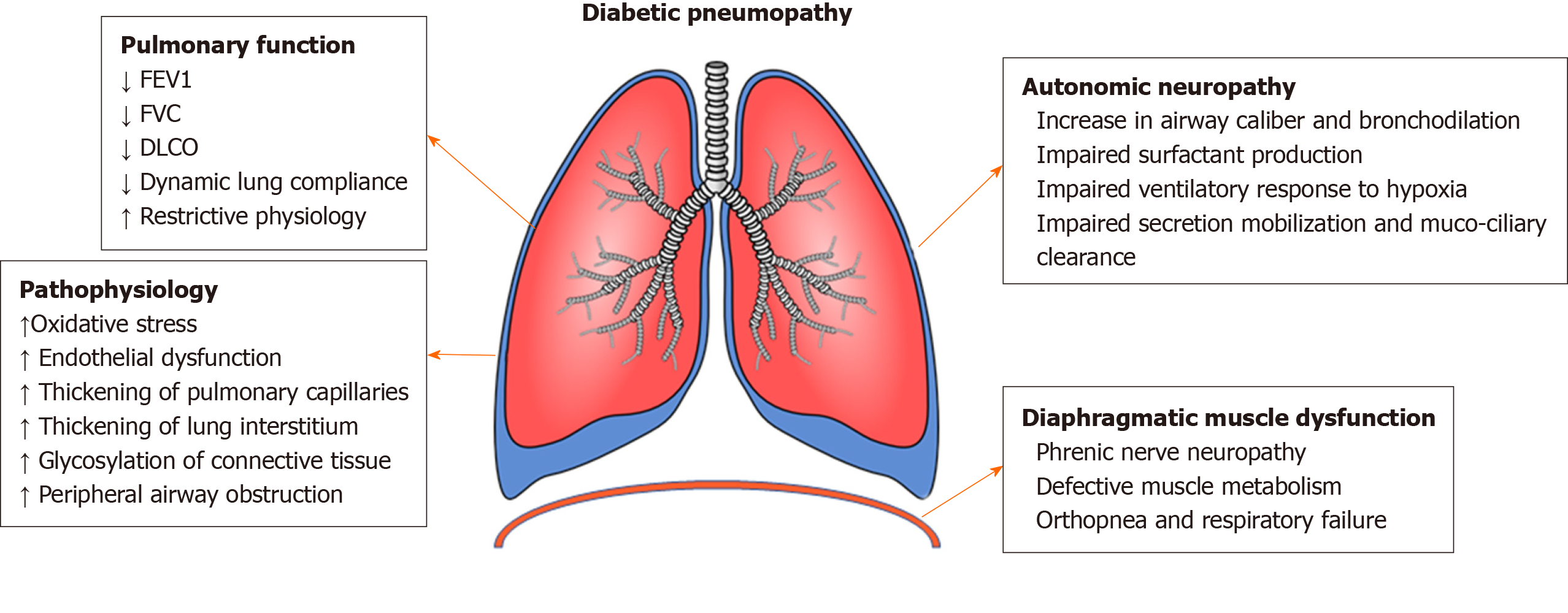
The pathogenesis of DM resulting in interstitial lung disease is multifactorial and highly complex (Figure 1)[5]. Hyperglycemia leads to oxidative stress and causes an imbalance between free radical generation and antioxidant activity and this contributes to lung dysfunction in DM. Diabetes often when poorly controlled leads to autonomic neuropathy which can affect the pulmonary vascular tone resulting in pulmonary hypertension and phrenic nerve neuropathy that can result in diaphragmatic dysfunction and these patients commonly present with unexplained dyspnea and orthopnea. Autonomic neuropathy also affects the pulmonary mechanoreceptors resulting in impaired airway smooth muscle tone and excess surfactant production[6]. Long standing hyperglycemia leads to non-enzymatic glycation of extracellular proteins in the pulmonary interstitium contributing to ILD in DM. DM induced microangiopathy of pulmonary capillaries along with the glycosylation of alveolar basement membrane proteins results in significant impairment of alveolar gas exchange[7].
