Published online Dec 15, 2020. doi: 10.4239/wjd.v11.i12.654
Peer-review started: June 27, 2020
First decision: September 18, 2020
Revised: October 9, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 15, 2020
Processing time: 168 Days and 14.7 Hours
Multiple studies demonstrate that fluctuating blood glucose level produces greater damage compared with sustained hyperglycemia. Flash glucose monitoring system is an effective method in documenting blood glucose variability, contributing to better glucose management and reduced hypoglycemic event occurrence.
To investigate the improvement in glycemic variability (GV), blood glucose level, and metabolic indexes of patients with type 2 diabetes mellitus after combined treatment of exenatide once weekly (EXQW) and metformin.
Twenty-five patients with type 2 diabetes mellitus suffering from poor blood glucose control under metformin treatment were recruited. The recruited patients were prescribed with oral metformin only (maintaining a dosage of metformin at ≥ 1500 mg/day) for 2 wk (screening period), and then given EXQW (2 mg, subcutaneous injection) for 12 wk (experimental period). The flash glucose monitoring system was used to document blood glucose values during the screening period and the last 2 wk of the experimental period.
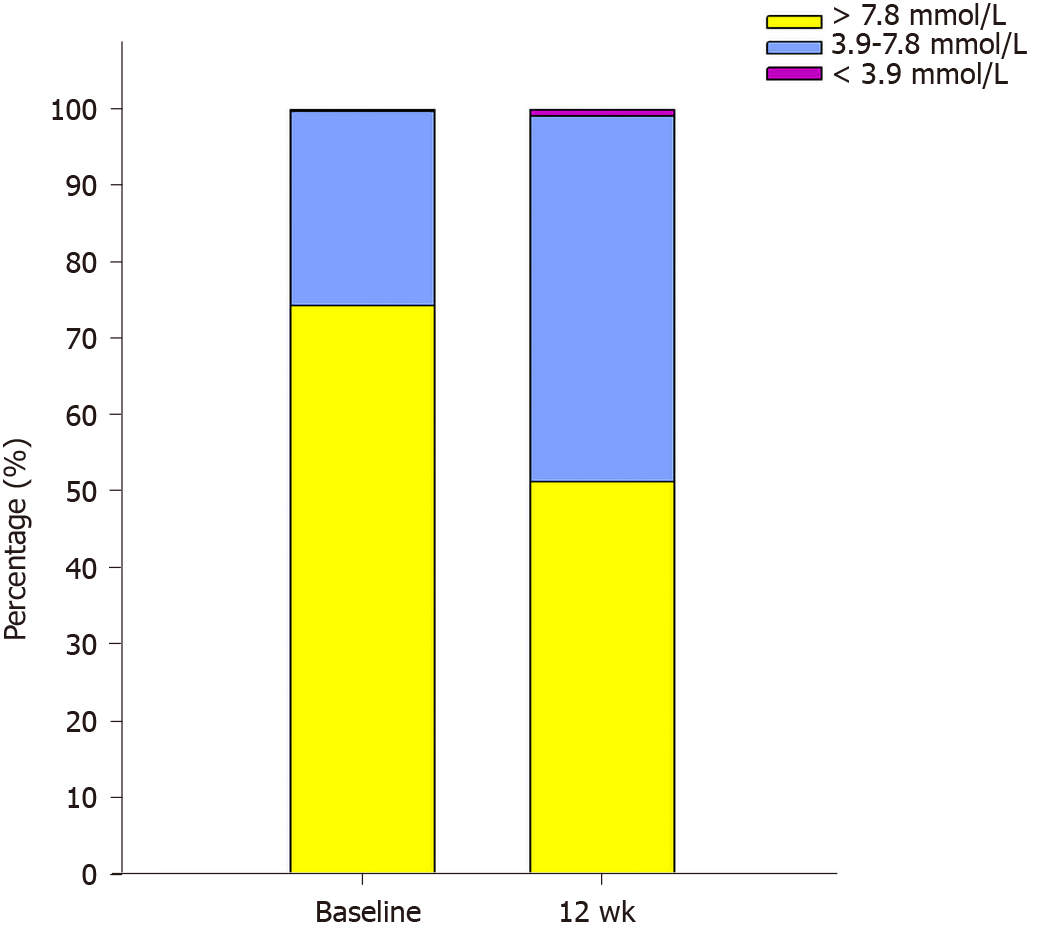
Four patients were excluded for various reasons, yielding a total of 21 patients, including 17 males and 4 females, with an average age of 48.8 years, who completed this study. The estimated glycated hemoglobin, mean blood glucose, fasting and postprandial blood glucose levels, and percentage of blood glucose above 7.8 mmol/L decreased compared to those at baseline (P = 0.003, 0.003, 0.008, 0.010, 0.014, 0.017, and 0.005, respectively), while the percentage of blood glucose between 3.9 and 7.8 mmol/L significantly increased (P = 0.005). Parameters of GV including standard deviation of blood glucose, mean amplitude of glycemic excursions, mean of daily difference, area under the curve difference between percentiles 25 and 75, and area under the curve difference between percentiles 10 and 90 were significantly lower compared to that of baseline (P = 0.017, 0.006, 0.000, 0.024, 0.036, respectively). The durations of blood glucose below 3.9 mmol/L during the day and nocturnal periods significantly increased after treatment (P = 0.041 and 0.028, respectively), but there was no significant increase in severe hypoglycemia (< 3.0 mmol/L) compared with that at baseline (P = 0.207). In addition, some metabolic indicators improved after EXQW treatment.
