Published online Jun 15, 2020. doi: 10.4239/wjd.v11.i6.252
Peer-review started: December 31, 2019
First decision: March 24, 2020
Revised: April 9, 2020
Accepted: April 24, 2020
Article in press: April 24, 2020
Published online: June 15, 2020
Processing time: 153 Days and 23.4 Hours
Bariatric surgery is an efficient strategy for body weight and type 2 diabetes mellitus (T2DM) management. Abnormal lipid deposition in visceral organs, especially the pancreas and liver, might cause beta-cell dysfunction and insulin resistance. Extracellular matrix (ECM) remodeling allows adipose expansion, and matrix metalloproteinases (MMPs) play essential roles in ECM construction. MMP-2 and MMP-9 are the substrates of MMP-7. Different studies have reported that MMP-2, -7, and -9 increase in patients with obesity and metabolic syndromes or T2DM and are considered biomarkers in obesity and hyperglycemia patients.
To prospectively investigate whether MMP-2, MMP-7, and MMP-9 differ after two bariatric surgeries: Gastric bypass (GB) and sleeve gastrectomy (SG).
We performed GB in 23 and SG in 19 obese patients with T2DM. We measured body weight, waist circumference, body mass index (BMI), and serum concentrations of total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, fasting blood sugar (FBS), hemoglobin A1c (HbA1c), C-peptide, homeostasis model assessments of insulin resistance, and MMP-2, MMP-7, and MMP-9 levels at baseline and at 3, 12, and 24 mo post-operation.
Twenty-three patients aged 44.7 ± 9.7 years underwent GB, and 19 patients aged 40.1 ± 9.1 years underwent SG. In the GB group, BMI decreased from 30.3 ± 3.4 to 24.4 ± 2.4 kg/m2, HbA1c decreased from 9.2% ± 1.5% to 6.7% ± 1.4%, and FBS decreased from 171.6 ± 65.0 mg/dL to 117.7 ± 37.5 mg/dL 2 years post-operation (P < 0.001). However, the MMP-2, MMP-7, and MMP-9 levels pre- and post-GB were similar even 2 years post-operation (P = 0.107, 0.258, and 0.466, respectively). The SG group revealed similar results: BMI decreased from 36.2 ± 5.1 to 26.9 ± 4.7 kg/m2, HbA1c decreased from 7.9% ± 1.7% to 5.8% ± 0.6%, and FBS decreased from 138.3 ± 55.6 mg/dL to 95.1 ± 3.1 mg/dL (P < 0.001). The serum MMP-2, -7, and -9 levels pre- and post-SG were not different (P = 0.083, 0.869, and 0.1, respectively).
Improvements in obesity and T2DM induced by bariatric surgery might be the result of MMP-2, -7, or -9 independent pathways.
Core tip: Bariatric surgery is a very effective strategy for managing obesity patients and those with type 2 diabetes mellitus. Matrix metalloproteinases play roles in extracellular matrix remodeling which consequently results in insulin resistance. Some authors reported higher levels of matrix metalloproteinases (MMP)-2, -7, and -9 in obese or diabetic patients. We measured plasma MMP-2, -7, and -9 concentrations in obese patients before and after bariatric surgeries; however, we did not identify any statistical differences in the MMP levels. We suggested that bariatric surgery reduces obesity and diabetes through MMP-2, -7, or -9 independent pathways.
- Citation: Wu WC, Lee WJ, Lee TH, Chen SC, Chen CY. Do different bariatric surgical procedures influence plasma levels of matrix metalloproteinase-2, -7, and -9 among patients with type 2 diabetes mellitus? World J Diabetes 2020; 11(6): 252-260
- URL: https://www.wjgnet.com/1948-9358/full/v11/i6/252.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i6.252
Type 2 diabetes mellitus (T2DM) and obesity raise concerns among global health issues[1-4]. Bariatric surgical procedures, including gastric bypass (GB) and sleeve gastrectomy (SG), have been generally acknowledged as some of the most effective methods to manage body weight and glycemic control in obese patients[5-8]. Matrix metalloproteinases (MMPs) are calcium-dependent and zinc-containing proteases involved in extracellular matrix (ECM) synthesis, basement membrane degradation, and growth factor stimulation[9,10], which further affect adipogenesis and adipose tissue growth[11]. MMPs are classified into six groups based on their substrate and homology: Collagenases, such as MMP-1 and MMP-8; gelatinases, such as MMP-2, and -9; stromelysins, such as MMP-3 and -11; matrilysins, including MMP-7 and -26; membrane type MMPs; and other MMPs[12]. In 2001, Bouloumié et al[13] first reported that human adipocytes and pre-adipocytes secrete MMP-2 and MMP-9, and in turn, these two MMPs serve as potential essential regulators in adipocyte differentiation.