Figure 1 Pathophysiology of diabetic pneumopathy.
FEV1: Forced expiratory volume in 1 s; FVC: Forced vital capacity; DLCO: Diffusing capacity of the lungs for carbon monoxide.
There is enough data to prove that the incidence of IPF increases with age and hence it is possible that age and lifestyle-related diseases including DM could be a risk factor for the development of IPF. It is also interesting to note that another potential link between IPF and DM is gastroesophageal reflux disease (GERD). Patients with DM are at a higher risk of GERD and studies suggest that GERD is a significant risk factor for IPF because of its association with micro-aspiration[8]. There have been many studies which have reported an accelerated decline in lung function in patients with DM and inadequate glycemic control. Diabetes related microvascular complications also lead to thickening of the basal lamina of pulmonary capillaries, reduced pulmonary capillary blood volume, cardiac autonomic nervous system dysfunction, glycosylation of connective tissue matrix and oxidative stress resulting in significant decline in diffusion capacity of the lung[9].
DM also leads to pulmonary autonomic neuropathy which affects the control of ventilation with an impaired ventilatory response to hypoxia but not to hypercapnia[10]. Dysfunction in parasympathetic tone leads to increase in airway caliber, reduced muco-ciliary clearance and increased susceptibility to lung infections[11]. The defective muscle metabolism leads to reduction in muscle strength. Endurance of respiratory muscles is reduced and is inversely proportional to hemoglobin A1c. DM leading to phrenic nerve neuropathy impairs respiratory neuromuscular function and results in reduction of lung volumes and accelerates the restrictive complications in diabetic patients[12]. Due to the large pulmonary reserve, micro and macrovascular dysfunctions due to DM develop later in lungs than other organs. Oxidative stress, endothelial micro-injuries and platelet activation with consecutive inflammation are considered important mechanisms in development of pulmonary fibrosis[13].
Studies have proposed two major mechanisms by which diabetes leads to lung disease. Thorax and lungs are rich in collagen and elastin and the non-enzymatic glycation of these compounds could result in stiffening of the thoracic cage and lung parenchymal tissue resulting in a restrictive physiology. The second mechanism by which DM causes lung damage is through microvascular damage in the lungs that runs parallel to nephropathy, retinopathy and neuropathy. This results in thickening of alveolar epithelium and pulmonary capillary basal laminae and reduced presence of pulmonary capillary blood volume[14]. The combination of thickening alveolar wall and reduced perfusion results in ventilation perfusion mismatch resulting in impaired diffusion capacity.
PREVALENCE OF ILD IN DM
It is interesting to note that both the international guidelines on ILD and DM do not mention DM as a risk factor for ILD. A German study screened 280 participants (18 to 75 years) at an outpatient clinic to investigate the incidence of restrictive lung disease and ILD in patients with prediabetes and type 2 DM[15]. In this study there were 48 nondiabetics, 68 patients with prediabetes, 29 patients who have been newly diagnosed with type 2 DM and 110 patients with long-term type 2 DM. Five participants with type 2 DM, dyspnea and restrictive lung disease underwent high-resolution computed tomography (CT) and 6-min walk test. Out of the 5, ILD was diagnosed in four patients and histological analysis revealed fibrosing ILD[15]. 9% of the patients who were prediabetes had restrictive lung disease, whereas it was seen in 20% and 27% in newly diagnosed and long-term DM, respectively. In patients with long-term diabetes, presence of albuminuria, nephropathy were independent risk factors for development of restrictive lung disease. The MMRC (Modified Medical Research Council) dyspnea scale showed increased breathlessness in patients with long-term type 2 DM compared to patients with prediabetes and nondiabetics. Increased fasting glucose was significantly associated with decreased forced vital capacity (FVC). Normal lung tissue which was obtained from patients during surgery for lung cancer (3 with and 4 without diabetes) showed increased fibrotic disease in patients with type 2 DM compared to nondiabetics. This study showed increased risk for dyspnea and ILD in patients with type 2 DM[15].