EXQW combined with metformin can effectively improve blood glucose levels, reduce GV, and improve metabolic indicators. However, there is still a risk of nocturnal hypoglycemia, and careful attention should be paid to patients with EXQW treatment.
Core Tip: In this study, flash glucose monitoring system was used not only to observe the hypoglycemic effect of and the improvement of glycemic variability by exenatide once weekly in combination with metformin, but also to accurately assess the specific time period for the improvement of blood glucose and the time when the occurrence of hypoglycemia was concentrated. The results indicated that the combination of these drugs significantly improved fasting blood glucose, but the risk of increasing hypoglycemia, especially at night, should be cautioned.
- Citation: Li Y, Han MM, He Q, Liu ZA, Liang D, Hou JT, Zhang Y, Liu YF. Exenatide once weekly combined with metformin reduced glycemic variability in type 2 diabetes by using flash glucose monitoring system. World J Diabetes 2020; 11(12): 654-665
- URL: https://www.wjgnet.com/1948-9358/full/v11/i12/654.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i12.654
Achieving and maintaining blood glucose levels within normal range is an integral part of diabetes mellitus (DM) management, in which blood glucose monitoring plays an indispensable role. Glycated hemoglobin (HbA1c) is a long-term blood glucose indicator, providing useful information about the most recent 2-3 mo blood glucose levels. However, patients with similar or identical HbA1c results may have different glucose variability (GV) profiles. GV condition in patients with DM has gained wide attention in recent years, because it is a more reliable indicator for a wide range of acute or chronic diabetes-related complications[1,2]. Current studies have demonstrated that compared to persistent hyperglycemia, fluctuating hyperglycemia can cause greater damage to the blood vessels and endothelial tissue.
Flash glucose monitoring system (FGMS) can be used to continuously measure interstitial fluid blood glucose level over a period of 10 to 14 d. Compared to the continuous glucose monitoring system, the FGMS is factory calibrated without the alert function for the risk of hyperglycemia and hypoglycemia[3], and available data revealed that the FGMS is an effective method in documenting blood glucose variability, contributing to better glucose management and reduced hypoglycemic event occurrence[4,5].
Glucagon-like peptide 1 receptor agonists (GLP-1RAs) have been demonstrated to significantly improve blood glucose level, blood lipid profile, and weight in patients with DM, which are a class of safe and effective hypoglycemic medications due to their glucose-dependent hypoglycemic property without increasing the risk of hypoglycemia. In addition, the safety of GLP-1RAs in the cardiovascular system has been verified, specifically, the LEADER study[6] has shown that liraglutide, as a kind of GLP-1RA, has a significant protective effect on the cardiovascular system. Exenatide once-weekly (EXQW), a long-acting GLP-1RA, can significantly improve metabolic disorders in vivo. Considering the reduced frequency of injections and discomfort experience, EXQW has become an effective and convenient therapy that can improve patients’ quality of life and compliance[7]. Therefore, we hypothesized that the combination of EXQW and metformin can bring significant clinical benefits for DM management.
As the aims of this study, the clinical and therapeutic effects of combined EXQW and metformin treatment in type 2 DM (T2DM) patients were evaluated by using dynamic glucose monitoring and metabolic indicators. Based on the FGMS data, we analyzed and compared the GV-related indices, and hypoglycemic incidence between baseline and end points.
This is a pre-post study involving the same group of patients, conducted at the First Hospital of Shanxi Medical University from June 2018 to March 2019. This study was approved by the Ethics Committee of the First Hospital of Shanxi Medical University (2018K007). All patients were informed of the objective of the study and signed a written informed consent form.
Twenty-five patients with T2DM, whose blood glucose was poorly controlled by metformin monotherapy, were recruited. These patients were selected according to the following criteria: (1) Patients were diagnosed with T2DM for 2 to 10 years, based on the DM diagnosis standards published by the World Health Organization in 1999, and their ages ranged from 18 to 70 years old; (2) Measured HbA1C ≥ 7.0 % but < 11.0%; (3) Patients receiving monotherapy of metformin for at least 3 mo (daily metformin dosage ≥ 1500 mg) with unsatisfactory blood glucose control; and (4) Patients with an estimated glomerular filtration rate > 60 mL/min/1.73 m2.