MMPs have been recognized as biomarkers of several disorders such as coronary artery diseases and heart failure. Plasma levels of MMPs have been reported to be significantly higher in obesity and T2DM patients[11]. MMP-2 and MMP-9 have both been reported to promote inflammation in high coronary risk events and plaque instability[14,15]. Both have also been reported to increase in obese patients, those with metabolic syndromes, and even patients with diabetes[16,17]. MMP-7 targets various substrates for ECM function, including MMP-2 and MMP-9[12,18]. Elevated MMP-7 levels in obese patients were reported to facilitate adipocyte differentiation[19]. Some authors considered MMP-7 as a marker for obesity, fat cell diameters, and obesity-related metabolic traits[20,21].
We hypothesized that having bariatric surgery would result in a decrease of plasma levels of MMP-2, -7, and -9. If correct, then those MMPs might represent biomarkers of the efficacy of bariatric surgeries. Furthermore, bariatric surgery might improve glycemic control through MMP-2, -7, or -9 independent pathways, and those MMPs could be novel therapeutic targets and prognostic biomarkers for obese patients with T2DM.
We conducted a prospective observational study using a hospital-based design. Overweight or obese patients with T2DM receiving either GB or SG surgery were enrolled in the study. The study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Institutional Review Board (IRB approval number: MSIRB2016006).
Eligible patients had been diagnosed with T2DM for more than 6 mo previously with a hemoglobin A1c (HbA1c) level > 8% and were receiving regular medical treatment, including therapeutic nutritional therapy, oral anti-diabetic agents, or insulin. The body mass index (BMI) in these patients ranged from 27.5-35 kg/m2, and these patients were willing to undergo additional treatment with lifestyle modifications, accepted follow-up visits, and provided written informed consent documents.
Patients with cancer within the last 5 years, human immunodeficiency virus infection, active pulmonary tuberculosis, cardiovascular instability within the previous 6 mo, pulmonary embolisms, serum creatinine levels > 2.0 mg/dL, chronic hepatitis B or C, liver cirrhosis, inflammatory bowel disease, acromegaly, organ transplantation, history of another bariatric surgery, alcoholic disorders, or drug abuse, or those who were uncooperative were excluded from the study.
Clinical anthropometry and routine laboratory assessments were performed on the day before surgery as baseline (M0) and at 3 mo (M3), 12 mo (M12), and 24 mo (M24) postoperatively. The participants were required to fast overnight prior to each blood sample collection. The samples were taken from the median cubital vein between 8 and 11 o’clock in the morning. Laboratory assessments included serum levels of total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, fasting blood sugar (FBS), hemoglobin A1c (HbA1c), C-peptide, homeostasis model assessments of insulin resistance, and MMPs-2, -7, and -9. Anthropometry measurements included body weight, waist circumference (WC), and BMI. The homeostasis model assessments of insulin resistance was calculated as plasma glucose (mmol/L) × insulin (μU/mL)[22].
Overall, there were 23 patients who received GB, and 19 patients who underwent SG in this study. There were 6 men and 17 women aged 44.7 ± 9.7 years in the GB group. There were 14 men and 5 women aged 40.1 ± 9.1 years in the SG group. The duration of T2DM was 4.0 ± 2.7 and 2.6 ± 2.8 years in the GB and SG groups, respectively (Table 1).
| Baseline characteristics | Gastric bypass | Sleeve gastrectomy |
| Patient numbers | 23 | 19 |
| Male | 6 | 14 |
| Female | 17 | 5 |
| Age (yr) | 44.7 ± 9.7 | 40.1± 9.1 |
| Duration of type 2 diabetes mellitus (yr) | 4.0 ± 2.7 | 2.6 ± 2.8 |
The blood samples were promptly injected into aprotinin-containing tubes (500 U/mL) once taken. After standardized centrifugation at 300 g and storage at −20 °C, the plasma was aliquoted into polypropylene tubes. Validated enzyme immunoassays for MMPs-2, -7, and -9 (QuickZyme Biosciences B.V., CK Leiden, The Netherlands) performed in a single batch and in a blinded fashion was used to measure the concentrations of MMP-2, -7, and -9.