A Japanese case control study analyzed 65 consecutive patients with IPF and 184 control subjects who presented to an outpatient clinic for routine medical examination. The prevalence of DM was 32.7% in patients with IPF and 11.4% in control subjects[16]. A retrospective longitudinal cohort study done in northern California concluded that patients with diabetes are at increased risk of developing IPF [HR-1.54 (1.31-1.81)]. The cohort included 77637 members with DM and 1733591 without diabetes. Incidence rate was 0.09 in nondiabetics and 0.14 in diabetics (per thousand person years)[17]. Suarez et al[18] reported higher prevalence of DM in patients with IPF, Non-specific interstitial pneumonitis (NSIP) and Hypersensitivity pneumonitis (HP). Abramowitz et al[19] investigated 2832 patients with IPF and concluded that 9% of them had DM. Suga et al[20] reported DM in 24% of patients with idiopathic interstitial pneumonias compared to 4.5% of patients with DM in control group.
Patients with certain ILDs such as cryptogenic organizing pneumonia, HP, NSIP, sarcoidosis are frequently treated with chronic oral corticosteroids and this can lead to steroid induced diabetes. A Korean study found that out of 125 patients with idiopathic interstitial pneumonitis who were treated with oral glucocorticoids, 27 patients (21.6%) developed steroid induced DM[21].
DM AND IPF
IPF is a chronic progressive fibrotic ILD of unknown etiology with a high fatality rate and is usually seen in adults over 50 years of age. Aging is an important risk factor for both DM and IPF. Increased thickness of alveolar capillary walls, alveolar walls and pulmonary arteriolar walls representing fibrotic histopathological changes have been reported in autopsied lungs from diabetic patients. The alveolar epithelial cells and endothelial capillary basal laminae are significantly thicker in diabetic patients[22]. A Danish study found that DM is the third most frequently observed comorbidity of IPF after cardiovascular disease and arterial hypertension[23]. The study also suggested DM significantly increases mortality in IPF patients. A multicenter Korean study investigated the relationship between DM and IPF[24]. Out of 1685 patients studied, 299 patients had DM (17.7%). The study found that IPF patients with DM were more likely to have a typical usual interstitial pneumonia pattern on high resolution CT chest including reticular and honeycombing pattern than were IPF patients without DM[24].
PULMONARY FUNCTION TEST IN DIABETES
Diabetes has been associated with decline in lung function and particularly shows a restrictive pattern of ventilatory defect on the pulmonary function testing. Studies have shown a decline in forced expiratory volume in 1 s (FEV1) in patients with DM at a rate 2-3 times faster than that of normal non-smoking subjects[25]. On an average FEV1 reduces by 25 to 30 mL/year in non-smoking healthy adults and around 71 mL per year decline in FEV1 is seen in subjects with DM[26]. In patients with DM, a decline in FVC and dynamic lung compliance which is probably related to peripheral airway obstruction has been reported[25]. Shah et al[27] analyzed pulmonary function parameters in 60 type 2 diabetic patients and 60 normal healthy controls aged 40 to 60 years. FVC, FEV1 were significantly reduced in patients with type 2 DM compared to healthy controls except FEV1/ FVC ratio which was similar in both.
In 2010, van den Borst et al[28] in their meta-analysis, studied the PFT data of 3182 patients with diabetes. They had 27080 control subjects in their meta-analysis. The results showed impaired pulmonary function with a restrictive pattern. FEV1, FVC and diffusing capacity of the lungs for carbon monoxide (DLCO) were significantly decreased in patients with DM when compared to the healthy subjects. The results were irrespective of BMI, smoking, diabetes duration and hemoglobin A1c levels. In a study done by Klein et al[29] 560 patients with DM were compared to nondiabetics and they were found to have a significant reduction in FEV1, FVC and DLCO.
METFORMIN AND IPF
There is ongoing research about the role of metformin in resolution of the fibrotic changes in patients with IPF. IPF is characterized by excessive accumulation of extracellular matrix and remodeling of lung architecture from dysfunctional tissue response to injury. Adenosine monophosphate activated protein kinase (AMPK) is a cellular energy sensor and metabolic regulator. Reduced activity of AMPK has been implicated in the development of organ fibrosis[30]. Pharmacological activation of AMPK in myofibroblasts has shown to exert protective effects on lung and mitigate the development of pulmonary fibrosis[30]. Metformin has shown to accelerate the resolution of bleomycin induced pulmonary fibrosis in mice in an AMPK dependent manner[31].