The exclusion criteria were as follows: (1) Patients with contraindications according to the instructions on EXQW, or who had a history of allergy to any other excipients; (2) Patients received insulin therapy for more than 7 d consecutively within 1 year before the screening (excluding those taking insulin therapy for acute diseases or operations); (3) Patients with a history of acute or chronic pancreatitis; (4) Patients with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2; (5) Patients suffering from serious gastrointestinal diseases; and (6) Patients who could not cooperate with the researcher because of poor compliance or mental disorders, unwilling to communicate, or having difficulty in expressing thoughts.
This study comprised of two periods, namely, a 2-wk screening period and a 12-wk experiment period. During the screening period, the recruited patients’ profiles comprising height, weight, waist circumference, and other basic information were compiled. Thereafter, all patients were provided with the standard diabetes health education and relevant training on the FGMS. It is recommended that all subjects have meals at 8:00, 13:00, and 18:00 each day. According to the Dietary Guidelines for Type 2 Diabetes in China and Guidelines for the Prevention and Control of Type 2 Diabetes in China, the patients were instructed to eat 50%-65% carbohydrate, 20%-30% fat, and 15%-20% protein every day, and they were given 150 min of moderate-intensity aerobic exercise every week.
During the 2-wk screening period, the patients were prescribed with oral metformin only (maintaining a dosage of metformin at ≥ 1500 mg/day). At the end of the screening period, EXQW was prescribed at 2 mg via subcutaneous injection, administered at a fixed time every week. The combined drug therapy continued for 12 wk.
FGMS was used in the screening period and the last 2 wk of the experimental period. The sensor of an FGMS device was placed under the skin of patient’s upper arm with the help of professional medical staff. The patients were asked to avoid strenuous exercise and long hours of bathing or swimming to prevent the sensor from detaching. Additionally, the patients were required to obtain complete blood glucose data at least once every 8 h using the separate touchscreen reader device.
The primary endpoint of this study was to evaluate the change of GV after 12-wk treatment relative to the baseline by using an FGMS, and the secondary end points were blood glucose control and the occurrence of hypoglycemia. In addition, changes in some biochemical indicators were also observed. Pancreatic islet function and insulin resistance (IR) were assessed using homeostasis model assessment (HOMA)[8]: HOAM-β = 20*FINS/(FBG-3.5), and HOMA-IR = FBG*FINS/22.5.
The FGMS records blood glucose values every 15 min, and the information has to be scanned at least once every 8 h for data compilation. Therefore, 96 continuous blood glucose level values could be obtained for every 24 h. After wearing the FGMS for 10 to 14 d, the patients’ baseline blood glucose data and automatically-generated ambulatory glucose profile (AGP) were captured by the commercial software in the FGMS device. Should there be missing values in the baseline blood glucose data, they will be replaced by the average blood glucose value nearing the missing value.
Based on the FGMS data, we calculated some parameters of GV, including mean amplitude of glycemic excursion (MAGE), mean of daily difference (MODD), coefficient of variation (CV), standard deviation of blood glucose (SDBG), large amplitude of glycemic excursion, area under the curve (AUC) difference between percentiles 25 and 75 (area of IQR), and AUC difference between percentiles 10 and 90 (area of IDR). The automatically generated AGPs were used as parameters for blood glucose control, including estimated HbA1c, mean blood glucose (MBG), time in range (TIR) (3.9-7.8 mmol/L), time out of range (TOR), percentage of time below 3.9 mmol/L, percentage of time between 3.9-7.8 mmol/L, and percentage of time above 7.8 mmol/L. At the same time, fasting (6:00-8:00) and postprandial (8:00-10:00, 13:00-15:00, and 18:00-20:00) blood glucose levels were extracted for specific analysis. The means of blood glucose in each time period were plotted into a line graph and the AUCs were calculated accordingly. In addition, two different hypoglycemic cutoffs, less than 3.9 mmol/L and 3.0 mmol/L, were used during the day (6:00-24:00) and nocturnal periods (0:00-6:00). The parameters of GV and blood glucose levels were obtained using SigmaPlot 12.5 version for Windows and SPSS version 23.0 software, except that some of them were provided by AGP.
SPSS version 23.0 software was used for statistical analyses. Data are reported as the mean ± SD for normally distributed continuous variables and median (inter-quartile range) for the non-normally distributed continuous variables. Paired-sample t-tests or Wilcoxon signed rank test were performed to compare the data between start and the end of this intervention study. P < 0.05 was considered statistically significant.