The comparison of baseline and postoperative variables was conducted using the Wilcoxon signed-rank test. Friedman’s one-way repeated measures analysis of variance on ranks and a post-hoc test were performed to analyze the difference in plasma levels of MMP-2, -7, and -9 at M0, M3, M12, and M24. Spearman’s correlation analysis was used to test the correlations between two parameters. The statistical package for Social Science, version 12.0 (SPSS, Inc., Chicago, Illinois, IL, United States) was used for all analyses.
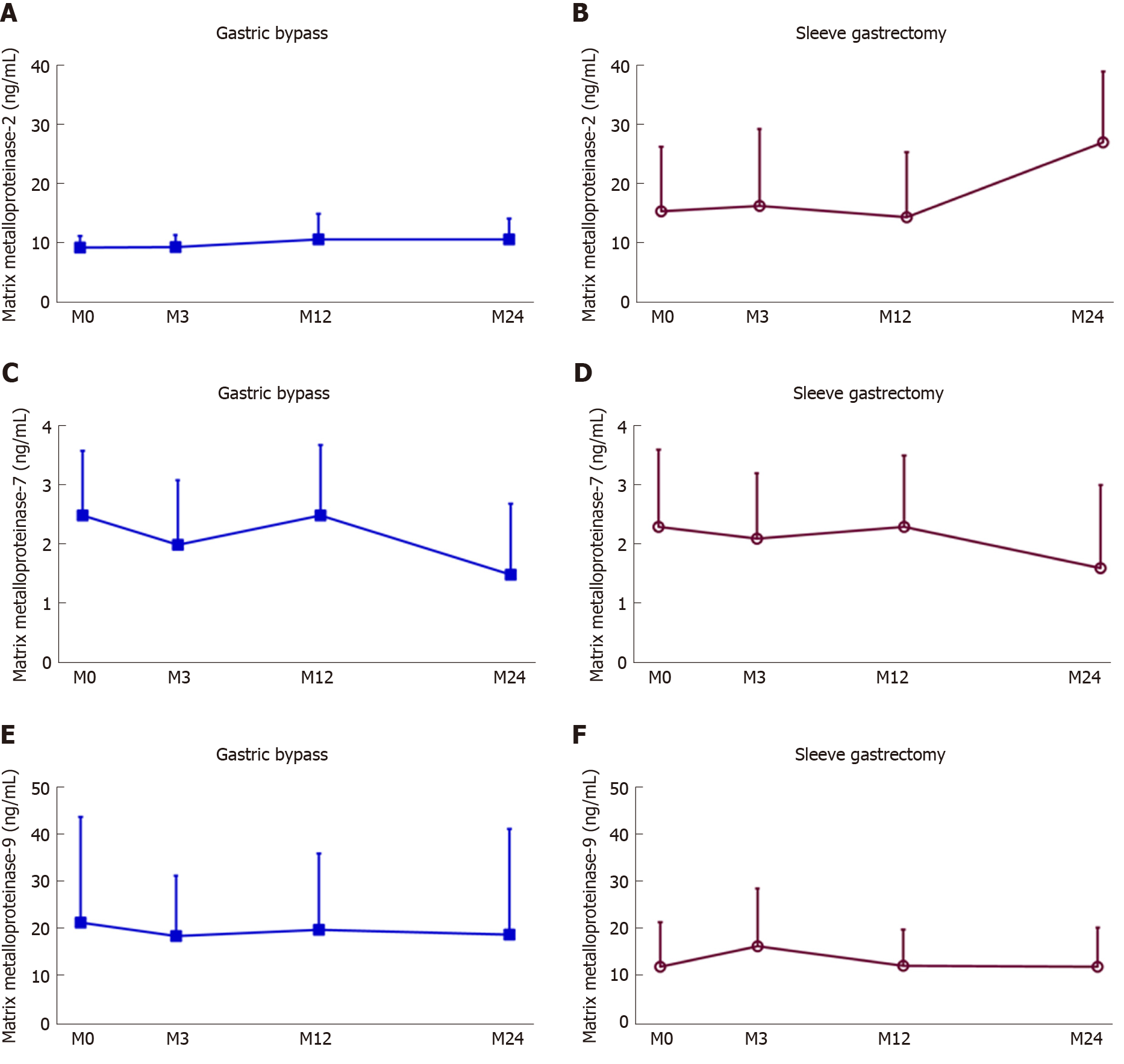
In the GB group, WC, BMI, HbA1c, and FBS were significantly decreased at 2 years postoperatively. WC decreased from 103.2 ± 10.3 to 84.2 ± 7.1 cm; BMI decreased from 30.3 ± 3.39 to 24.4 ± 2.4 kg/m2; HbA1c decreased from 9.2% ± 1.5% to 6.7% ± 1.4%; and FBS decreased from 171.6 ± 65.0 to 117.7 ± 37.5 mg/dL; and all were statistically significant (P < 0.001). However, the MMP-2, MMP-7, and MMP-9 levels were similar before and after GB even 2 years postoperatively (P = 0.107, 0.258, and 0.466, respectively) (Table 2).
| BMI (kg/m2) | Waist circumference (cm) | HbA1c (%) | FBS (mg/dL) | MMP-2 (ng/mL) | MMP-7 (ng/mL) | MMP-9 (ng/mL) | ||||||||
| mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | |
| M0 | 30.3 | 3.4 | 103.2 | 10.3 | 9.2 | 1.5 | 171.6 | 65.0 | 9.22 | 1.9 | 2.5 | 1.2 | 21.7 | 22.3 |
| M3 | 26.0 | 2.8 | 90.4 | 8.0 | 7.1 | 1.6 | 127.0 | 46.7 | 9.28 | 2.0 | 2.1 | 1.1 | 18.8 | 12.8 |
| M12 | 24.2 | 2.2 | 82.3 | 5.3 | 6.5 | 1.2 | 114.1 | 31.1 | 10.65 | 4.3 | 2.5 | 1.2 | 20.6 | 16.2 |
| M24 | 24.4 | 2.4 | 84.2 | 7.1 | 6.7 | 1.41 | 117.7 | 37.5 | 10.59 | 3.5 | 1.5 | 1.2 | 19.1 | 22.4 |
| P | < 0.0011 | < 0.0011 | < 0.0011 | < 0.0011 | 0.107 | 0.258 | 0.466 | |||||||
The SG group revealed similar results. WC decreased from 109.4 ± 10.5 to 87.7 ± 11.3 cm; BMI decreased from 36.2 ± 5.1 to 26.9 ± 4.7 kg/m2; HbA1c decreased from 7.9% ± 1.7% to 5.8% ± 0.6%; and FBS decreased from 138.3 ± 55.6 mg/dL to 95.1 ± 3.1 mg/dL; and all were statistically significant (P < 0.001), although serum MMP-2, -7, and -9 levels before and after SG were not statistically significant (P = 0.083, 0.869, and 0.1, respectively) (Table 3). The serum MMP-2, MMP-7, and MMP-9 concentration trends of GB and SG are shown in Figure 1.
| BMI (kg/m2) | Waist circumference (cm) | HbA1c (%) | FBS (mg/dL) | MMP-2 (pg/mL) | MMP-7 (pg/mL) | MMP-9 (pg/mL) | ||||||||
| mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | mean | SD | |
| M0 | 36.2 | 5.1 | 109.4 | 10.5 | 7.9 | 1.7 | 138.3 | 55.6 | 15.4 | 10.8 | 2.3 | 1.3 | 12.1 | 9.4 |
| M3 | 30.6 | 4.6 | 92.5 | 9.9 | 5.9 | 0.5 | 91.0 | 2.2 | 16.3 | 12.9 | 2.1 | 1.2 | 16.4 | 12.2 |
| M12 | 26.8 | 4.4 | 87.1 | 12.7 | 5.7 | 0.5 | 90.6 | 3.5 | 14.4 | 10.9 | 2.3 | 1.2 | 12.3 | 7.6 |
| M24 | 26.9 | 4.7 | 87.7 | 11.3 | 5.8 | 0.6 | 95.1 | 3.1 | 27.0 | 12.0 | 1.6 | 1.4 | 12.1 | 8.3 |
| P | < 0.0011 | < 0.0011 | < 0.0011 | < 0.0011 | 0.083 | 0.869 | 0.100 | |||||||
Obesity results from more lipid storage in adipose tissues causing further ECM accumulation[23]. ECM remodeling and reshaping are necessary to allow for new adipose tissue to grow[24]. In obesity, oxidative stress such as hypoxia and inflammation leads to pathological expansion of the ECM, macrophage aggregation, and collagen expression. Collagen accumulation might further induce lipid deposition[25]. Excessive ECM in white adipose tissue causes necrosis and apoptosis, cell death, accumulation of macrophages, and, consequently, insulin resistance[26]. Ectopic lipid deposition in the liver and pancreas might further result in beta-cell dysfunction and additional insulin resistance[27,28]. ECM changes in adipose tissue are considered to be related to T2DM[29].