Reactive oxygen species exacerbates transforming growth factor (TGF)-β induced myofibroblast differentiation which plays a key role in the pathogenesis of pulmonary fibrosis[32]. Increased NOX4 (NADPH oxidase) expression were observed in fibroblasts isolated from IPF patients[33]. Metformin has shown to inhibit TGF-β induced NOX4 expression and thereby regulate myofibroblast differentiation in human lung fibroblasts as well as bleomycin induced pulmonary fibrosis in mice[34].
OTHER ANTI-DIABETIC MEDICATIONS ASSOCIATED WITH ILD
Few isolated cases of ILD from dipeptidyl peptidase-4 inhibitors have been reported in literature[35-37]. These cases have been secondary to vildagliptin and patients fully recovered after withdrawal of the offending drug and administration of glucocorticoids. A single case of sitagliptin induced diffuse alveolar hemorrhage presenting as bilateral pulmonary infiltrates was reported in Japan[38].
Pioglitazone which belongs to thiazolidinedione group of antidiabetic medications is also a peroxisome proliferator activated receptor gamma ligand. Pioglitazone with its potent anti-inflammatory action has shown to ameliorate bleomycin-induced acute inflammatory response and fibrotic changes in rats and is being studied as a potential treatment for pulmonary fibrosis[39]. One case of pioglitazone induced acute lung injury has been reported in Japan[40]. Glibenclamide is a commonly used sulfonylurea drug in the treatment of type 2 DM. By downregulating pro-inflammatory cytokines and reactive oxygen species it also exerts a protective role in inflammatory disorders like pulmonary fibrosis[41]. Liraglutide, which is a glucagon like peptide-1 receptor agonist that belongs to incretin mimetic group has both preventive and therapeutic effects on pulmonary arterial hypertension by increasing nitric oxide synthetase expression in smooth muscle cells[42].
EFFECT OF BLOOD SUGAR CONTROL AND LIFESTYLE CHANGES
The effect of strict blood sugar control on progression of interstitial lung disease has not been studied. Studies however have found a significant inverse correlation of lung function with fasting blood glucose levels. The association of blood glucose levels and lung function was analyzed in 3254 participants of the Framingham heart cohort and it was found that participants in the highest quartile (102-305 mg/dL) of fasting blood glucose had FEV1 and FVC that were on an average 85 mL and 94 mL lower than subjects in the lowest quartile (48-88 mg/dL)[43]. Hemoglobin A1c and fasting blood glucose were found to have negative association with FVC, DLCO and TLC in a prospective study of over 280 participants with prediabetes and type-2 diabetes[15]. These studies indicate that a strict blood sugar control may potentially improve the lung function and symptoms in patients with ILD. Direct impact of lifestyle modification on progression of interstitial lung disease is not known. But studies have shown that in patients with IPF, those who practice healthy lifestyle behaviors like walking, eat healthy and exercise on a regular basis were found to have improved quality of life with less symptoms, hospitalizations and mortality from IPF[44].
CONCLUSION
Diabetic microangiopathy targets lung and pneumopathy is a late complication of DM, and it should be more frequently screened in patients with type 2 DM and dyspnea. Pulmonary system remains relatively spared from exhibiting signs of severe dysfunction until later part of life when compared to the other organs because of its wide microvascular beds and large reserve. Early detection, screening and awareness about diabetes related interstitial lung disease could significantly reduce the pulmonary comorbidity from hyperglycemia. More studies are needed to better understand the pathophysiological mechanisms by which DM leads to ILD especially idiopathic pulmonary fibrosis and role of metformin in treatment of ILD. Both the DM and IPF societies should consider including diabetes as a risk factor for IPF in their guidelines because of its strong association.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Saisho Y S-Editor: Wang JL L-Editor: A E-Editor: Ma YJ