Twenty-five subjects were recruited for this study, of whom two failed to complete the study and two failed to obtain FGMS data. Finally, a total of 21 subjects were involved in this study, including 17 males and 4 females. The mean age of participants was 48.8 ± 8.4 years (range, 33–66 years). The mean duration of diabetes was 5.3 ± 2.1 years (range, 2–10 years). All patients wore the FGMS devices for a total of 284 d for the baseline period and 309 d after 12-wk treatment. Baseline characteristics of the participants are shown in Table 1.
| Variable | Total (21) |
| Age (yr) | 48.8 ± 8.4 |
| Duration of diabetes (yr) | 5.3 ± 2.1 |
| Gender (female/male) | 4/17 |
| BMI (kg/m2) | 26.7 ± 1.6 |
| Smoking (%) | 71.4 |
| Hypertension (%) | 66.7 |
| Macrovascular complications (%) | 19.0 |
| Microvascular complications (%) | 38.1 |
Overall, after 12 wk of combined drug therapy, blood glucose levels improved significantly compared with baseline values, as shown in Table 2. The estimated HbA1c, MBG, and TOR decreased after treatment (P = 0.003, 0.003, and 0.005, respectively), while TIR increased (P = 0.005).
| Variable | Baseline | 12 wk | P value |
| eHbA1c (%) | 8.4 ± 1.4 | 7.0 ± 1.3 | 0.003 |
| MBG (mmol/L) | 10.8 ± 2.3 | 8.6 ± 2.0 | 0.003 |
| TIR (h) | 6.1 ± 5.9 | 11.5 ± 5.8 | 0.005 |
| TOR (h) | 17.9 ± 5.9 | 12.5 ± 5.8 | 0.005 |
As shown in Figure 1, the percentage of time below 3.9 mmol/L and percentage of time above 7.8 mmol/L significantly decreased (P = 0.035 and 0.005, respectively), while percentage of time between 3.9-7.8 mmol/L significantly increased (P = 0.005) after 12 wk of combination therapy compared with that at baseline.
After intervention treatment, GV improved significantly compared to baseline value. Parameters representing within-day GV (SDBG and MAGE) and day-to-day GV (MODD) decreased significantly (P = 0.017, 0.006, and 0.000, respectively). Further extraction and analysis of GV parameters for daytime and night showed that the values of MAGE and MODD were reduced during the daytime as well as at night (P = 0.010, 0.020, 0.001, and 0.007, respectively). Moreover, AUC difference between percentiles 25 and 75 (area of IQR) and AUC difference between percentiles 10 and 90 (area of IDR) narrowed (P = 0.024 and 0.036, respectively). However, there was no significant difference in CV or large amplitude of glycemic excursion. Detailed information is shown in Table 3.