Molecules other than MMPs such as integrins, collagens, a disintegrin and metalloproteinase domain-containing proteins, osteopontin, and tissue inhibitors of metalloproteinases are crucial players in ECM remodeling and adipose tissue rearrangement[30]. Integrins are the major adhesion receptors of the ECM, which transduce signals across the cell membrane and influence intracellular signaling[31]. Rodent studies have demonstrated that integrins might modulate glucose transporter 4 in adipose tissue, impair skeletal muscle glucose uptake, and aggravate insulin resistance[32,33]. Integrins take part in mechanical stimulation of insulin signaling, membrane insulin receptor localization, and insulin sensitivity[34]. Integrin subgroup β2 impacts glucose balance under high fat consumption by activating the immune system, increasing neutrophil growth, and allowing infiltration of leukocytes into the tissue, which improves insulin resistance[35]. This mechanism was corroborated in a study by Roumans et al[36] who demonstrated changes in integrin gene activity and ECM remodeling in obesity patients whose therapy was diet-control.
Collagens affect cell adhesion, migration, and differentiation in adipose tissue[37], and their accumulation results in the formation of adipose tissue and insulin resistance[38]. In patients with obesity, collagen V1 was suppressed in adipose tissue and surgery-induced weight loss increased collagen VI in subcutaneous tissue[39]. Other authors found that plasma osteopontin was significantly elevated in T2DM patients. They also concluded that osteopontin might serve a key role in insulin resistance and help to predict 3-year diabetic remission rates in patients undergoing bariatric surgery[40-42].
Although some studies demonstrated higher levels of MMP-2 and MMP-9 in patients with obesity, metabolic syndrome, or diabetes compared to controls[16], other studies showed no difference in the levels of MMP-2 and -9 in patients with similar disorders[43]. Additionally, two studies have shown that MMP-2 and MMP-9 activity decreased in white adipose tissue, but not in the plasma from animals with insulin resistance[44,45]. According to the 2019 report by García-Prieto et al[46], MMP-2 activity, measured by gelatin zymography, was initially similar in both obesity and non-obesity patients, but then decreased significantly after bariatric surgery. Similarly, although MMP-9 levels were higher in obesity patients than in non-obesity control patients, it decreased after bariatric surgery[46]. Boumiza et al[47] found that MMP-7 polymorphisms had only a non-significant association with BMI, and both systolic and diastolic blood pressures, triglycerides, total cholesterol, and high-density lipoprotein cholesterol plasma levels were not influenced by MMP-7 polymorphisms.
Metabolically unhealthy people with normal body weight are susceptible to cardiovascular diseases and DM due to hyperinsulinemia, insulin resistance, and hypertriglyceridemia[23,48]. Therefore, the mechanism of how bariatric surgery improves DM might not be related to its ability to control weight. Furthermore, a previous study also demonstrated that while long-term aerobic training attenuated MMP-2 levels, it also increased MMP-9 levels[12]. The interactions and associations among MMP-2, MMP-7, MMP-9, and weight management are still ambiguous and require additional research.
Our study has some limitations. First, the study population was relatively small. Second, more women received GB than men and more men received SG than women. Third, a type-2 statistical error might occur due to the selected study populations. Furthermore, neither the MMP levels in adipose tissue nor their activities in plasma or adipose tissue were measured. Lastly, the study was conducted in a single-center and was open-labeled.
In the present study we investigated the effects of two bariatric surgeries, GB and SG, on MMP-2, -7, and -9 plasma concentrations. The plasma levels of the three MMPs did not differ before and after the two surgeries. We suggested that bariatric surgery helps improve glucose in obese patients with T2DM via the MMP-2, -7, and -9 independent pathways, and that it might be the adipose tissue, rather than the plasma concentrations of MMP-2, -7, and -9, or the plasma and adipose tissue MMP activities, that influences T2DM. Our results augment the current evidence of how bariatric surgery affects glycemic control in obesity and T2DM patients. Further trials determining whether MMPs could be potential markers for the efficacy of bariatric surgeries and how bariatric surgeries can affect diabetic control in obese patients are warranted.
Bariatric surgeries, including gastric bypass and sleeve gastrectomy, are generally accepted to be effective in controlling body weight and blood glucose in obese patients. Researchers have found matrix metalloproteinases (MMPs) as biomarkers in many disorders. The levels of MMPs were reported to be increased in obese and type 2 diabetes mellitus (T2DM) patients.
Previous research reported decreased MMPs, along with reduced body weight, in the exercise group rather than the control group. We hypothesized that the MMP-2, -7, -9 levels would decrease in patients who underwent bariatric surgeries and further explained the mechanism of body weight loss and blood sugar control caused by bariatric surgeries.
The results disclosed that the MMP-2, -7, and -9 levels did not differ before or after bariatric surgery. Bariatric surgeries are helpful for weight loss and blood sugar control without significantly affecting MMP-2, -7, and -9 levels. How bariatric surgeries regulate body weight and blood sugar in obese T2DM patients needs further investigation. Whether MMPs other than MMP-2, -7, and -9 play roles demands further study.
Overall, 6 men and 17 women who received gastric bypass (GB), and 14 men and 5 women who received sleeve gastrectomy (SG) were included. All of the above subjects had a hemoglobin A1c (HbA1c) level > 8% under regular medication by endocrinologists and a body mass index (BMI) ranging from 27.5-35 kg/m2. We measured their clinical anthropometry and serum levels of total cholesterol, triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, fasting blood sugar, HbA1c, C-peptide, homeostasis model assessments of insulin resistance, and MMPs-2, -7, and -9 on the day before surgery as the baseline (M0 and at 3 mo (M3), 12 mo (M12), and 24 mo (M24) postoperatively. We use the validated enzyme immunoassays (QuickZyme Biosciences B.V., CK Leiden, The Netherlands) for the concentration of MMPs-2, -7, and -9. The procedure was performed in a blinded manner. For data analyses, the statistical package for Social Science, version 12.0 (SPSS, Inc., Chicago, Illinois, IL, United States) was used. The statistical methods included the Wilcoxon signed-rank test, Friedman’s one-way repeated measures analysis of variance on ranks followed by a post-hoc test, and Spearman’s correlation analysis.
In both the GB and SG groups, waist circumference, BMI, HbA1c, and fasting blood sugar were significantly decreased 2 years postoperatively. However, serum MMP-2, -7, and -9 levels did not significantly change after both surgeries. Our study added on the knowledge about the relationship between the biomarkers MMP-2, -7, and -9 and GB and SG surgeries.
Our study demonstrated that the MMP-2, -7, and -9 levels did not differ before or after the bariatric surgeries, which indicated that bariatric surgeries might be helpful for body weight and glucose management without altering MMP-2, -7, and -9 levels. The mechanism of weight loss and glucose management by bariatric surgeries in obese T2DM patients needs more exploration.
The study population was relatively small, and there were more women than men who received GB, and more men than women who received SG. Also, neither of the MMP levels nor their activities in adipose tissue were measured. In future studies, the sex ratio should be kept balanced in both groups. Furthermore, the MMP levels and activities in adipose tissue should be taken into consideration.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Carter WG, Exbrayat JM, Hamaguchi M S-Editor: Wang J L-Editor: Wang TQ E-Editor: Ma YJ
| 1. | GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5669] [Cited by in RCA: 5064] [Article Influence: 633.0] [Reference Citation Analysis (2)] |
| 2. | Unnikrishnan R, Pradeepa R, Joshi SR, Mohan V. Type 2 Diabetes: Demystifying the Global Epidemic. Diabetes. 2017;66:1432-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 217] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 3. | Morris MJ. Cardiovascular and metabolic effects of obesity. Clin Exp Pharmacol Physiol. 2008;35:416-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Bhatt L, Addepalli V. Matrix metalloproteinases in diabesity. Diabesity. 2015;1:18-20. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Kang JH, Le QA. Effectiveness of bariatric surgical procedures: A systematic review and network meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96:e8632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 6. | Koliaki C, Liatis S, le Roux CW, Kokkinos A. The role of bariatric surgery to treat diabetes: current challenges and perspectives. BMC Endocr Disord. 2017;17:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 7. | Wang W, Fann CSJ, Yang SH, Chen HH, Chen CY. Weight loss and metabolic improvements in obese patients undergoing gastric banding and gastric banded plication: A comparison. Nutrition. 2019;57:290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Huang HH, Lee WJ, Chen SC, Chen TF, Lee SD, Chen CY. Bile Acid and Fibroblast Growth Factor 19 Regulation in Obese Diabetics, and Non-Alcoholic Fatty Liver Disease after Sleeve Gastrectomy. J Clin Med. 2019;8:pii: E815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Medeiros NI, Gomes JAS, Fiuza JA, Sousa GR, Almeida EF, Novaes RO, Rocha VLS, Chaves AT, Dutra WO, Rocha MOC, Correa-Oliveira R. MMP-2 and MMP-9 plasma levels are potential biomarkers for indeterminate and cardiac clinical forms progression in chronic Chagas disease. Sci Rep. 2019;9:14170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3186] [Cited by in RCA: 3284] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 11. | Derosa G, Ferrari I, D'Angelo A, Tinelli C, Salvadeo SA, Ciccarelli L, Piccinni MN, Gravina A, Ramondetti F, Maffioli P, Cicero AF. Matrix metalloproteinase-2 and -9 levels in obese patients. Endothelium. 2008;15:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Jaoude J, Koh Y. Matrix metalloproteinases in exercise and obesity. Vasc Health Risk Manag. 2016;12:287-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Bouloumié A, Sengenès C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001;50:2080-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 242] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 1789] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 15. | Thorp EB. Contrasting Inflammation Resolution during Atherosclerosis and Post Myocardial Infarction at the Level of Monocyte/Macrophage Phagocytic Clearance. Front Immunol. 2012;3:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Hopps E, Lo Presti R, Montana M, Noto D, Averna MR, Caimi G. Gelatinases and their tissue inhibitors in a group of subjects with metabolic syndrome. J Investig Med. 2013;61:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Kosmala W, Plaksej R, Przewlocka-Kosmala M, Kuliczkowska-Plaksej J, Bednarek-Tupikowska G, Mazurek W. Matrix metalloproteinases 2 and 9 and their tissue inhibitors 1 and 2 in premenopausal obese women: relationship to cardiac function. Int J Obes (Lond). 2008;32:763-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood). 2006;231:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 312] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 19. | Ress C, Tschoner A, Ciardi C, Laimer MW, Engl JW, Sturm W, Weiss H, Tilg H, Ebenbichler CF, Patsch JR, Kaser S. Influence of significant weight loss on serum matrix metalloproteinase (MMP)-7 levels. Eur Cytokine Netw. 2010;21:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes. 2002;51:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Yang PJ, Ser KH, Lin MT, Nien HC, Chen CN, Yang WS, Lee WJ. Diabetes Associated Markers After Bariatric Surgery: Fetuin-A, but Not Matrix Metalloproteinase-7, Is Reduced. Obes Surg. 2015;25:2328-2334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Lee WJ, Chen CY, Chong K, Lee YC, Chen SC, Lee SD. Changes in postprandial gut hormones after metabolic surgery: a comparison of gastric bypass and sleeve gastrectomy. Surg Obes Relat Dis. 2011;7:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Ruiz-Ojeda FJ, Méndez-Gutiérrez A, Aguilera CM, Plaza-Díaz J. Extracellular Matrix Remodeling of Adipose Tissue in Obesity and Metabolic Diseases. Int J Mol Sci. 2019;20:pii: E4888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 184] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 24. | Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol. 2018;221:pii: jeb162958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 25. | Hammarstedt A, Gogg S, Hedjazifar S, Nerstedt A, Smith U. Impaired Adipogenesis and Dysfunctional Adipose Tissue in Human Hypertrophic Obesity. Physiol Rev. 2018;98:1911-1941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 311] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 26. | Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 733] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 27. | Bobulescu IA, Lotan Y, Zhang J, Rosenthal TR, Rogers JT, Adams-Huet B, Sakhaee K, Moe OW. Triglycerides in the human kidney cortex: relationship with body size. PLoS One. 2014;9:e101285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Catanzaro R, Cuffari B, Italia A, Marotta F. Exploring the metabolic syndrome: Nonalcoholic fatty pancreas disease. World J Gastroenterol. 2016;22:7660-7675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 142] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 29. | Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455:479-492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 30. | Lin, Chun TH, Kang L. Adipose extracellular matrix remodelling in obesity and insulin resistance. Biochem Pharmacol. 2016;119:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 167] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 31. | Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6323] [Cited by in RCA: 6566] [Article Influence: 285.5] [Reference Citation Analysis (0)] |
| 32. | Zong H, Bastie CC, Xu J, Fassler R, Campbell KP, Kurland IJ, Pessin JE. Insulin resistance in striated muscle-specific integrin receptor beta1-deficient mice. J Biol Chem. 2009;284:4679-4688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Kang L, Ayala JE, Lee-Young RS, Zhang Z, James FD, Neufer PD, Pozzi A, Zutter MM, Wasserman DH. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes. 2011;60:416-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 34. | Kim J, Bilder D, Neufeld TP. Mechanical stress regulates insulin sensitivity through integrin-dependent control of insulin receptor localization. Genes Dev. 2018;32:156-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Meakin PJ, Morrison VL, Sneddon CC, Savinko T, Uotila L, Jalicy SM, Gabriel JL, Kang L, Ashford ML, Fagerholm SC. Mice Lacking beta2-Integrin Function Remain Glucose Tolerant in Spite of Insulin Resistance, Neutrophil Infiltration and Inflammation. PLoS One. 2015;10:e0138872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Roumans NJ, Vink RG, Fazelzadeh P, van Baak MA, Mariman EC. A role for leukocyte integrins and extracellular matrix remodeling of adipose tissue in the risk of weight regain after weight loss. Am J Clin Nutr. 2017;105:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277-1292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 399] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 38. | Buechler C, Krautbauer S, Eisinger K. Adipose tissue fibrosis. World J Diabetes. 2015;6:548-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 39. | McCulloch LJ, Rawling TJ, Sjöholm K, Franck N, Dankel SN, Price EJ, Knight B, Liversedge NH, Mellgren G, Nystrom F, Carlsson LM, Kos K. COL6A3 is regulated by leptin in human adipose tissue and reduced in obesity. Endocrinology. 2015;156:134-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 40. | Zhang Q, Wang C, Tang Y, Zhu Q, Li Y, Chen H, Bao Y, Xue S, Sun L, Tang W, Chen X, Shi Y, Qu L, Lu B, Zheng J. High glucose upregulates osteopontin expression by FoxO1 activation in macrophages. J Endocrinol. 2019;242:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Barchetta I, Ceccarelli V, Cimini FA, Bertoccini L, Fraioli A, Alessandri C, Lenzi A, Baroni MG, Cavallo MG. Impaired bone matrix glycoprotein pattern is associated with increased cardio-metabolic risk profile in patients with type 2 diabetes mellitus. J Endocrinol Invest. 2019;42:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Carbone F, Adami G, Liberale L, Bonaventura A, Bertolotto M, Andraghetti G, Scopinaro N, Camerini GB, Papadia FS, Cordera R, Dallegri F, Montecucco F. Serum levels of osteopontin predict diabetes remission after bariatric surgery. Diabetes Metab. 2019;45:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Papazafiropoulou A, Perrea D, Moyssakis I, Kokkinos A, Katsilambros N, Tentolouris N. Plasma levels of MMP-2, MMP-9 and TIMP-1 are not associated with arterial stiffness in subjects with type 2 diabetes mellitus. J Diabetes Complications. 2010;24:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Miksztowicz V, Morales C, Zago V, Friedman S, Schreier L, Berg G. Effect of insulin-resistance on circulating and adipose tissue MMP-2 and MMP-9 activity in rats fed a sucrose-rich diet. Nutr Metab Cardiovasc Dis. 2014;24:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Berg G, Barchuk M, Miksztowicz V. Behavior of Metalloproteinases in Adipose Tissue, Liver and Arterial Wall: An Update of Extracellular Matrix Remodeling. Cells. 2019;8:pii: E158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 46. | García-Prieto CF, Gil-Ortega M, Vega-Martín E, Ramiro-Cortijo D, Martín-Ramos M, Bordiú E, Sanchez-Pernaute A, Torres A, Aránguez I, Fernández-Alfonso M, Rubio MA, Somoza B. Beneficial Effect of Bariatric Surgery on Abnormal MMP-9 and AMPK Activities: Potential Markers of Obesity-Related CV Risk. Front Physiol. 2019;10:553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Boumiza S, Bchir S, Ben Nasr H, Abbassi A, Jacob MP, Norel X, Tabka Z, Chahed K. Role of MMP-1 (-519A/G, -1607 1G/2G), MMP-3 (Lys45Glu), MMP-7 (-181A/G), and MMP-12 (-82A/G) Variants and Plasma MMP Levels on Obesity-Related Phenotypes and Microvascular Reactivity in a Tunisian Population. Dis Markers. 2017;2017:6198526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Mathew H, Farr OM, Mantzoros CS. Metabolic health and weight: Understanding metabolically unhealthy normal weight or metabolically healthy obese patients. Metabolism. 2016;65:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 128] [Article Influence: 14.2] [Reference Citation Analysis (0)] |