| Variable | Baseline | 12 wk | P value | |
| SDBG (mmol/L) | 2.8 ± 0.8 | 2.3 ± 0.6 | 0.017 | |
| CV (%) | 26.3 ± 5.4 | 26.3 ± 3.5 | 0.997 | |
| MAGE (mmol/L) | Overall | 5.7 ± 1.3 | 4.5 ± 1.1 | 0.006 |
| During day | 5.8 ± 1.2 | 4.6 ± 1.3 | 0.010 | |
| At night | 4.7 ± 1.8 | 3.6 ± 1.2 | 0.020 | |
| MODD (mmol/L) | Overall | 2.8 ± 1.2 | 1.6 ± 0.4 | 0.000 |
| During day | 2.9 ± 1.1 | 1.8 ± 0.5 | 0.001 | |
| At night | 1.8 (1.1-3.9 ) | 1.0 (0.6-1.5 ) | 0.007 | |
| LAGE (mmol/L) | Overall | 15.2 ± 2.6 | 13.5 ± 3.2 | 0.092 |
| During day | 14.8 ± 2.8 | 13.1 ± 3.3 | 0.127 | |
| At night | 9.7 ± 3.6 | 8.5 ± 2.4 | 0.258 | |
| AUC of IQR | 5017.5 ± 1932.8 | 3877.1 ± 1001.1 | 0.024 | |
| AUC of IDR | 9713.2 ± 2782.2 | 8015.9 ± 2208.2 | 0.036 |
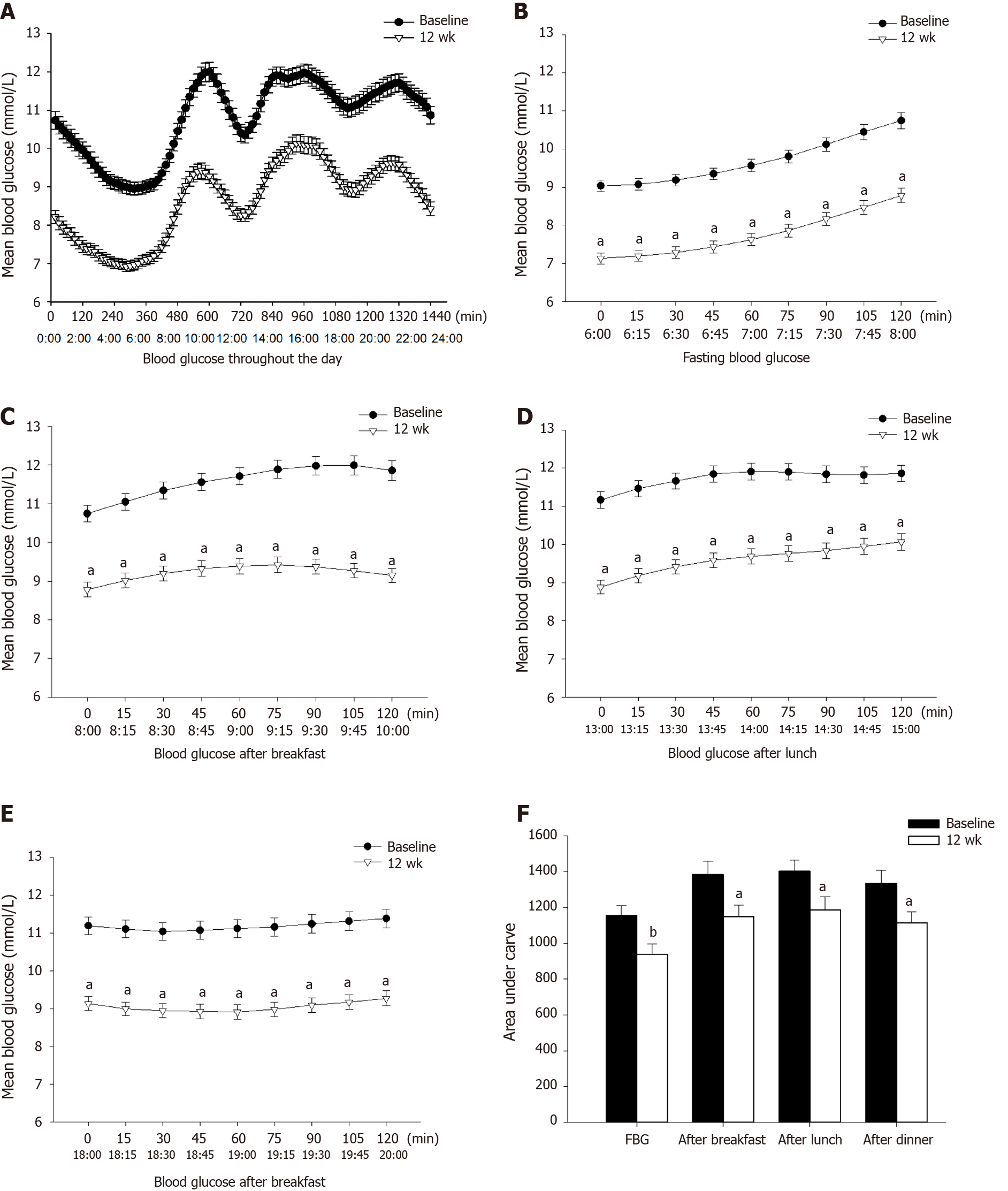
The results are shown in Figure 2. Fasting and postprandial glucose levels significantly decreased after treatment compared with those at baseline (P = 0.008, 0.010, 0.014, and 0.017, respectively).
As shown in Table 4, when the interstitial fluid blood glucose less than 3.9 mmol/L was considered as hypoglycemia, the durations of hypoglycemia during the whole day and the nocturnal period (0:00-6:00) increased significantly compared with those at baseline (P = 0.041 and 0.028, respectively), while the duration of hypoglycemia during the daytime (6:00-24:00) showed no significant difference (P = 0.157). For the hypoglycemic cutoff point of 3.0 mmol/L, the durations of hypoglycemia did not increase throughout the day, during the daytime (6:00-24:00), or during the nocturnal period (0:00-6:00).
| Variable | Before trial | After trial | P value |
| Hypoglycemia cutoff: 3.9 mmol/L | |||
| Throughout the day (min) | 0 (0-2.3) | 1.1 (0-22.0) | 0.041 |
| During day (min) | 0 (0-1.3) | 0 (0-3.0) | 0.157 |
| At night (min) | 0 (0-0) | 1.1 (0-10.0) | 0.028 |
| Hypoglycemia cutoff: 3.0 mmol/L | |||
| Throughout the day (min) | 0 (0,0) | 0 (0, 0) | 0.207 |
| During day (min) | 0 (0,0) | 0 (0, 0) | 0.197 |
| At night (min) | 0 (0-0) | 0 (0, 0) | 0.465 |
After 12-wk combined therapy, mean weight of patients decreased from 78.1 ± 8.3 to 77.3 ± 8.5 kg (P = 0.008), and mean waist circumference decreased from 100.8 ± 9.2 to 99.5 ± 8.5 cm (P = 0.021). Besides, the significantly decreased HOMA-IR and significant increased HOMA-β than those at baseline indicated an improvement in insulin resistance and pancreatic islets function. Results are shown in Table 5.
| Variable | Baseline | 12 wk | P value |
| Weight (kg) | 78.1 ± 8.3 | 77.3 ± 8.5 | 0.008 |
| Waist circumference (cm) | 100.8 ± 9.2 | 99.5 ± 8.5 | 0.021 |
| HOMA-IR | 4.8 ± 2.2 | 4.0 ± 1.9 | 0.033 |
| HOMA-β | 41.2 ± 24.1 | 64.4 ± 51.6 | 0.046 |
This study explored the effects of EXQW combined with metformin treatment on GV, blood glucose levels, hypoglycemia, and some metabolic indicators in patients with T2DM. A recent study has revealed that exenatide twice daily and metformin could jointly improve GV profile[9]. The present study extended this prior research by demonstrating that the combination of EXQW and metformin could significantly improve GV and blood glucose control. Additionally, the therapeutic effects on weight loss, waist circumference reduction, and improvement of islet function were also evident.
In this research, an FGMS was used to acquire continuous blood glucose levels in 10 to 14 d to assess GV, blood glucose control, and duration of hypoglycemia in T2DM patients with combined EXQW and metformin treatment. This new-type glucose monitoring system consists of a subcutaneous sensor and an independent reader. It is easy to wear and is almost pain free. Besides, its invisibility and portability have provided much convenience for patients who have trouble in monitoring blood glucose levels consistently. A prospective study applied FGMS in adults with T2DM and suggested that FGMS usage could improve blood glucose control, reduce the incidence of hypoglycemia, and improve patients’ quality of life[10]. Besides, FGMS has high user satisfaction and is a convenient option for patients requiring intensive glucose monitoring.
EXQW is a long-acting GLP-1RA, which is usually injected subcutaneously at 2 mg per week. A prospective study observed the therapeutic effect of EXQW combined with a standard dosage of metformin, suggesting that this treatment can effectively decrease HbA1c and fasting blood glucose levels, and improve carotid intima-media thickness[11].
According to this study, after 12-wk EXQW and metformin combination treatment, estimated HbA1c, MBG, and TOR significantly decreased, while TIR significantly increased, indicating that the overall blood glucose control was better compared to that at baseline. TIR is an important index for the effectiveness in blood glucose control. When TIR is lowered, it suggests the existence of hypoglycemia or hyperglycemia. The relationship between TIR and macro- and micro-vascular complications has been confirmed by previous researchers[12,13]; hence, the reduction of TIR helps to delay the progression of diabetes.
In recent years, evidence-based medicine has achieved significant progress; there are new findings suggesting that unstable GV is more dangerous than the mere hyperglycemia[14,15]. GV is considered unstable when the blood glucose levels fluctuate between peaks and troughs, increasing the risks of inflammation, oxidative stress, and damage to the endothelial cells[16]. Therefore, GV is closely related to multiple complications in diabetes and is also a risk factor independent of blood glucose level. Our study revealed that the combination treatment of EXQW and metformin for 12 wk could improve the general GV (MBG and CV), within-day GV (SDBG and MAGE), and day-to-day GV (MODD). Besides, the AUC difference between percentiles 25 and 75 (area of IQR) and AUC difference between percentiles 10 and 90 (area of IDR) narrowed. By comparing the changes in MAGE and MODD at daytime and at night to those at baseline, we found that these indicators improved significantly. A randomized placebo-controlled research using continuous glucose monitoring system to assess GV found that EXQW could not only improve blood glucose level but also reduce GV[17], which is consistent with the results of this study.
Researchers have demonstrated that GV is a powerful independent predicting factor for patients with T2DM and acute coronary syndrome[18]. Another study has also found that long-term follow-up of GV is probably a more reliable index compared to HbA1c in assessing the future risk of macro- and micro-vascular complications in patients with T2DM[19]. Owing to the relatively short period of this study, follow-up on the occurrence of relevant complications could not be carried out. However, it can be inferred that EXQW in combination with metformin slows down the progression of diabetes-related complications by improving GV.
We specifically analyzed the blood glucose levels and AUCs at four different time periods. It revealed that EXQW can significantly reduce fasting blood glucose and achieve satisfactory blood glucose after breakfast, lunch, and dinner. Therefore, as a long-acting GLP-1RA, EXQW has a significant effect on the control of fasting blood glucose and exerts a certain effect on the control of postprandial blood glucose.
In this research, we calculated the durations of hypoglycemia below 3.9 mmol/L and 3.0 mmol/L, respectively. Results show that EXQW combined with metformin increased the durations of hypoglycemia (< 3.9 mmol/L) throughout the day and during the night (0:00-6:00), without any obvious increase of hypoglycemia during daytime (6:00-24:00). Further analysis of the duration of blood glucose below 3.0 mmol/L showed that severe hypoglycemia did not increase for the day, at daytime or night. Hypoglycemia causes anxiety and fear in patients, and severe hypoglycemia tends to occur at night. However, nocturnal hypoglycemia is usually asymptomatic and difficult to monitor, which often results in patients not getting timely and appropriate severe hypoglycemia management. GLP-1RA promotes insulin secretion in a glucose-dependent manner and inhibits glucagon secretion, contributing to a low risk of hypoglycemia. However, we should still be vigilant against the occurrence of nocturnal hypoglycemia, though the risk of hypoglycemia due to GLP-1RA is relatively low.
In addition to lowering blood glucose, we also found that HOMA-IR decreased after treatment, while HOMA-β increased. This indicates that EXQW could not only lower blood glucose, but also contribute to the repair of damaged pancreatic islet function and reduce the resistance of peripheral tissues to insulin, which is consistent with the findings of Bunck et al[20].
In this study, changes in the body weight and waist circumference of patients were recorded. After 12 wk of treatment, both body weight and waist circumference were reduced compared to those at baseline, which is consistent with previous findings on using EXQW to improve body weight and waist circumference[12,21,22]. We are informed that EXQW reduces body weight and waist circumference mainly through inhibition of the appetite, extended digestion in the stomach, and reduction in calorie intake.
Nevertheless, this study has several limitations, one of which is the lack of a control group. Moreover, it was a single-center study involving a small number of patients and a short observation period that limited the evaluation of certain essential treatment indicators. Meanwhile, the poor compliance of some patients or force majeure resulted in some missing data on blood glucose levels. To overcome these study limitations, a multi-center study could be carried out, with higher number of subjects, extended period of observation, and enhanced education to improve patient compliance.
In summary, EXQW in combination with metformin could effectively improve blood glucose levels and reduce GV. It has a remarkable effect on the control of fasting blood glucose and also exerts certain effect on the blood glucose levels after three meals in a day. While GV is more stable throughout the day, we also found that the GV parameters of day and night times were reduced. Although the risk of severe hypoglycemia in EXQW is relatively low, there is still a risk of nocturnal hypoglycemia, which requires careful attention. In addition, EXQW has a variety of effects other than lowering blood glucose, such as alleviating insulin resistance, improving islet function, and reducing body weight and waist circumference. Therefore, it has a good clinical application prospect for the treatment of patients with T2DM.
Multiple studies demonstrate that fluctuating blood glucose level produces greater damage compared with sustained hyperglycemia. Flash glucose monitoring system (FGMS) is an effective method in documenting blood glucose variability, contributing to better glucose management and reduced hypoglycemic event occurrence.
A dynamic blood glucose monitoring system was used to observe the blood glucose characteristics of type 2 diabetes patients during the use of exenatide once weekly (EXQW) combined with metformin.
As the aims of this study, the clinical and therapeutic effects of combined EXQW and metformin treatment in type 2 diabetes patients were evaluated by using FGMS and metabolic indicators. Based on the FGMS data, we analyzed and compared the glycemic variability-related indices, and hypoglycemic incidence between baseline and end points.
This study is a pre-post study involving the same group of patients. Patients wore FGMS twice during the screening period (oral metformin monotherapy) and the experimental period (EXQW combined with metformin), respectively, and the changes of blood glucose characteristics and related metabolic indexes at baseline and endpoint were observed.
After 12 wk of combined treatment, hyperglycemia was controlled, especially fasting blood glucose, and postprandial blood glucose levels were also reduced. The percentage of time in range (3.9-7.8 mmol/L) increased and the percentage of time out of range decreased. The fluctuation of blood glucose was more stable than baseline, and there were statistically significant differences in standard deviation of blood glucose, mean amplitude of glycemic excursion, mean of daily difference, area under the curve of IQR, and area under the curve of IDR. After treatment of EXQW combined with metformin, the duration of hypoglycemia (< 3.9 mmol/L) increased, mainly at night, but the duration of severe hypoglycemia (< 3.0 mmol/L) did not increase. In addition, metabolic indexes such as body weight and waist circumference were improved.
The combination of EXQW and metformin can effectively control hyperglycemia, reduce glycemic variability, and improve metabolic indexes. By analyzing FGMS data during different time periods, EXQW was found to significantly improve fasting and postprandial glucose without increasing the incidence of severe hypoglycemia (< 3.0 mmol/L) events, but still with an increased risk of nocturnal hypoglycemia.
This study has its limitations, and we expect that a multi-center, large-sample study should be carried out.
We are grateful to all the study subjects for their participation.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Athyros VG, Koch T, Nakajima K S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Prázný M, Škrha J, Šoupal J, Škrha J Jr. [Glycemic variability and microvascular complications of diabetes]. Cas Lek Cesk. 156:308-313. [PubMed] |
| 2. | Nusca A, Tuccinardi D, Albano M, Cavallaro C, Ricottini E, Manfrini S, Pozzilli P, Di Sciascio G. Glycemic variability in the development of cardiovascular complications in diabetes. Diabetes Metab Res Rev. 2018;34:e3047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 3. | Heinemann L, Freckmann G. CGM Versus FGM; or, Continuous Glucose Monitoring Is Not Flash Glucose Monitoring. J Diabetes Sci Technol. 2015;9:947-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Rouhard S, Buysschaert M, Alexopoulou O, Preumont V. Impact of flash glucose monitoring on glycaemic control and quality of life in patients with type 1 diabetes: A 18-month follow-up in real life. Diabetes Metab Syndr. 2020;14:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Kublin O, Stępień M. Effect of Using Additional Readers for Flash Glucose Monitoring System on Metabolic Control, Safety, and the Incidence of Complications in Patients With Diabetes Mellitus. J Diabetes Sci Technol. 2020;1932296819900257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4164] [Cited by in RCA: 4921] [Article Influence: 546.8] [Reference Citation Analysis (0)] |
| 7. | Fifer S, Rose J, Hamrosi KK, Swain D. Valuing injection frequency and other attributes of type 2 diabetes treatments in Australia: a discrete choice experiment. BMC Health Serv Res. 2018;18:675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3281] [Cited by in RCA: 3716] [Article Influence: 177.0] [Reference Citation Analysis (0)] |
| 9. | Xu S, Liu X, Ming J, Ji Q. Comparison of exenatide with biphasic insulin aspart 30 on glucose variability in type 2 diabetes: study protocol for a randomized controlled trial. Trials. 2016;17:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Dover AR, Stimson RH, Zammitt NN, Gibb FW. Flash Glucose Monitoring Improves Outcomes in a Type 1 Diabetes Clinic. J Diabetes Sci Technol. 2017;11:442-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Patti AM, Nikolic D, Magan-Fernandez A, Giglio RV, Castellino G, Chianetta R, Citarrella R, Corrado E, Provenzano F, Provenzano V, Montalto G, Rizvi AA, Rizzo M. Exenatide once-weekly improves metabolic parameters, endothelial dysfunction and carotid intima-media thickness in patients with type-2 diabetes: An 8-month prospective study. Diabetes Res Clin Pract. 2019;149:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Lu J, Ma X, Shen Y, Wu Q, Wang R, Zhang L, Mo Y, Lu W, Zhu W, Bao Y, Vigersky RA, Jia W, Zhou J. Time in Range Is Associated with Carotid Intima-Media Thickness in Type 2 Diabetes. Diabetes Technol Ther. 2020;22:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 13. | Lu J, Ma X, Zhou J, Zhang L, Mo Y, Ying L, Lu W, Zhu W, Bao Y, Vigersky RA, Jia W. Association of Time in Range, as Assessed by Continuous Glucose Monitoring, With Diabetic Retinopathy in Type 2 Diabetes. Diabetes Care. 2018;41:2370-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 351] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 14. | Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care. 2008;31:2198-2202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 322] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 15. | Šoupal J, Škrha J Jr, Fajmon M, Horová E, Mráz M, Škrha J, Prázný M. Glycemic variability is higher in type 1 diabetes patients with microvascular complications irrespective of glycemic control. Diabetes Technol Ther. 2014;16:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Saisho Y. Glycemic variability and oxidative stress: a link between diabetes and cardiovascular disease? Int J Mol Sci. 2014;15:18381-18406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 142] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 17. | Frías JP, Nakhle S, Ruggles JA, Zhuplatov S, Klein E, Zhou R, Strange P. Exenatide once weekly improved 24-hour glucose control and reduced glycaemic variability in metformin-treated participants with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19:40-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Gerbaud E, Darier R, Montaudon M, Beauvieux MC, Coffin-Boutreux C, Coste P, Douard H, Ouattara A, Catargi B. Glycemic Variability Is a Powerful Independent Predictive Factor of Midterm Major Adverse Cardiac Events in Patients With Diabetes With Acute Coronary Syndrome. Diabetes Care. 2019;42:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 19. | Cardoso CRL, Leite NC, Moram CBM, Salles GF. Long-term visit-to-visit glycemic variability as predictor of micro- and macrovascular complications in patients with type 2 diabetes: The Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc Diabetol. 2018;17:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 20. | Bunck MC, Diamant M, Cornér A, Eliasson B, Malloy JL, Shaginian RM, Deng W, Kendall DM, Taskinen MR, Smith U, Yki-Järvinen H, Heine RJ. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 21. | Gorgojo-Martínez JJ, Gargallo-Fernández MA, Brito-Sanfiel M, Lisbona-Catalán A. Real-world clinical outcomes and predictors of glycaemic and weight response to exenatide once weekly in patients with type 2 diabetes: The CIBELES project. Int J Clin Pract. 2018;72:e13055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Jones KL, Huynh LQ, Hatzinikolas S, Rigda RS, Phillips LK, Pham HT, Marathe CS, Wu T, Malbert CH, Stevens JE, Lange K, Rayner CK, Horowitz M. Exenatide once weekly slows gastric emptying of solids and liquids in healthy, overweight people at steady-state concentrations. Diabetes Obes Metab. 2020;22:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |