Published online Dec 15, 2020. doi: 10.4239/wjd.v11.i12.622
Peer-review started: August 31, 2020
First decision: October 5, 2020
Revised: October 12, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: December 15, 2020
Processing time: 103 Days and 18 Hours
Benzylamine and methylamine activate glucose uptake in adipocytes. For tyramine, this effect has even been extended to cardiomyocytes.
To investigate the effects of catecholamines and other amines on glucose uptake.
A screening compared 25 biogenic amines on 2-deoxyglucose (2-DG) uptake activation in rat adipocytes. Pharmacological approaches and transgenic mouse models were then used to decipher the mode of action of several hits.
In rat adipocytes, insulin stimulation of 2-DG uptake was reproduced with catecholamines. 100 µmol/L or 1 mmol/L adrenaline, noradrenaline, dopamine and deoxyepinephrine, maximally activated hexose transport only when sodium orthovanadate was added at 100 µmol/L. Such activation was similar to that already reported for benzylamine, methylamine and tyramine, well-recognized substrates of semicarbazide-sensitive amine oxidase (SSAO) and monoamine oxidase (MAO). Several, but not all, tested agonists of β-adrenoreceptors (β-ARs) also activated glucose transport while α-AR agonists were inactive. Lack of blockade by α- and β-AR antagonists indicated that catecholamine-induced 2-DG uptake was not mediated by AR stimulation. Adipocytes from mice lacking β1-, β2- and β3-ARs (triple KO) also responded to millimolar doses of adrenaline or noradrenaline by activating hexose transport in the presence of 100 µmol/L vanadate. The MAO blocker pargyline, and SSAO inhibitors did not block the effects of adrenaline or noradrenaline plus vanadate, which were blunted by antioxidants.
Catecholamines exert unexpected insulin-like actions in adipocytes when combined with vanadium. For limiting insulin resistance by activating glucose consumption at least in fat stores, we propose that catecholamine derivatives combined with vanadium can generate novel complexes that may have low toxicity and promising anti-diabetic properties.
Core Tip: In rat and mouse fat cells, the combination of catecholamines with vanadium reproduces the sugar entry activation already reported for benzylamine, methylamine or tyramine. Glucose transport stimulation is observed only with catecholamines at millimolar doses and in the presence of a vanadium dose that is ineffective on its own. The synergism between adrenaline or noradrenaline and vanadate is not mediated by adrenoceptors and resists amine oxidase inhibitors; while it is sensitive to antioxidants. Since vanadium exhibits antidiabetic properties, but with toxicological concerns, it is proposed that the combination of catecholamine derivatives plus vanadate salts might generate complexes with safer blood glucose-lowering properties.
- Citation: Fontaine J, Tavernier G, Morin N, Carpéné C. Vanadium-dependent activation of glucose transport in adipocytes by catecholamines is not mediated via adrenoceptor stimulation or monoamine oxidase activity. World J Diabetes 2020; 11(12): 622-643
- URL: https://www.wjgnet.com/1948-9358/full/v11/i12/622.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i12.622
Type 2 diabetes is one of the morbid complications of obesity. The hypertrophied adipose tissues (AT) do not play only a quantitative but also a qualitative role in glucose handling. Due to the elevated inflammation state and the oxidative stress found in pathological adipose tissue, its constitutive cell types (adipocytes, precursor cells and immune cells) modulate the adipokines they release in the organism, leading to a state of insulin-resistance. With the exception of adiponectin, which facilitates the action of insulin, most of the adipokines are proinflammatory or impair insulin antihyperglycemic actions: interleukin (IL)-6, tumor necrosis factor α, and many others, which can be exemplified by one of them, the well-named resistin.
To treat such diabetic insulin resistant- and insulin deficient-states, the search for molecules that reproduce totally or partially the insulin activation of glucose transport has been included in the antidiabetic strategies developed so far. In the search for activators of glucose uptake that may replace insulin, the adipocytes themselves constitute a valuable cell model since their glucose transport capacity is highly responsive to insulin, at least in non-obese, non-diabetic rodents.
Glucose entry can be measured as an acute response after short-term exposure of adipocytes to the tested molecules followed by a transport assay consisting of the measurement of the intracellular uptake of a non-metabolizable analogue of glucose, such as 2-deoxyglucose (2-DG). Using this model, it was previously observed that the reactive oxygen species (ROS), especially hydrogen peroxide, partially mimic the insulin stimulation of glucose uptake[1] while an excess of pro-oxidant agents, leading to oxidative stress hampers insulin action[2]. Notably, the combination of hydrogen peroxide with vanadium, known to generate peroxovanadate, results in a more potent stimulation of glucose uptake as it acts downstream of the insulin receptor directly on the turnover of glucose transporters (GLUT4) between intracellular vesicles and extracellular surface[3].
Similarly, it has been reported that hexose transport stimulation can be obtained by incubating fat cells with benzylamine and sodium orthovanadate[4,5]. Indeed, the amine is oxidized by amine oxidases that generate benzaldehyde, ammonium and hydrogen peroxide during the oxidative deamination. Among these end-products, it is hydrogen peroxide that reacts with vanadate to generate peroxovanadate thereby inhibiting tyrosine phosphatases involved in the turn-off of glucose carrier translocation to the cell surface[6]. Since benzylamine is a reference substrate for a class of amine oxidases that is abundant in adipocytes, the semicarbazide-sensitive amine oxidase (SSAO)[7,8], we expected to extend such 'insulin like' property to other substrates. As SSAO has been renamed primary amine oxidase (PrAO) because it is blocked by many other inhibitors in addition to semicarbazide and due to its activity on a wide range of primary amines[9], the number of potential insulin-mimicking agents was largely increased among the SSAO/PrAO substrate candidates. This was the case for methylamine, another SSAO/PrAO substrate of reference, which was demonstrated to activate glucose transport in rat adipocytes[10] and in human fat cells[11]. Methylamine is also active in mouse fat cells[12]. Both benzylamine and methylamine have revealed anti-hyperglycemic effects in mice[13,14], rats[15], and rabbits[10], and these effects are abolished in mice invalidated for SSAO/PrAO, also known as vascular adhesion protein-1 (VAP-1)[16].
Alongside SSAO/PrAO, other amine oxidases are present in adipocytes, which also release hydrogen peroxide when oxidating their substrates. Among them, monoamine oxidases (MAO) have been reported to be involved in tyramine stimulated glucose transport in rodent adipocytes and cardiomyocytes[17], which is also enhanced by vanadium[18]. Similar to benzylamine, tyramine also exhibits long-term insulin-like actions such as the stimulation of adipose differentiation of preadipocytes[19].
In this context, we aimed to verify in adipocytes whether the insulin-like properties of benzylamine plus vanadium could be reproduced or improved by exposing the fat cells to other amines, in order to retrieve novel insulin-mimickers on the basis of glucose transport activation, and candidates leading to potential antidiabetic strategies. We thus performed a screening of various amines, with a focus on testing them with and without vanadium in the incubation medium of freshly isolated rat adipocytes. The addition of sodium orthovanadate (Na3VO4) at 100 µmol/L in the incubation medium was chosen for this comparative approach as this has been successfully performed in rat adipocytes[18]. This led us to use the diminutive term vanadate throughout this study although vanadyl and vanadate forms can coexist together with multiple oxidation states or oligovanadate forms in biological systems[20]. Our screening was mainly limited to naturally occurring amines, since we previously reported insulin-like properties of non-natural compounds[21], arylalkylamines[22,23] and the hexaquis (benzylammonium) decavanadate[24], which have not been approved for further studies due to toxic side-effects.
The following results will provide evidence for the novel, insulin-like effect of catecholamines. Catecholamines are monoamine neurotransmitters composed of a catechol cycle (benzene with two hydroxyl side groups) and a side-chain amine: dopamine (with NH2), noradrenaline (with OH and NH2, also known as norepinephrine), and adrenaline (also named epinephrine, with OH and NH-CH3). Catecholamines, under certain conditions, are able to activate glucose transport in fat cells in an unexpected manner, different to that of the reference SSAO/PrAO and MAO substrates: benzylamine, methylamine and tyramine.
(+/-)-Adrenaline (also named epinephrine), (-)-noradrenaline (also named norepinephrine or arterenol), dopamine, tyramine, benzylamine, sodium orthovanadate, (-)-isoprenaline (also named isoproterenol), collagenase, dimethyl sulfoxide and most of the other reagents were from Sigma-Aldrich-Merck (Saint Quentin Fallavier, France). Brimonidine was a gift from the late Dr. Paris H. (Toulouse, France), while CL 316243 was kindly given by Dr. Lafontan M. (Toulouse, France). [3H]-2-deoxyglucose was from Perkin Elmer (Boston, MA, United States).
Male Wistar rats from Charles River (L’Arbresle, France) were euthanized after overnight fasting when 2- to 3-mo old, according to Inserm guidelines. The generation of "β-less" mice was performed at the Centre Médical Universitaire (Genève, Switzerland). β-less (β1-/-β2-/-β3-/-) and wild-type (WT) (β1+/+β2+/+β3+/+) strains were obtained by intercrossing β3-AR knockout mice[25] and β1/β2-AR knockout mice[26], kindly provided by Dr. Kobilka B.K. (Howard Hughes Medical Institute, Stanford, CA, USA). β1+/- β2+/- β3+/- offspring were crossed to generate established WT and β-less colonies on the same, mixed genetic background (129 Sv/ev, 129 Sv/J, FVB/N, C57BL/6J, and DBA/2). After being transferred by shipment adapted for genetically modified organisms, the mice were bred under SOPF status at the animal unit of UMS006 (Toulouse, France); genotypes were determined by Southern blot as described elsewhere[27]. Studies were performed on 4 β-less males aged 32 wk, while 13 WT mice with a mean age of 22 wk were studied in parallel. All the rodents were treated in accordance with the ARRIVE guidelines (Animal Research: Reporting of In Vivo Experiments)[28].
Adipocyte preparations were obtained by collagenase digestion of perigonadic, retroperitoneal, perirenal and inguinal fat pads as described previously[24]. The same procedure was applied for rat and mouse adipocyte preparations, as previously reported[29]. Briefly, adipose tissues were minced with scissors in Krebs-Ringer salt solution pH 7.5 containing 15 mmol/L sodium bicarbonate, 10 mmol/L HEPES and 3.5% fat-depleted bovine serum albumin. As the only source of glucose for the cell preparations tested for glucose uptake assays was the non-metabolizable analogue described below, 2 mmol/L pyruvate was present in the medium for energy supply. After digestion with 1 mg/mL collagenase type II for approximately 45 min under agitation, buoyant adipocytes were separated by filtration through nylon mesh and washed twice with medium to eliminate collagenase and obtain functional adipocyte suspensions, ready for use in the initial screening or the complementary approaches. Suspensions of freshly isolated rat adipocytes were used at approximately 15 mg lipids/400 µL unless otherwise stated.
An isotopic dilution of the non-metabolizable glucose analogue [3H]-2-DG[30] was prepared to reach a final concentration of 0.1 mmol/L when added to 400 µL of fat cell suspension as described previously[31]. This radioactive tracer was added after 45 min preincubation with the tested or reference agents. Then, the fat cells were incubated for additional 10 min in the presence of [3H]-2-DG (approximately 1300000 dpm/vial) and stopped with 100 µL of 100 µmol/L cytochalasin B. 200 µL of cell suspension were immediately transferred to plastic centrifugation microtubes prefilled with dinonyl phthalate (density 0.98 g/mL) before a 40 s spin, to separate the buoying adipocytes from the medium as described previously[10]. The upper part of the tubes, containing radiolabelled hexose internalized in intact fat cells above the silicon layer was then counted in scintillation vials. The extracellular [3H]-2-DG present in this upper part of the tubes, and which was not internalized in cells was determined with adipocytes whose transport activity was previously blocked by cytochalasin B at time 0. It did not exceed 1% of the maximum 2-DG uptake in response to insulin and was subtracted from all assays. No radioactivity was found in the silicon layer separating the upper and lower part of the microtubes (not shown), in accordance with the entirely hydrophilic nature of 2-DG[30].
Irrespective of the size of the molecular library used for a screening to test the properties of chemicals on hexose uptake it is mandatory to define the baseline and the maximal level of the transport of a non-metabolizable analogue of glucose, and to compare the unknowns to these negative and positive cut-offs. To perform a pilot approach comparing a total of 25 amines, we used a method previously validated, based on the above described uptake of [3H]-2-deoxyglucose, which has been revealed to be efficient in detecting insulin-like agents, such as vanadium derivatives[24] or inhibitors such as β-AR agonists[31]. The negative and positive controls were simply normalized, by setting baseline uptake at 0%, while maximal response to 100 nmol/L insulin was set at 100%. Each of five subsets of five amines was tested for 45 min with rat adipocytes, in parallel with the above-mentioned controls. Since each subset was tested on three independent adipocyte preparations, a total of fifteen adipocyte preparations isolated from fifteen rats was required for this first comparative approach. In the subsequent studies aiming at deciphering the mode of action of the best hits, the same protocol and data processing were used except that, alongside all the required negative and positive controls, pharmacological agents were added during the 45-min preincubation period and remained present during 2-DG uptake assays. As each step of these mechanistic investigations was performed on groups of 4 to 6 rats, 38 rats were necessary for testing successively MAO and PrAO inhibitors, their interplay with vanadium, as well as the influence of different doses of dopaminergic and β- or α1-/α2-adrenergic receptor antagonists or agonists. Thus, a total of 53 rats were used for both the pilot and mechanistic halves of the screening.
Tested agents were added to 400 µL of fat cell suspension as above for glucose uptake, with the following differences. The agents were incubated with the fat cells at 37°C under gentle shaking for 90 min in a buffer containing 5.5 mmol/L glucose instead of pyruvate and 2-DG. Incubations were stopped on ice. Lipolysis was determined by using glycerol release as an index as already documented, considering that free fatty acid release exhibits parallel variations in our experimental conditions[32].
Results are presented as means ± SE of (n) observations. All the statistical analyses for comparisons between parameters obtained in the presence of 100 µmol/L vanadium and their respective control used the paired Student’s t test. Other statistical comparisons used ANOVA followed by post-hoc Dunnett's multiple comparisons test, analyzed with Prism 6 for Mac OS X (from GraphPad software, Inc).
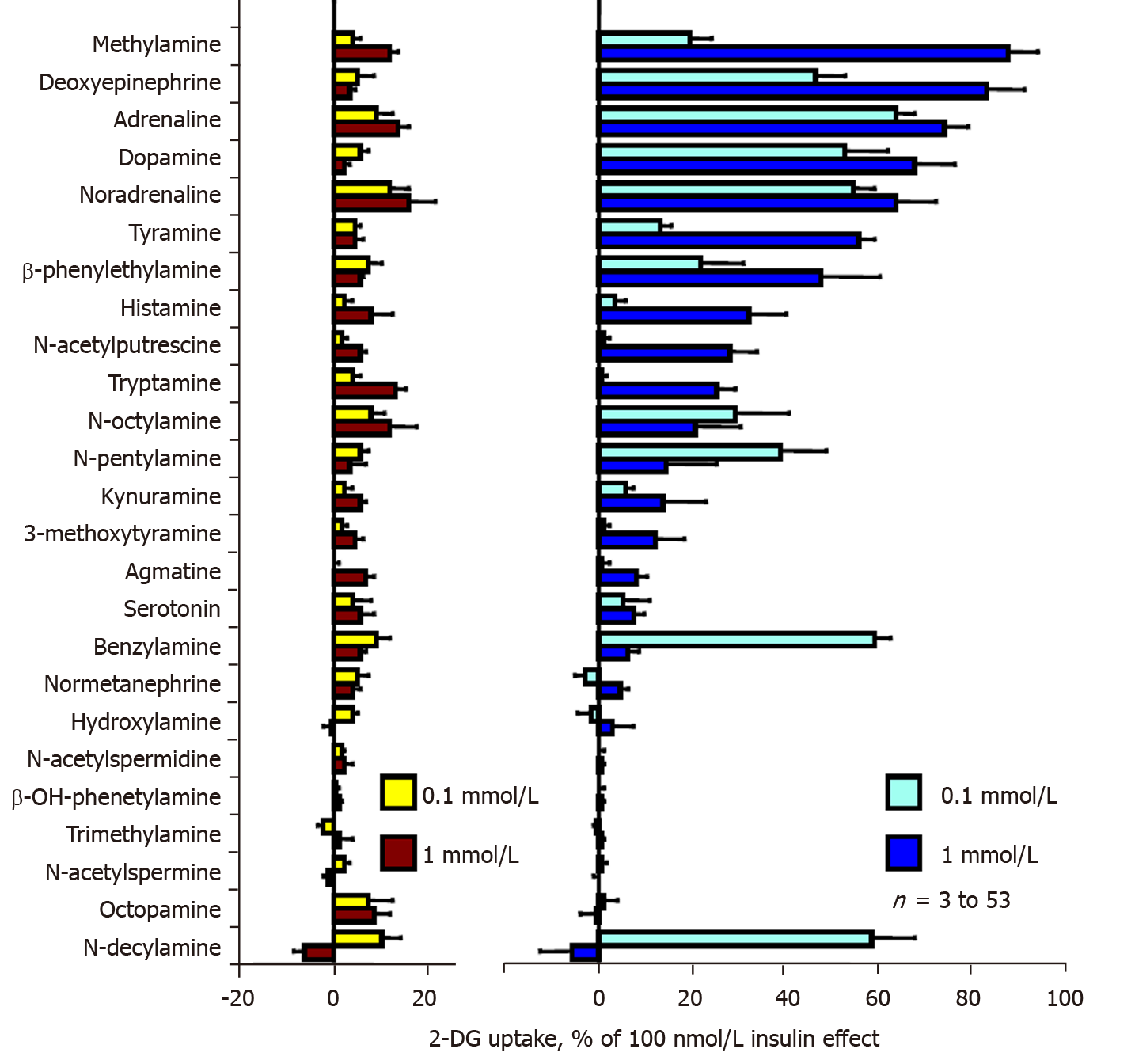
Our preliminary screening approach aimed to detect naturally occurring amines that can mimic the previously reported effects of benzylamine, tyramine and methylamine on the uptake of the non-metabolizable glucose analogue 2-DG in fat cells. Figure 1 displays data retrieved from 25 natural amines tested. The left panel of Figure 1 shows that none of the amines increased hexose transport activity more than 20% of the maximal stimulation elicited by 100 nmol/L insulin (positive control, set at 100%). When tested alone, several amines even inhibited basal 2-DG uptake (baseline, set at 0%), leading to negative percentages. This was the case for n-decylamine, N-acetyl spermidine and trimethylamine. In contrast, methylamine, adrenaline, noradrenaline and tryptamine tended to slightly activate 2-DG uptake when tested at 0.1 and 1 mmol/L. Although such weak activations were detected, they were not considered positive hits since they never exceeded 20% of the maximal insulin effect, which was the threshold considered for the screening.
More importantly, when sodium orthovanadate was added at 100 µmol/L at the beginning of the 45-min incubation period with amines, a totally different pattern was obtained. The chosen dose of vanadate, already reported to potentiate tyramine[18] and benzylamine[5] actions in rodent adipocytes was unable on its own to modify basal or insulin stimulated uptake, but facilitated the activation of glucose uptake by most of the tested amines. Several of them almost reached the maximal activation obtained with insulin itself. The powerful insulin-mimicking effect of methylamine on 2-DG uptake[11] was confirmed since it was the best hit of the screening (Figure 1, right panel). Ranking the tested amines according to the response they elicited at 1 mmol/L in the presence of 100 µmol/L vanadate showed that the millimolar dose of deoxyepinehrine, adrenaline, dopamine, and noradrenaline reproduced more than 75% of the insulin-induced maximal stimulation of glucose transport (Figure 1, right panel). A large proportion of the tested amines enhanced glucose uptake in a dose-dependent manner, except for benzylamine, n-decylamine and n-pentylamine, which were more efficient at 0.1 mmol/L than at 1 mmol/L. Since 100 nmol/L insulin increased the 2-DG uptake of isolated rat adipocytes by more than ten-fold (Figure 2), the stimulation reached after 45 min exposure to millimolar doses of these natural amines has to be definitely considered as substantial and highly significant, since the basal uptake was increased more than ten times. For tyramine and benzylamine (reference substrates of MAO and PrAO already demonstrated to activate 2-DG uptake in rodent adipocytes[4,5,18]), the stimulatory property on glucose entry was also confirmed. As our aim was to detect amines that might reproduce or even overpass the previously described synergism between vanadium and benzylamine[33], tyramine[18], or methylamine[34], such confirmatory observations totally supported our chosen screening approach and amines of reference. Unfortunately, the screening did not allow the detection of a molecule that behaves more efficiently than the reference amines in stimulating glucose consumption. Nevertheless, such an approach identified various catecholaminergic neurotransmitters as potent activators of glucose uptake when present at 0.1 mmol/L together with vanadium. In fact, it was not so amazing to find adrenaline, noradrenaline and dopamine among the best hits of this preliminary screening since they share with the reference amines the capacity to be substrates of amine oxidases. However, a different readout was obtained with histamine, a well-known substrate of diamine oxidase, which was hardly more active at 1 than at 0.1 mmol/L, and with serotonin (a MAO-A substrate also known as 5-hydroxytryptamine), which was almost ineffective.
Noradrenaline and adrenaline were consequently considered as the major novel hits of the screening. On the large number of observations obtained during the primary screening and its further exploration steps, these two catecholamines clearly reproduced approximately 70%-80% of insulin stimulation on glucose entry when present at 0.1-1 mmol/L in combination with 0.1 mmol/L vanadate (cumulated number of preparations was n = 53 for each catecholamine). Although we were aware of the apparently extra-physiological nature of such an in vitro effect, we further explored its putative mechanisms of action, keeping in mind that these neurotransmitters are well-known substrates of central MAOs, which deaminate them into the corresponding aldehydes and hydrogen peroxide[35].
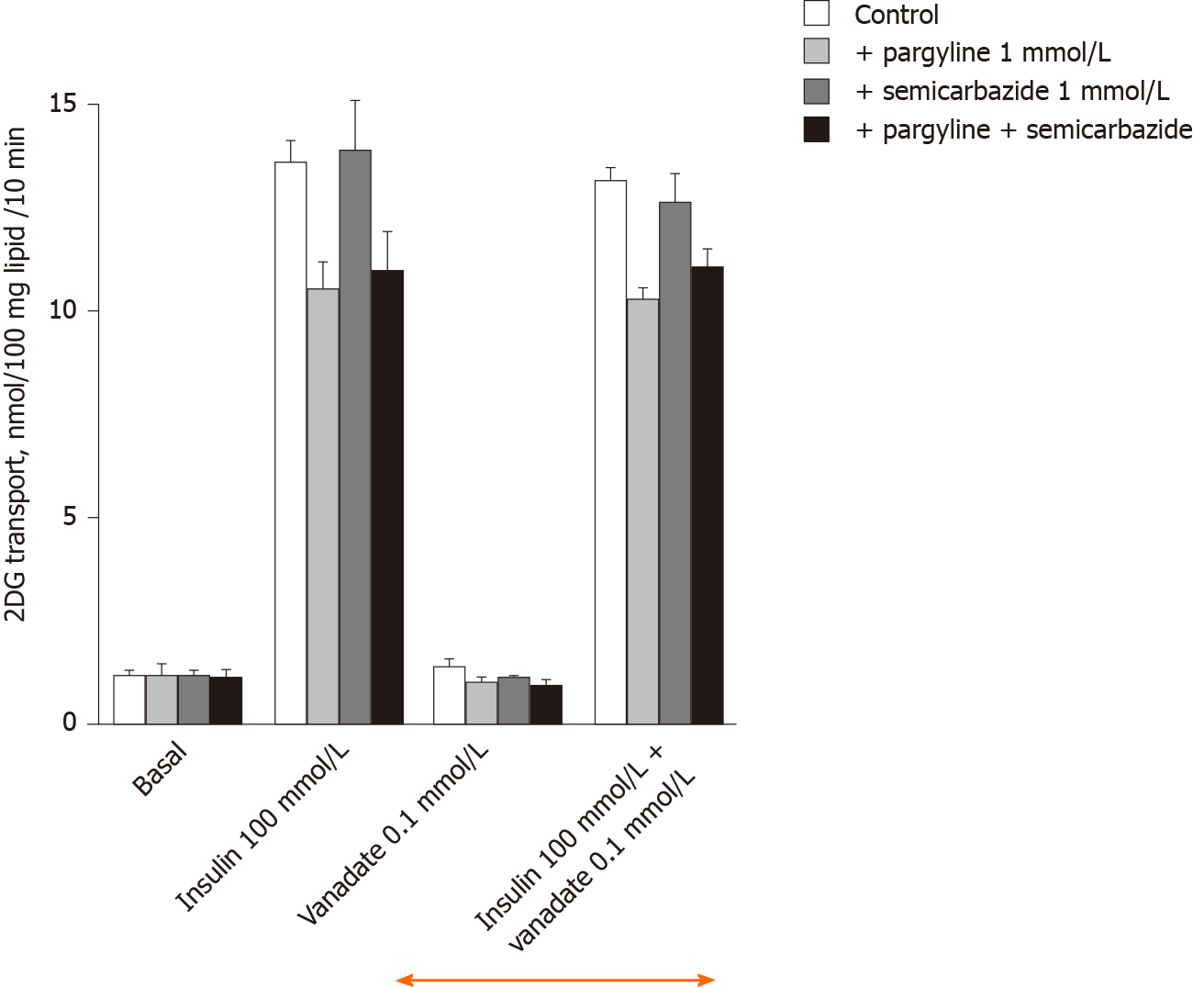
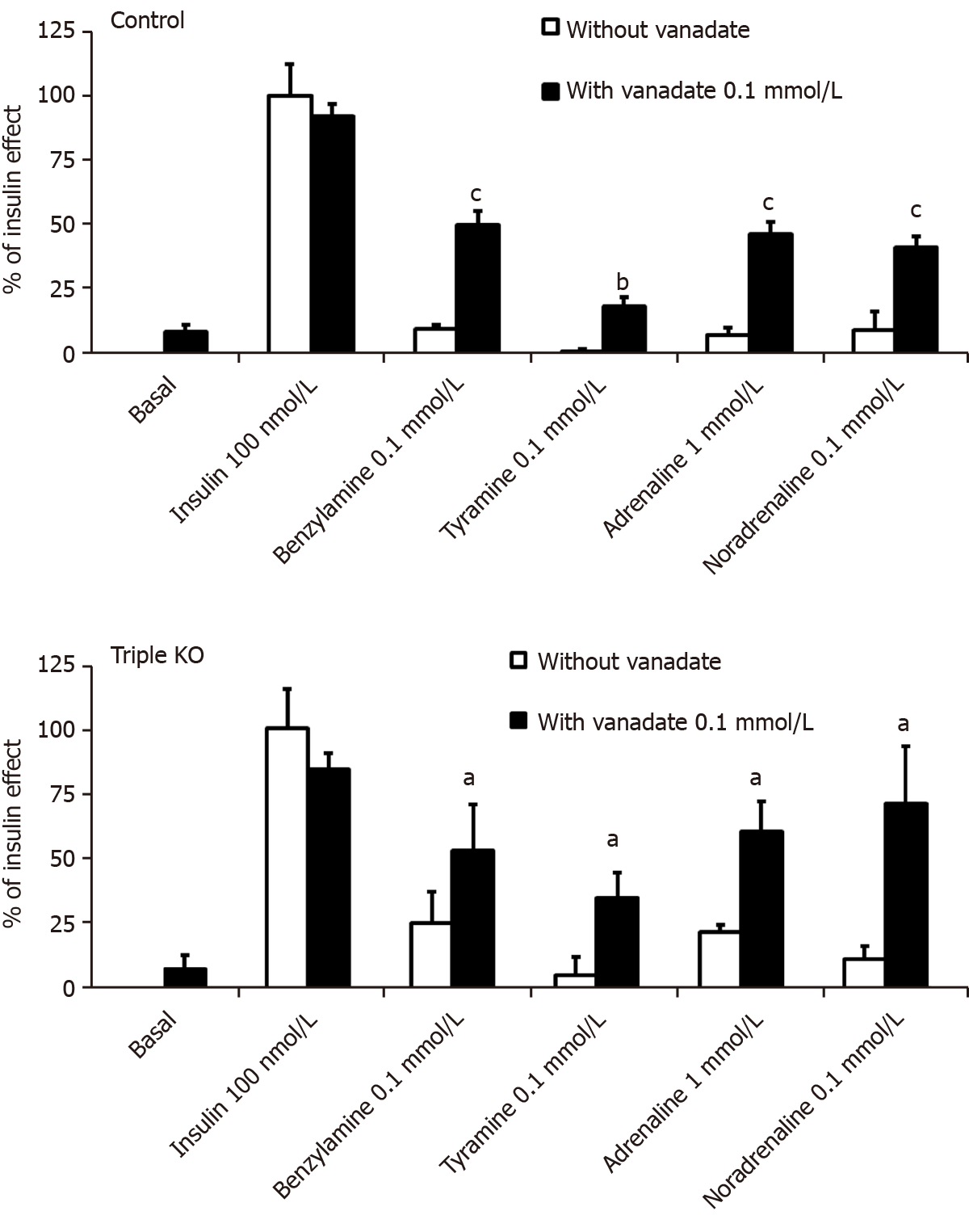
100 µmol/L vanadate did not alter basal 2-DG uptake in rat adipocytes in these mechanistic investigations, as during the preliminary screening experiments. This was also the case for 1 mmol/L pargyline (MAO inhibitor) or 1 mmol/L semicarbazide (SSAO/PrAO inhibitor) (Figure 2). The maximal effect of the high insulin dose (100 nmol/L) was also unaffected by 100 µmol/L vanadate or by the inhibitors, either when tested separately or in combination (Figure 2). These observations fully support why the dose of 0.1 mmol/L vanadate is described as 'ineffective on its own' since it did not alter basal or insulin-stimulated uptake whereas higher doses of vanadium clearly activate glucose transport[36].
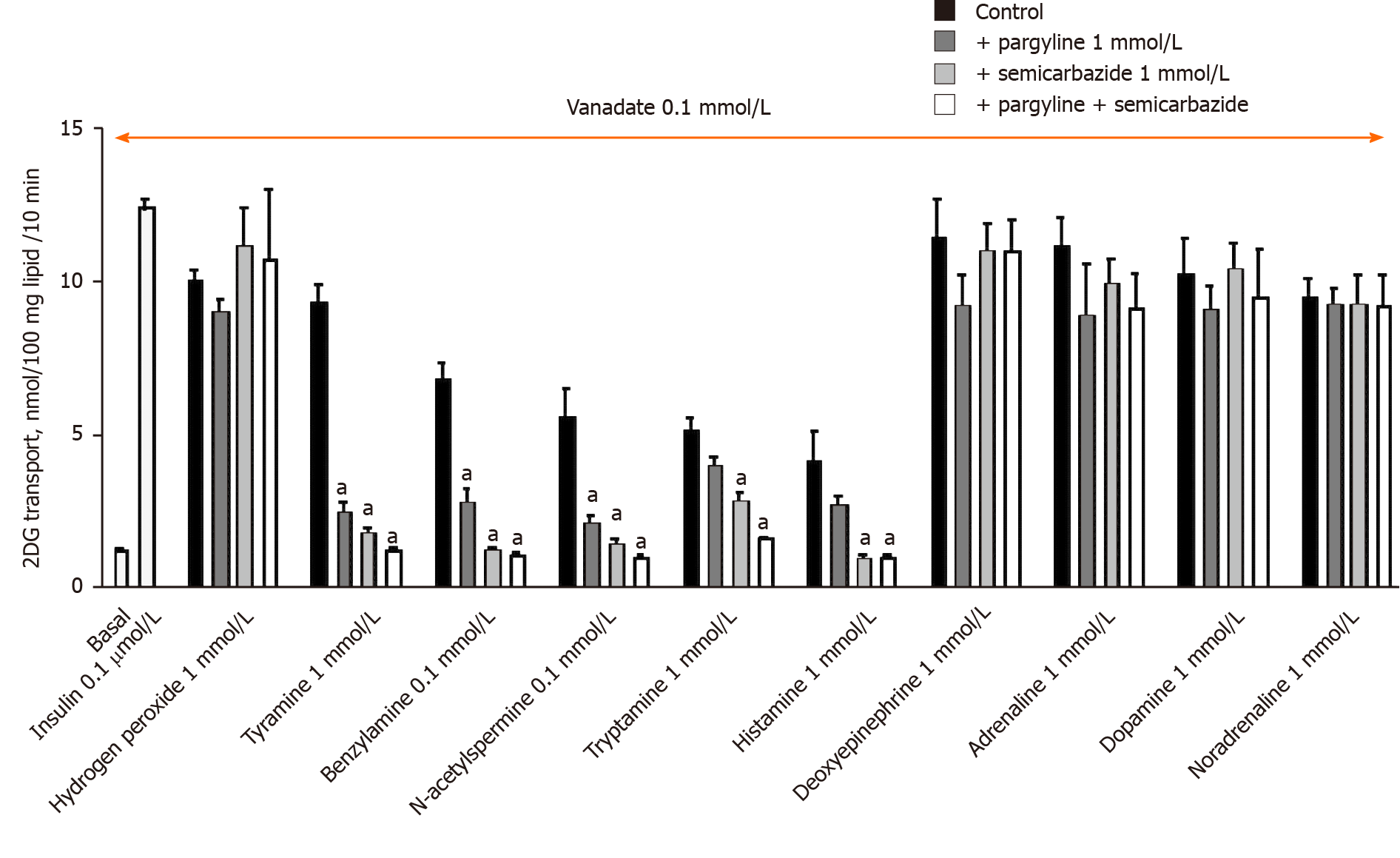
The stimulation of 2-DG uptake induced by the combination of 1 mmol/L hydrogen peroxide and 100 µmol/L vanadium corresponds to a well-recognized potentiation of the separate effects of ROS and metal ions[20,37], and results from the non-enzymatic generation of peroxovanadate, a potent insulin mimicker[38], the efficacy of which was confirmed here (Figure 3). Notably, the effect of hydrogen peroxide + vanadium was not impaired by the MAO and PrAO inhibitors. In contrast, the blockade of MAO and/or PrAO by pargyline and/or semicarbazide prevented tyramine, benzylamine, N-acetylputrecine, tryptamine and histamine from activating glucose transport (Figure 3). A complete inhibition of the latter amines was observed with the combination of pargyline and semicarbazide. However, for benzylamine and histamine, maximal inhibition was attained with semicarbazide alone, without any further inhibition obtained by its combination with pargyline (Figure 3). Surprisingly, the amine oxidase inhibitors, used either separately or in combination, were totally unable to alter the activation of glucose uptake by deoxyepinephrine, adrenaline, dopamine or noradrenaline (Figure 3). This finding was unexpected as it was initially supposed that catecholamines activated 2-DG uptake in rat adipocytes in a manner similar to that of other widely recognized substrates of MAO or PrAO[7,17].
In fact, noradrenaline, adrenaline, dopamine and deoxyepinephrine can be considered as "false hits" in our screening. Elevated concentrations of these catecholamines stimulated hexose uptake in a manner that resembled that of hydrogen peroxide, but which was basically distinct from that of other MAO or PrAO substrates; including those used as internal references (benzylamine, methylamine, tyramine). In other words, their mechanism of action appeared independent from MAO or PrAO activity.
At this point, it is worth mentioning that the "insulin-like" effect of several catecholamines could not be qualified as non-specific since not observed with most of the other amines tested at 1 mmol/L. This was probably not a toxic effect, as noxious chemical species are rather inhibiting than facilitating or mimicking insulin action[39].
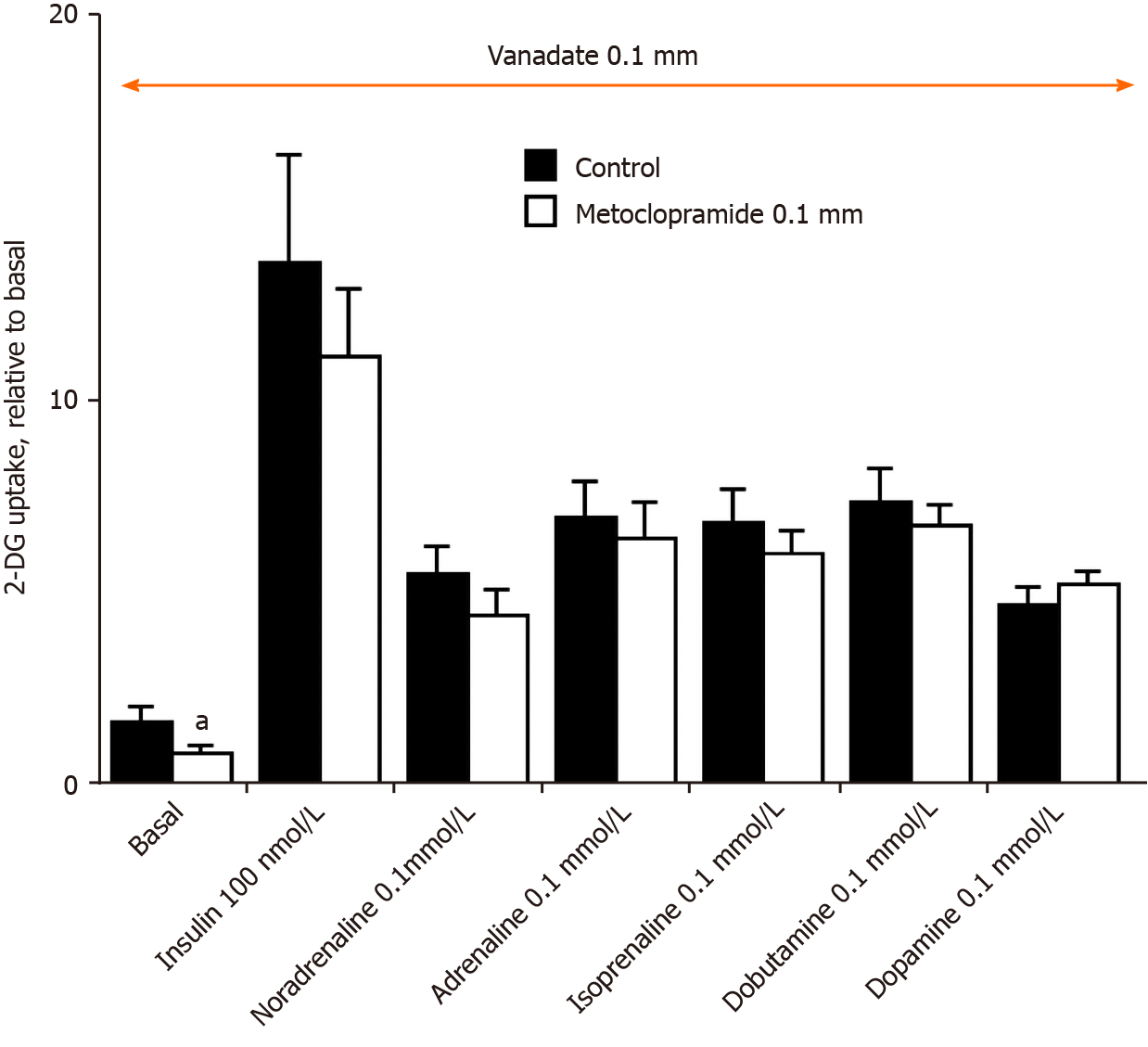
Regarding the effect of dopamine, its resistance to the blockade by the dopaminergic antagonist metoclopramide (Figure 4) did not suggest any mediation via dopaminergic receptor activation. Similarly, dopaminergic receptors were apparently not involved in the stimulation of 2-DG uptake by adrenaline or noradrenaline (Figure 4). Thus, further exploration consisted of testing whether adrenergic receptors, which bind catecholamines, were involved in their stimulatory action on hexose uptake. Hence, diverse β-adrenoreceptor (β-AR) agonists have already been reported to modulate glucose transport activity in adipocytes, but with contradictory effects, from stimulatory[40-42] to inhibitory[31,43] depending on the nature (white, beige, brown) of the adipocytes, the animal species[44], and the experimental conditions tested.
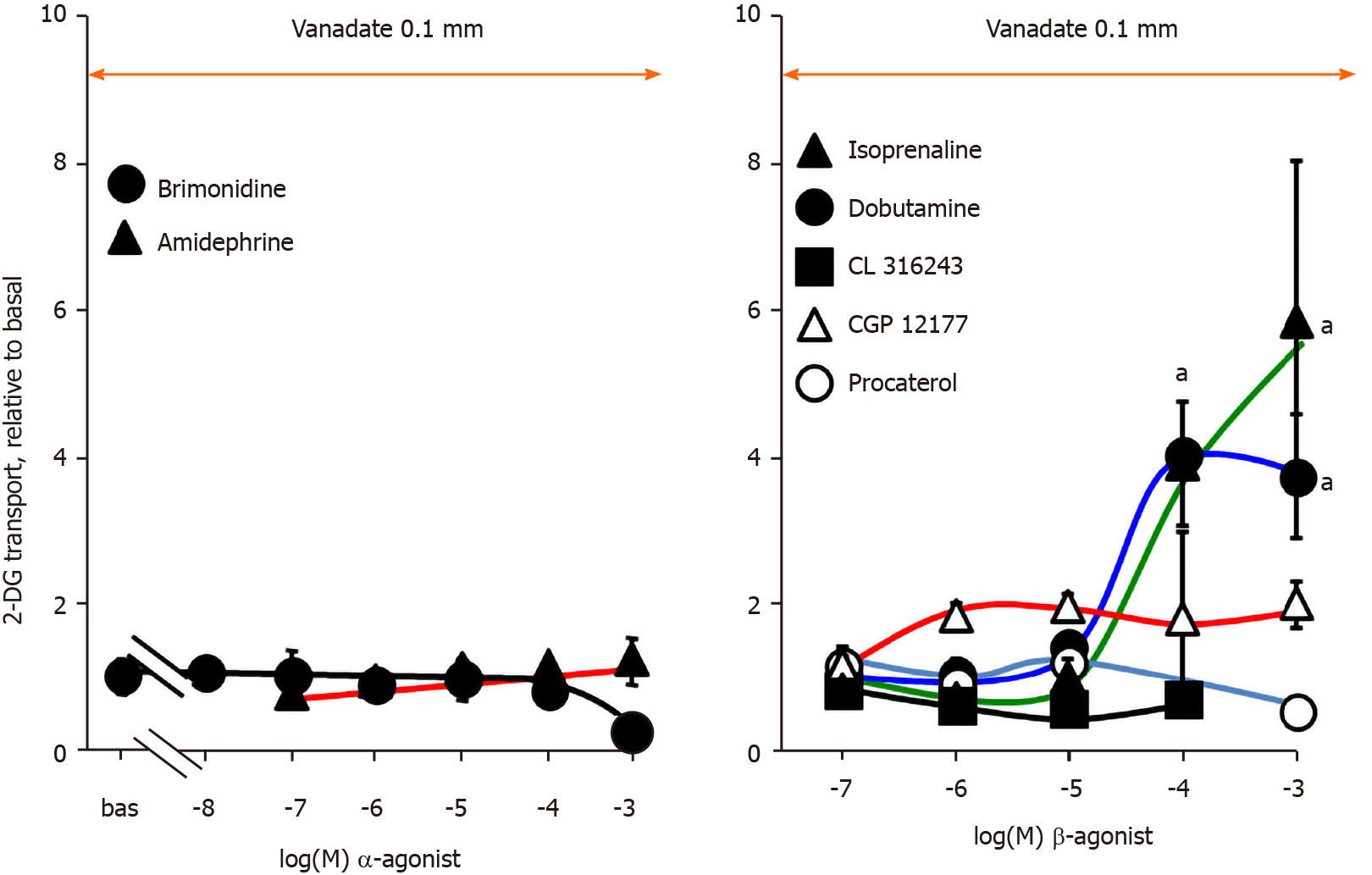
Further pharmacological tests required to study the activation of hexose transport by high doses of catecholamines were performed with increasing doses of α-adrenoreceptor (α-AR) agonists in rat adipocytes, still in the presence of 100 µmol/L vanadate: amidephrine, a α1-AR agonist, and brimonidine, an α2-AR agonist. Figure 5 indicates that stimulation with millimolar doses of catecholamines was not reproduced with these selective activators of α1- and α2-ARs in rat adipocytes, at least after short-term exposure to doses from 10 nmol/L up to 1 mmol/L.
Studies performed with β-AR agonists also indicated that several of them were totally unable to immediately activate glucose uptake, from micromolar to millimolar doses: procaterol (β2-AR agonist), CGP 12177 and CL 316243 (β3-AR agonists) (Figure 5, right panel). Only the β1-receptor agonist dobutamine and the pan-agonist (active on β1-, β2-, β3-ARs) isoprenaline reproduced at submillimolar and millimolar concentrations the hexose uptake stimulation observed with catecholamines. As these β-AR agonists are secondary amines structurally close to catecholamines, they can undergo similar amine oxidation, and thereby stimulate uptake independently from β-AR activation, either via a MAO-, or PrAO-dependent mechanism (as depicted for tyramine and tryptamine in Figure 3) or via an auto-oxidation process, as proposed for adrenaline.
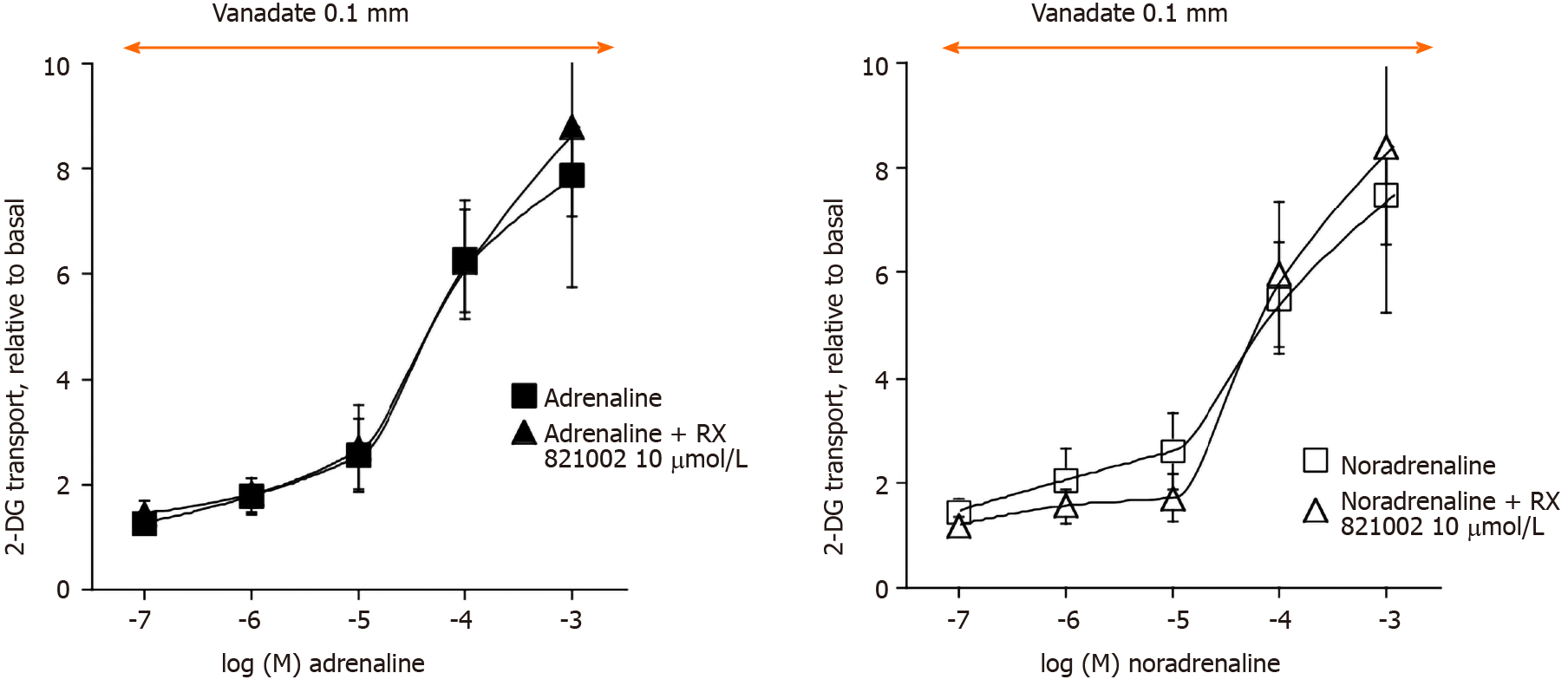
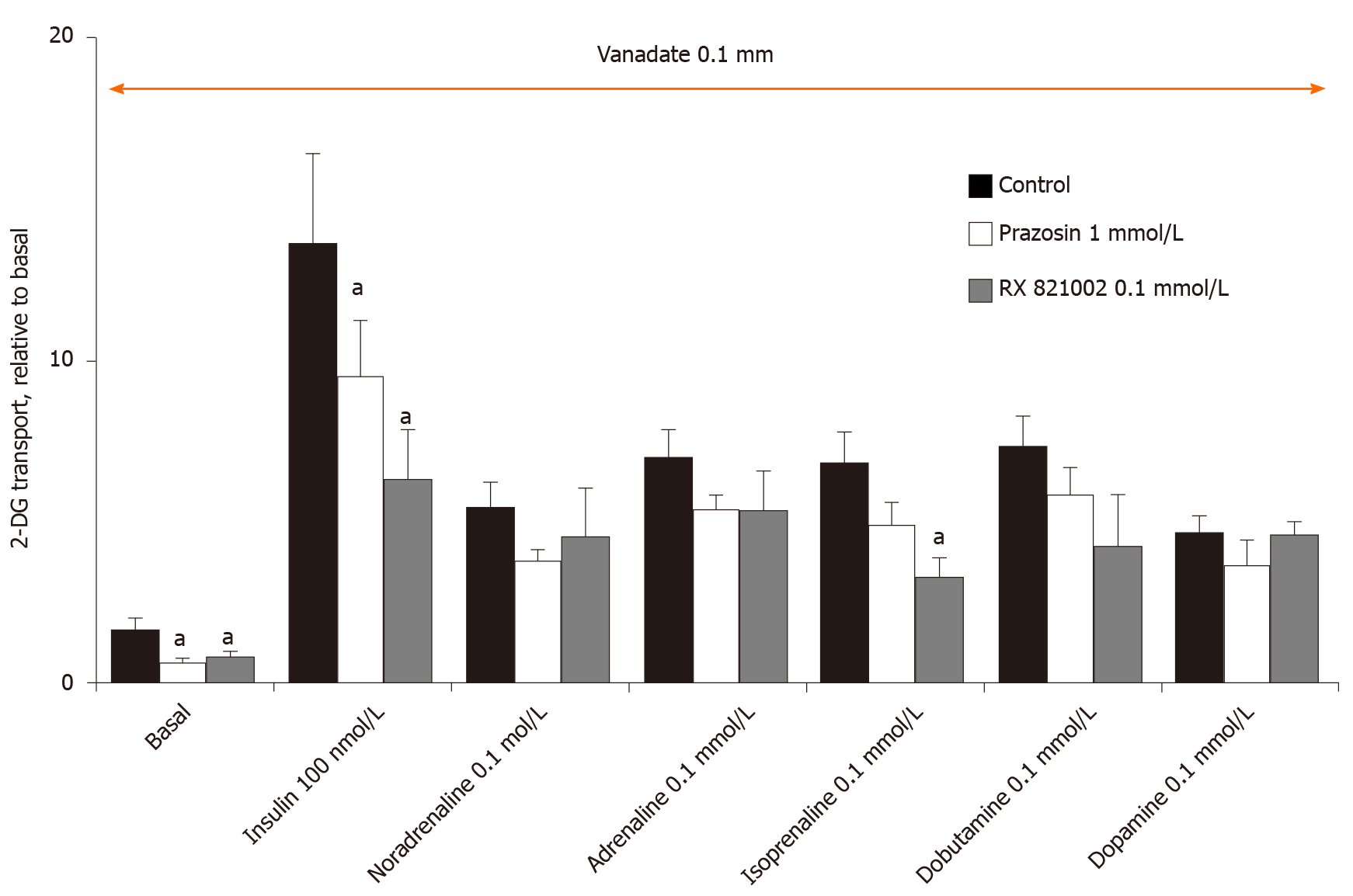
At this step, the use of several AR-antagonists was necessary to study whether the effects of adrenaline and noradrenaline, but also those of isoprenaline, dobutamine and dopamine were resistant to adrenergic blockade. The selective α2-AR antagonist, methoxy-idazoxan (also known as RX 821002), did not influence at 10 µmol/L the effects of adrenaline or noradrenaline on glucose transport in rat adipocytes in the presence of vanadate (Figure 6). However, this dose-dependent experiment definitely showed that a substantial activation of hexose uptake is detected only with 0.1 and 1 mmol/L of noradrenaline and adrenaline, while lower doses (e.g., 10 µmol/L) only tend to moderately enhance the transport activity (Figure 6). No clear-cut effect of α2-AR blockade could be detected on any of these two halves of the dose-response curves (Figure 6). Similar observations were made with the α1-AR antagonist prazosin at 10 µmol/L (not shown). Increasing the doses of these two α-AR antagonists did not provide more conclusive findings (Figure 7). In addition, these high doses of α-AR antagonists did not hamper clearly the uptake stimulation by noradrenaline and adrenaline. As 0.1 mmol/L RX 821002 also altered the activation by the β-AR agonist isoprenaline, no definitive interpretation could be established from these tests, save that a lack of selectivity is often an expected pitfall of the use of large concentrations of recognized selective agonists and antagonists. Such loss of selectivity is often associated with cytotoxic effects and impairment of integrated biological responses. This was likely the case for RX 821002, which remained ineffective at 10 µmol/L in most of the studied conditions (Figure 6), while it was inhibitory at 0.1 mmol/L irrespective of the stimulation studied (Figure 7). This also applied to the millimolar dose of prazosin, but was not the case for noradrenaline and adrenaline since their complete dose-response curves exhibited an almost sigmoid shape in the range of studied concentrations without any inhibition at the higher doses tested (Figure 6).
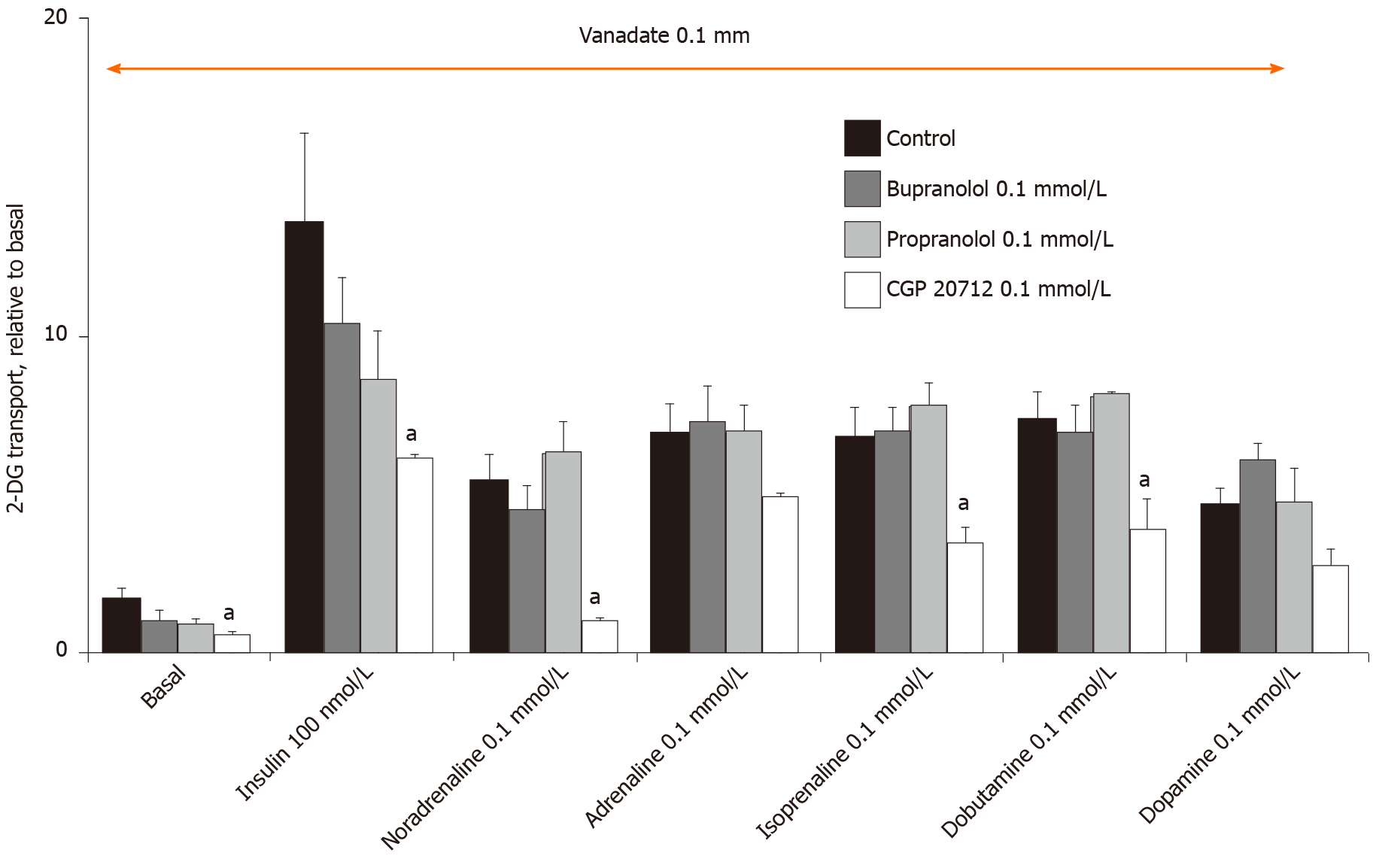
In this context, the β-AR antagonists were tested in order to delineate whether they could block the effects of high doses of catecholamines plus vanadate on 2-DG uptake at a maximal dose limited to 0.1 mmol/L for managing selectivity. At this dose, bupranolol or propranolol did not hamper basal or insulin-stimulated hexose uptake. They also did not affect the tested amines (Figure 8). They were therefore discarded in in the predominant participation of β-adrenergic receptors in the catecholamine-induced activation of hexose uptake. Again, a high dose of another β-AR antagonist, CGP 20712, did not bring further clear information since this selective β1-AR blocker inhibited basal and insulin-stimulated uptake (Figure 8). Taken together, these findings did not support a major contribution of α-ARs or β-ARs in the effects of high doses of adrenaline. The complex influence of several β-AR agonists and β-AR-blockers on hexose transport did not permit us to demonstrate whether totality or a portion of the insulin-like effects of catecholamines plus vanadate were independent from β-AR activation.
Instead of performing more in-depth investigations in the same animal model, we took advantage of the availability of mice from the "β-less" lineage, i.e., mice devoid of β-adrenergic receptors with triple knock-out of β1-, β2-, β3-adrenoceptors[27]. Our hypothesis-driven research was: if adipocytes from "β-less" mice, which are genetically invalidated for the three β-AR subtypes expressed in adipocytes, still respond to high doses of catecholamines by increasing glucose uptake, this should signify that β-ARs are not involved in the action of high doses of neurotransmitters.
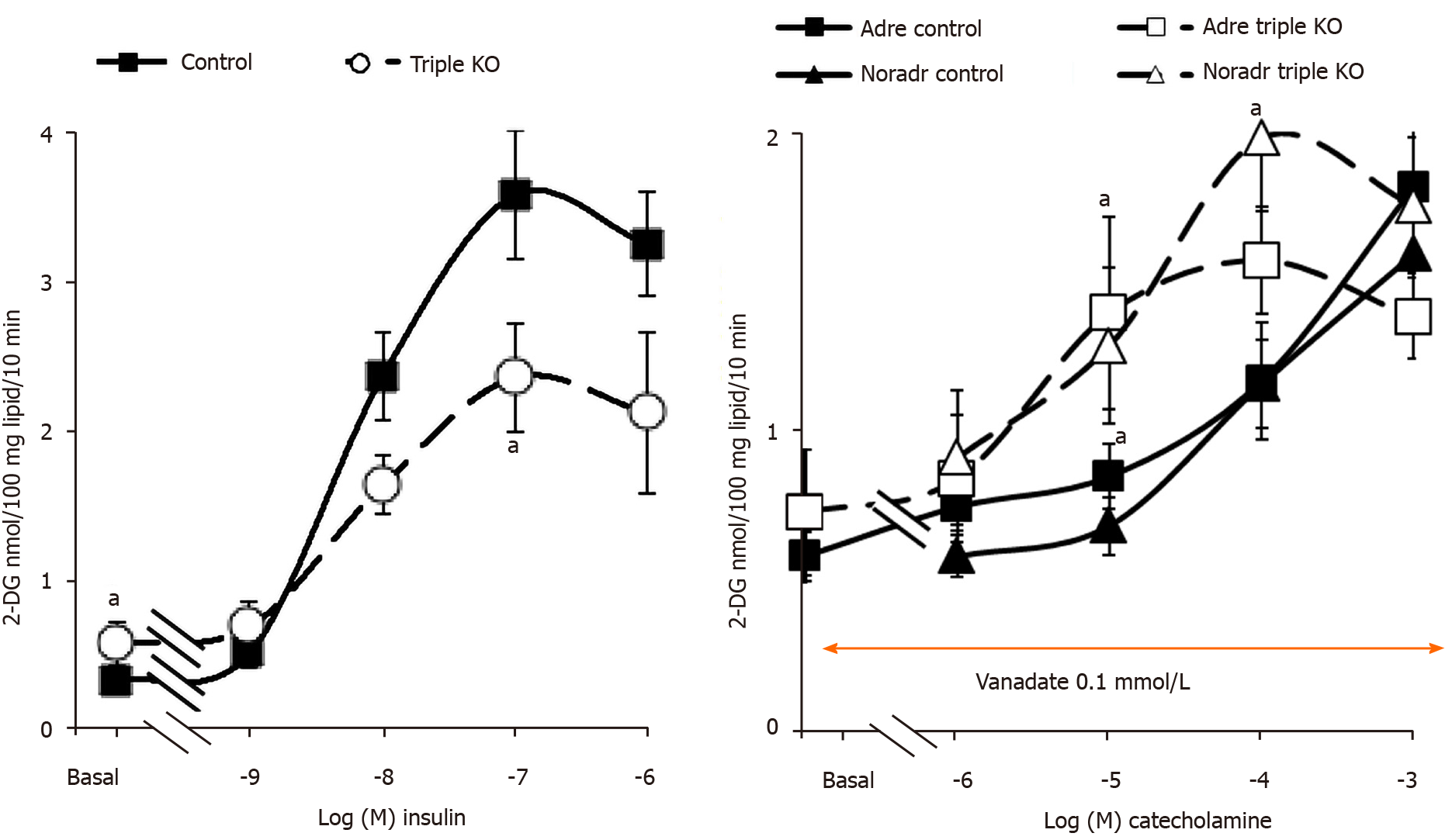
Glucose transport in adipocytes from "β-less" mice was sensitive to insulin as was the case for control WT mice. While basal uptake was slightly higher in the triple KO strain, the dose-dependent response of fat cells to insulin stimulation was not dramatically blunted (Figure 9). As for rat adipocytes, maximal activation of 2-DG uptake was obtained with 100 nmol/L insulin, although its magnitude was slightly reduced in "β-less" mice. When tested in the presence of 100 µmol/L vanadate, increasing doses of adrenaline and noradrenaline activated hexose uptake, leading to a similar degree of activation at 1 mmol/L in both genotypes (Figure 9). The activation by lower doses of catecholamines was even higher in "β-less" mice than in controls. These findings indicated that β-AR stimulation was not necessary for the activation of glucose transport by high doses of catecholamines. However, since 100 µmol/L vanadate abolished the small difference between basal uptake in control and triple KO mice (Figure 9), further verifications were performed. In fact, the dose of 100 µmol/L vanadium chosen from previous dose-response studies performed in rats[18] appeared suitable for studies on mouse adipocytes since it did not alter the response to insulin while it allowed the detection of an "insulin-like" response to tyramine or benzylamine in both genotypes (Figure 10). The use of mouse adipocytes also validated the relevance of a synergism between vanadate and millimolar doses of catecholamines, when considering short-term activation of glucose uptake. Again, the comparison of all our tested conditions indicated that adrenaline and noradrenaline, when tested alone at high doses without vanadium, did not notably stimulate glucose transport in fat cells. These results also indicated that the response to insulin could be slightly altered by the lack of β-ARs, although the exact mechanism involved and the occurrence of compensatory mechanisms could not be established.
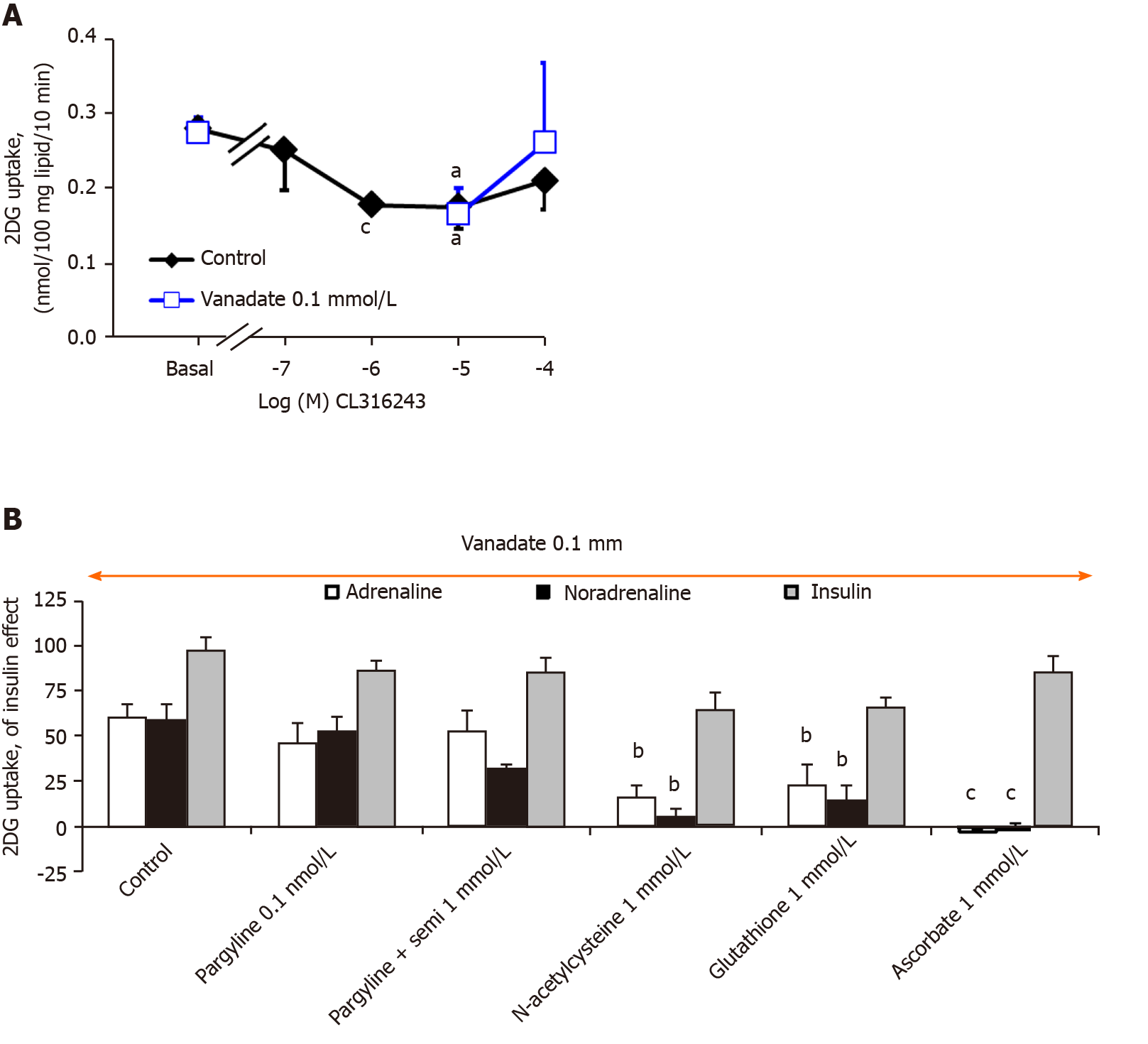
As a detailed examination of the dose-response to catecholamines in triple KO mice showed that 2-DG uptake was even more readily activated by 10 and 100 µmol/L noradrenaline in β-less than in WT mice (Figure 9), it was suggested that β-AR activation could even limit the effect observed in WT mice or rats. In keeping with this was the tendency of the β3-AR agonist CL 3162343 to inhibit basal 2-DG uptake in WT mice, which occurred at relatively low doses: 1-10 µmol/L (Figure 11). Moreover, CL 316423 did not exhibit any potentiation with vanadate and definitely behaved differently from the catecholamines regarding glucose transport regulation (Figure 11). Nonetheless, it was verified that this β3-AR agonist was devoid of noticeable lipolytic activity in adipocytes from "β-less" mice: at 10 µmol/L it hardly reproduced a fifth of the glycerol release found in WT mice (not shown), as previously reported[27]. Such a defect in lipid mobilization was in entire agreement with the increased fatness we observed in the triple KO group, since the inguinal fat stores weighed 0.67 ± 0.12 g vs 0.20 ± 0.05 g in four "β-less" and control mice (P < 0.02).
At this stage, rather that deciphering the exact role of β-ARs in the regulation of glucose handling by mouse adipocytes, it was the possible influence of hydrogen peroxide in the observed synergism between vanadate and amines that was further analyzed. As with rat adipocytes, 1 mmol/L hydrogen peroxide activated 2-DG uptake to only 14.7% ± 3.4% of the maximal insulin effect when tested alone, while when combined with 100 µM vanadate, this ROS reproduced 85.0% ± 6.0% of the insulin effect in WT mouse adipocytes (n = 8, P < 0.001). To determine whether amine oxidase inhibitors and/or antioxidants were able to prevent the synergism between adrenaline or noradrenaline and vanadate in mouse adipocytes, we tested pargyline, semicarbazide, N-acetylcysteine, glutathione and ascorbic acid. Again, insulin 100 nmol/L was used as a reference for glucose transport maximal activation. The insulin-induced stimulation almost remained unaltered by the tested agents (Figure 11). In contrast, the effects of adrenaline and noradrenaline were inhibited by antioxidants (notably abolished by 1 mmol/L ascorbate) while both resisted pargyline plus semicarbazide.
Together, these findings indicated that, in mouse and in rat, the catecholamines were active at high dose in the presence of vanadium in a manner that was: (1) Not predominantly reproduced by α-AR or β-AR activation; (2) Not mediated by MAO or PrAO activity; and (3) Dependent on redox mechanisms, probably via the generation of ROS that mimicked the synergism found between hydrogen peroxide and vanadium.
Although the in vitro effects of the combination of catecholamines and vanadium were observed at relatively high concentrations, they were of considerable magnitude and activated glucose uptake in a rapid and reproducible manner in rodent adipocytes, and therefore could be qualified as insulin-like. All these observations probably do not have a direct physiological relevance for a better knowledge of glucose homeostasis, but they might add a novel insight on the development of vanadium-based antidiabetic approaches, as discussed below.
A stimulatory effect of high doses of the catecholamines dopamine, noradrenaline and adrenaline was constantly observed on glucose uptake when rodent adipocyte preparations were incubated in the presence of vanadium. The effect of these catecholamines cannot be classified as artifactual since not mandatorily obtained with other biogenic amines or sympathomimetic agents. Among the other most active biogenic amines was deoxyepinephrine, also known as epinine or N-methyldopamine, which is also a catecholamine. The catecholamine effect on glucose entry is relatively rapid and of high magnitude and can be termed an “insulin-like effect”. Although not totally deciphered, its mechanism of action is different to other recognized substrates of MAO or SSAO/PrAO since the synergism between catecholamines and vanadium on glucose transport activation resists blockade by amine oxidase inhibitors, by contrast to previous observations made in similar conditions with tyramine[18], benzylamine[33] or methylamine[10,11]. This catecholamine effect is also distinct from the noxious effects of the highest doses of other tested agents, which hamper rather than activate glucose transport in insulin-sensitive cells. In fact, inhibitory effects have been observed under identical conditions with supra-therapeutic doses of pharmacological agents (prazosin, CGP 20712, RX 821002) or with a millimolar dose of the polyamine n-decylamine. These inhibitions were much less interesting than the clear-cut activation of glucose transport provoked by the synergism between 0.1 or 1 mmol/L catecholamines and 0.1 mmol/L sodium orthovanadate.
Curiously, the catecholamine-dependent stimulation of glucose transport is not mimicked by β1-, β2-, β3-, α1- or α2-AR agonists (except those having a chemical structure close to that of catecholamines; e.g., isoprenaline), even in the presence of vanadium, and is not clearly altered by adrenergic or dopaminergic antagonists. In addition, noradrenaline and adrenaline activate hexose transport in adipocytes from "β-less" mice, lacking β-ARs, demonstrating that such activation is adrenoceptor-independent. Sensitive to antioxidants, it likely results from a synergism between a still undefined oxidation or auto-oxidation product(s) of catecholamines reacting with vanadium, a metal known for decades to improve insulin sensitivity and to facilitate glucose handling[23,45]. Indeed, potentiation between other amines and vanadium has already been reported to depend on peroxovanadate generation[38]. It is widely recognized that metal ions favour the auto-oxidation of catecholamines and ROS generation[46]. However, in the case of the interaction between catecholamine and vanadium studied here, the transition metal appears in turn to be changed to a novel state of oxidation by a redox-sensitive interplay. As vanadium can adapt multiple oxidation states, the forms of oxyanions generated in our preparations when sodium orthovanadate was mixed together with catecholamines and adipocytes remain undefined. Nevertheless, our experiments suggest that, after being oxidized, either spontaneously or enzymatically, catecholamines trigger a better signalling role for vanadium forms, thereby activating redox-sensitive processes such as glucose carrier recruitment and glucose transport activation. In this view, complex forms such as metavanadate and decavanate have been reported to be more active than other vanadium forms[23,47,48].
The fact that ROS mediate some of dopamine signalling has been well characterized in various cell types, such as in the renal proximal tubules[49], but the exact cascade of events triggered by this and other catecholamines is still a matter of debate. It was out of the scope of the present study to totally decipher the mechanism underlying the synergism between catecholamines and vanadium, but it can be proposed that an auto-oxidation, or an oxidation independent of MAO and SSAO/PrAO activity, readily occurs at high doses of these neurotransmitters, and modifies the bioavailability of vanadium, improving its efficiency as an insulin mimicker, likely by turning off the activity of protein phosphatases[45,50], especially those involved in the termination of insulin signalling[23]. Unfortunately, we cannot attest whether the glucose uptake stimulated by catecholamines plus vanadate is a response to an increased energy demand of the fat cells, or is a direct consequence of insulin-sensitive glucose carrier recruitment to the membrane surface.
The lack of a clear α-adrenergic component in this catecholamine effect was evidenced by the use of several selective α1- and α2-AR agonists and antagonists, and confirms previous observations made in cultured mouse adipocytes[51]. However, our in vitro observations are in apparent contrast with the decrease of interstitial glucose concentration induced by the α1-AR agonist norfenefrine and by noradrenaline in human abdominal subcutaneous adipose tissue during microdialysis experiments[52]. In this clinical investigation, increased glucose uptake was accompanied by decreased blood flow. Both parameters were reversed upon pretreatment with the α1-AR antagonist urapidil[52], and it has been proposed that adrenergic agents were mimicking the stimulatory effect of sympathetic nerves. Although a large contribution of α1-AR is not confirmed here, this clinical study argued as our findings for a noradrenaline activation of glucose entry into adipocytes that was independent of insulin action.
The inhibitory effect of the β3-AR agonist CL 316243 observed in adipocytes from WT mice and reproduced to a lesser extent in rat adipocytes is in perfect agreement with previous reports on glucose transport inhibition in adipocytes by β-AR stimulation[31,51,53,54]. This also explains the slightly enhanced basal uptake found in the "β-less" mice, but contrasts with other previous studies claiming that CL 316243 stimulates glucose uptake in mouse brown adipocytes in culture[55] as well as β-AR activation under in-vivo conditions[42]. Indeed, such controversy does not appear to have great relevance for the regulation of glucose uptake in human adipocytes, the β3-adrenergic component of which is remarkably weak[56], and in which short-term CL 316243 administration neither induces alterations in the early insulin signalling cascade nor changes the basal level of glucose uptake[57].
Another previous study that is in agreement with our unexpected observation of a catecholamine stimulation of glucose transport is the elegant demonstration performed by the Canadian group of Bukowiecki, showing that noradrenaline infusion in awake rodents stimulates glucose uptake in adipose tissue, especially brown fat depots[58]. Obviously, the doses were lower in these in vivo experiments (noradrenaline was administered at 25 nmol/kg body weight/min) than in our in vitro studies. However, the activator effects of noradrenaline found in this study converge with those obtained with the microdialysis technique in human subcutaneous adipose tissue[52] to support the functional relevance of our current observations.
Catecholamines are widely described to increase blood glucose and are termed catabolic hormones in Physiology, as they increase heart rate, blood pressure, and blood glucose levels; therefore, providing the organism with the capacity to better "fight or flight" under stress conditions; whilst the opposite is observed for the anabolic hormone insulin. Therefore, both the recognized hyperglycemic actions and the circulating levels of catecholamines appear to contrast strikingly with the "insulin-like" effects we report here. Before discussing the putative physiological relevance of our findings, it is necessary to reassess the range of the so-called physiological concentrations of catecholamines in biological systems, or at least in humans. It must be reminded briefly that the highest concentrations of catecholamines found in the granules of chromaffin cells of the adrenal glands can reach 600 mmol/L, while the circulating levels in blood are highly variable, but average the nanomolar range in man. The millimolar concentration of adrenaline required to observe synergism with vanadium on glucose uptake is therefore within these limits. If one considers only the catecholamine plasma levels, then the effects we report can be qualified as extra-physiological since high levels of catecholamines (also known as catecholamine toxicity) occur in pathological conditions only: Extreme stress, brainstem trauma, pheochromocytoma, and neuroblastoma. Nevertheless, adipocytes are not simply surrounded by plasma, and to the best of our knowledge, the range of catecholamine physiological levels found in the intercellular matrix neighbouring adipocytes is still not well established, not only due to the catecholamine short half-life and the high efficiency of their reuptake systems, but also when considering that the post-ganglionic orthosympathetic nerves can liberate some of their neurotransmitter vesicles in synaptic clefts very close to those adipocytes that are innervated. It can therefore be proposed that the mechanism described at high doses of catecholamines, may occur in discrete areas of adipose depots, in which local catecholamines are released. This aspect also relies on the influence of noradrenaline and innervation on adipose tissue development[59], a research topic that has led to controversial findings regarding the origin of noradrenaline in this tissue during cold exposure. As the proposed local production of noradrenaline by activated macrophages of adipose tissue[60] does not seem to have been confirmed in recent studies[61], the debate regarding the relevance or the extraphysiological nature of the catecholamine doses used in our studies will only lead to speculative issues. For the sake of brevity, such debate needs to be closed and will give way to another discussion related to the multiplicity of catecholaminergic effects in adipocytes.
Adrenaline exerts multiple metabolic effects: it increases blood glucose at doses higher than those required for tachycardia induction; its stimulation of α1-ARs is glycogenolytic in liver and also contributes to hepatic neoglucogenesis, while β2-AR activation stimulates muscle glycogenolysis. Moreover, another widely known adrenergic effect contributes to increased blood glucose: The α2-AR mediated inhibition of insulin secretion in the endocrine pancreas. Therefore, how can a receptor-independent mechanism be required to increase glucose entry in several cell types when surrounding catecholamine levels are high?
One of our proposed answers is based on a functional point of view. Faced with overstimulation by catecholamines, adipocytes will strongly activate their lipolytic activity, thereby releasing huge amounts of fatty acids (FA) that can be used by the body if energy expenditure is required or that can re-accumulate ectopically, a phenomenon known as lipotoxicity, involved in the onset of obesity complications. Indeed, it is conceivable that catecholamines stimulate both the mobilization of stored lipids and the utilization of glucose in adipocytes. This might facilitate a finely tuned management of triacylglycerol synthesis and breakdown, that varies rapidly between bouts of energy intake (post-prandial periods) or energy dissipation (exercise, fasting, cold exposure). Besides providing FA for other tissues via their lipolytic effect, catecholamines have recently been described to also increase FA oxidation rate in the adipocytes themselves, via the phosphorylation of signal transducer and activator of transcription 3 (STAT3), which, in turn regulates FA metabolism in a non-genomic manner[62]. Alongside these two fates of released FA, mobilization and oxidation, there is also a third way, consisting in FA reesterification, which is predominant in adipocytes, since it limits excessive FA release and subsequent lipotoxicity. We therefore propose that, while increasing FA release and oxidation at low doses, catecholamines also increase FA reesterification when overstimulating adipocytes and when (dietary) vanadium is available. To esterify FA, adipocytes require a glycerol-P template, which is mainly provided from glucose since most of the fat cells are devoid of glycerokinase. Thus, we suggest that the effect we report here belongs to a possible feedback preventing adipocytes from excessive lipid mobilization when locally subjected to high doses of catecholamines, and this feedback requires enhanced glucose metabolism.
Another explanation of this unexpected effect of catecholamines relies on oxidative stress management. The autoxidation of catecholamines has been proposed to be a source of oxygen radicals that are noxious for cardiomyocytes, especially during ischemia-reperfusion injury[63]. However, spontaneous catecholamine autoxidation is slow, but is considerably increased by enzymatic or metal catalysis, which results in the generation of the superoxide-free radicals, quinones and aminochromes. This process has been implicated in neurotoxicity. Catechol-O-methyltransferase (COMT) and MAO are the major enzymes involved in catecholamine metabolism and are supposed to prevent such cytotoxic species. However, when MAO, abundant in adipocytes[64], oxidates amine substrates, it generates reactive aldehydes and hydrogen peroxide, i.e., products which are as toxic as the substrates. The concomitant presence of vanadium together with catecholamines probably helps to circumvent the toxicological effects of each species. Their interaction, either spontaneous or enzymatically driven, seems to generate intermediates leading to a substantial increase of glucose uptake in a ROS-dependent manner. Peroxovanadate is known to be more efficient than vanadate in the stimulation of hexose uptake[23]. Changes in the vanadium oxidation state generated in the presence of catecholamines might reproduce such a gain in insulin-like potency, resulting in uptake activations equivalent to those previously observed with benzylamine, being SSAO/PrAO-dependent or with tyramine, being mainly MAO-dependent.
By paying attention to the fact that overeating often accompanies the onset of insulin resistance, it is conceivable that the dietary intake of vanadium is sufficient to contribute to an alternative pathway for glucose uptake in the developing fat stores of obese subjects with insulin resistance, providing that stress conditions led to a rise in catecholamines to sufficient amounts. Indeed, regarding the stimulation of glucose uptake, it has already been described that vanadate is more efficient in insulin-resistant cells[20].
Although the activation of glucose transport in rodent adipocytes up to maximal effects obtained with benzylamine and vanadate[22,23] has not been overtly overpassed, our results open novel research aimed at improving the antidiabetic action of vanadium derivatives by limiting their cytotoxicity[48,65,66]. One of the limitations of our study lies with the limited number of screened amines, since many aromatic or aliphatic amines—either naturally occurring or not—were not tested (e.g., amphetamines). It is difficult to predict the behaviour of such amines not tested so far. Of note, trimethylamine, which is essentially a product of intestinal microbiota, and a precursor of trimethylamine N-oxide, currently associated with deleterious complications of obesity and diabetes[67,68], was without any notable effect in the present screening, even at 1 mmol/L. However, evidencing several catecholamines as hits regarding glucose entry can be extended to many other amines susceptible to interplay with vanadium. In this view, the drug metformin, which resembles a polyamine, has been successfully coupled with vanadium complexes[69] and one of the resulting compounds reported to exhibit hypoglycemic and lipid lowering properties after low dose administration in insulin-deficient or insulin-resistant diabetic rats[70,71].
This study demonstrates, for the first time, that the combination of vanadium plus catecholamines strongly stimulates hexose uptake in rodent adipocytes and appears more promising than simply extraphysiological, in view of the efforts made recently to improve the benefits/risk ratio of vanadium-based antidiabetic approaches. This might open an additional, unexpected possibility to develop vanadium-containing antidiabetic agents that could become more active, less toxic and "drugable".
Catecholamines have been demonstrated to induce oxidative stress by generating excessive reactive oxygen species (ROS) via amine oxidase-catalyzed oxidative deamination and via autoxidation. Like other metals, vanadium has been speculated to contribute to catecholamine oxidation. While catecholamines are recognized to mobilize lipids, vanadium is known to facilitate glucose consumption in fat cells. However, the effects of the combination of catecholamines plus vanadium on glucose utilization by adipose cells require clarification.
Vanadium is a potential antidiabetic agent, the use of which is limited by toxicological issues, since several vanadate salts tend to unselectively replace phosphate in many biological reactions. The search for other forms of biological vanadium complexes with either natural compounds or with designed molecules aims at developing novel less toxic and more potent antihyperglycemic agents.
To evaluate the impact of various biogenic amines, including the catecholamines, dopamine, adrenaline and noradrenaline, without and with vanadium, on glucose transport in adipose cells. To decipher the mode of action of the synergism between amines and vanadate regarding glucose transport activation.
Research methods included animal husbandry, especially rats and mice deficient in all the β-adrenoceptor subtypes, preparations of freshly isolated adipocytes, quantitative exploration of glucose transport using uptake assays of its non-metabolizable analogue 2-deoxyglucose, together with appropriate use of pharmacological agents.
In adipose cells, we confirmed the strong stimulatory action of insulin on glucose transport, leading to a tenfold increase over baseline. This was not altered by the addition of 100 µmol/L sodium orthovanadate or clearly reproduced by any of the 25 biogenic amines tested. However, when some amines were added at millimolar doses together with vanadium, they strongly increased glucose uptake up to 70% of the maximal response to insulin. This was the case for methylamine, benzylamine and tyramine, already demonstrated elsewhere to produce hydrogen peroxide when oxidized by monoamine oxidase (MAO) or semicarbazide-sensitive amine oxidase (SSAO) highly expressed in adipocytes. In addition to these amines of reference, known to exert insulin-like effects when combined with vanadate, the catecholamines dopamine, adrenaline and noradrenaline also stimulated glucose transport in a vanadium-dependent manner. Contrarily to reference amines, the stimulation by catecholamines was resistant to MAO and SSAO inhibition. Not all the tested α- and β-adrenergic agonists displayed a clear-cut stimulation of glucose uptake, and the effects of catecholamines were not inhibited by dopaminergic or adrenergic antagonists. These latter effects were even detected in mice genetically invalidated for β-adrenergic receptors. Only antioxidants, such as ascorbate, impaired the stimulation of glucose uptake by the combination of catecholamines plus vanadate.
It is likely an interaction between vanadium and catecholamine autoxidation that generates intermediates activating in a ROS-dependent manner glucose transport in adipose cells.
The observed synergism provides the basis for possible future research of novel vanadium/amine complexes with antidiabetic properties.
We thank the staff of animal unit CREFRE, currently headed by Xavier Collet, and especially its Rangueil satellite for housing wild type and transgenic rodents, and Anne Bouloumié for helpful discussions.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Choi MR S-Editor: Gao CC L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Czech MP. Differential effects of sulfhydryl reagents on activation and deactivation of the fat cell hexose transport system. J Biol Chem. 1976;251:1164-1170. [PubMed] |
| 2. | Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3370] [Cited by in RCA: 3834] [Article Influence: 191.7] [Reference Citation Analysis (0)] |
| 3. | Lönnroth P, Eriksson JW, Posner BI, Smith U. Peroxovanadate but not vanadate exerts insulin-like effects in human adipocytes. Diabetologia. 1993;36:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Enrique-Tarancón G, Marti L, Morin N, Lizcano JM, Unzeta M, Sevilla L, Camps M, Palacín M, Testar X, Carpéné C, Zorzano A. Role of semicarbazide-sensitive amine oxidase on glucose transport and GLUT4 recruitment to the cell surface in adipose cells. J Biol Chem. 1998;273:8025-8032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Enrique-Tarancón G, Castan I, Morin N, Marti L, Abella A, Camps M, Casamitjana R, Palacín M, Testar X, Degerman E, Carpéné C, Zorzano A. Substrates of semicarbazide-sensitive amine oxidase co-operate with vanadate to stimulate tyrosine phosphorylation of insulin-receptor-substrate proteins, phosphoinositide 3-kinase activity and GLUT4 translocation in adipose cells. Biochem J. 2000;350 Pt 1:171-180. [PubMed] |
| 6. | Zorzano A, Abella A, Marti L, Carpéné C, Palacín M, Testar X. Semicarbazide-sensitive amine oxidase activity exerts insulin-like effects on glucose metabolism and insulin-signaling pathways in adipose cells. Biochim Biophys Acta. 2003;1647:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Morin N, Lizcano JM, Fontana E, Marti L, Smih F, Rouet P, Prévot D, Zorzano A, Unzeta M, Carpéné C. Semicarbazide-sensitive amine oxidase substrates stimulate glucose transport and inhibit lipolysis in human adipocytes. J Pharmacol Exp Ther. 2001;297:563-572. [PubMed] |
| 8. | Shen SH, Wertz DL, Klinman JP. Implication for functions of the ectopic adipocyte copper amine oxidase (AOC3) from purified enzyme and cell-based kinetic studies. PLoS One. 2012;7:e29270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Olivieri A, Tipton KF, O'Sullivan J. Characterization of the in vitro binding and inhibition kinetics of primary amine oxidase/vascular adhesion protein-1 by glucosamine. Biochim Biophys Acta. 2012;1820:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Iglesias-Osma MC, Bour S, Garcia-Barrado MJ, Visentin V, Pastor MF, Testar X, Marti L, Enrique-Tarancon G, Valet P, Moratinos J, Carpéné C. Methylamine but not mafenide mimics insulin-like activity of the semicarbazide-sensitive amine oxidase-substrate benzylamine on glucose tolerance and on human adipocyte metabolism. Pharmacol Res. 2005;52:475-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Carpéné C, Mauriège P, Boulet N, Biron S, Grolleau JL, Garcia-Barrado MJ, Iglesias-Osma MC. Methylamine Activates Glucose Uptake in Human Adipocytes Without Overpassing Action of Insulin or Stimulating its Secretion in Pancreatic Islets. Medicines (Basel). 2019;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Yu PH, Wang M, Fan H, Deng Y, Gubisne-Haberle D. Involvement of SSAO-mediated deamination in adipose glucose transport and weight gain in obese diabetic KKAy mice. Am J Physiol Endocrinol Metab. 2004;286:E634-E641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Stolen CM, Madanat R, Marti L, Kari S, Yegutkin GG, Sariola H, Zorzano A, Jalkanen S. Semicarbazide sensitive amine oxidase overexpression has dual consequences: insulin mimicry and diabetes-like complications. FASEB J. 2004;18:702-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Iffiú-Soltész Z, Wanecq E, Lomba A, Portillo MP, Pellati F, Szöko E, Bour S, Woodley J, Milagro FI, Alfredo Martinez J, Valet P, Carpéné C. Chronic benzylamine administration in the drinking water improves glucose tolerance, reduces body weight gain and circulating cholesterol in high-fat diet-fed mice. Pharmacol Res. 2010;61:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Carpéné C, Bour S, Visentin V, Pellati F, Benvenuti S, Iglesias-Osma MC, García-Barrado MJ, Valet P. Amine oxidase substrates for impaired glucose tolerance correction. J Physiol Biochem. 2005;61:405-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Bour S, Prévot D, Guigné C, Stolen C, Jalkanen S, Valet P, Carpéné C. Semicarbazide-sensitive amine oxidase substrates fail to induce insulin-like effects in fat cells from AOC3 knockout mice. J Neural Transm (Vienna). 2007;114:829-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Morin N, Visentin V, Calise D, Marti L, Zorzano A, Testar X, Valet P, Fischer Y, Carpéné C. Tyramine stimulates glucose uptake in insulin-sensitive tissues in vitro and in vivo via its oxidation by amine oxidases. J Pharmacol Exp Ther. 2002;303:1238-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Marti L, Morin N, Enrique-Tarancon G, Prevot D, Lafontan M, Testar X, Zorzano A, Carpéné C. Tyramine and vanadate synergistically stimulate glucose transport in rat adipocytes by amine oxidase-dependent generation of hydrogen peroxide. J Pharmacol Exp Ther. 1998;285:342-349. [PubMed] |
| 19. | Subra C, Fontana E, Visentin V, Testar X, Carpéné C. Tyramine and benzylamine partially but selectively mimic insulin action on adipose differentiation in 3T3-L1 cells. J Physiol Biochem. 2003;59:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Lu B, Ennis D, Lai R, Bogdanovic E, Nikolov R, Salamon L, Fantus C, Le-Tien H, Fantus IG. Enhanced sensitivity of insulin-resistant adipocytes to vanadate is associated with oxidative stress and decreased reduction of vanadate (+5) to vanadyl (+4). J Biol Chem. 2001;276:35589-35598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Marti L, Abella A, De La Cruz X, García-Vicente S, Unzeta M, Carpéné C, Palacín M, Testar X, Orozco M, Zorzano A. Exploring the binding mode of semicarbazide-sensitive amine oxidase/VAP-1: identification of novel substrates with insulin-like activity. J Med Chem. 2004;47:4865-4874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | García-Vicente S, Yraola F, Marti L, González-Muñoz E, García-Barrado MJ, Cantó C, Abella A, Bour S, Artuch R, Sierra C, Brandi N, Carpéné C, Moratinos J, Camps M, Palacín M, Testar X, Gumà A, Albericio F, Royo M, Mian A, Zorzano A. Oral insulin-mimetic compounds that act independently of insulin. Diabetes. 2007;56:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Zorzano A, Palacín M, Marti L, García-Vicente S. Arylalkylamine vanadium salts as new anti-diabetic compounds. J Inorg Biochem. 2009;103:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Carpéné C, Garcia-Vicente S, Serrano M, Marti L, Belles C, Royo M, Galitzky J, Zorzano A, Testar X. Insulin-mimetic compound hexaquis (benzylammonium) decavanadate is antilipolytic in human fat cells. World J Diabetes. 2017;8:143-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Revelli JP, Preitner F, Samec S, Muniesa P, Kuehne F, Boss O, Vassalli JD, Dulloo A, Seydoux J, Giacobino JP, Huarte J, Ody C. Targeted gene disruption reveals a leptin-independent role for the mouse beta3-adrenoceptor in the regulation of body composition. J Clin Invest. 1997;100:1098-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Rohrer DK, Desai KH, Jasper JR, Stevens ME, Regula DP Jr, Barsh GS, Bernstein D, Kobilka BK. Targeted disruption of the mouse beta1-adrenergic receptor gene: developmental and cardiovascular effects. Proc Natl Acad Sci. 93:7375-7380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 212] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Tavernier G, Jimenez M, Giacobino JP, Hulo N, Lafontan M, Muzzin P, Langin D. Norepinephrine induces lipolysis in beta1/beta2/beta3-adrenoceptor knockout mice. Mol Pharmacol. 2005;68:793-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Kilpatrick IC, Traut M, Heal DJ. Monoamine oxidase inhibition is unlikely to be relevant to the risks associated with phentermine and fenfluramine: a comparison with their abilities to evoke monoamine release. Int J Obes Relat Metab Disord. 2001;25:1454-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Iffiú-Soltész Z, Prévot D, Carpéné C. Influence of prolonged fasting on monoamine oxidase and semicarbazide-sensitive amine oxidase activities in rat white adipose tissue. J Physiol Biochem. 2009;65:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Yamamoto N, Ueda-Wakagi M, Sato T, Kawasaki K, Sawada K, Kawabata K, Akagawa M, Ashida H. Measurement of Glucose Uptake in Cultured Cells. Curr Protoc Pharmacol 2015; 71: 12.14.1-12.14. 26;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Carpéné C, Chalaux E, Lizarbe M, Estrada A, Mora C, Palacin M, Zorzano A, Lafontan M, Testar X. Beta 3-adrenergic receptors are responsible for the adrenergic inhibition of insulin-stimulated glucose transport in rat adipocytes. Biochem J. 1993;296 (Pt 1):99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Les F, Deleruyelle S, Cassagnes LE, Boutin JA, Balogh B, Arbones-Mainar JM, Biron S, Marceau P, Richard D, Nepveu F, Mauriège P, Carpéné C. Piceatannol and resveratrol share inhibitory effects on hydrogen peroxide release, monoamine oxidase and lipogenic activities in adipose tissue, but differ in their antilipolytic properties. Chem Biol Interact. 2016;258:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Marti L, Abella A, Carpéné C, Palacín M, Testar X, Zorzano A. Combined treatment with benzylamine and low dosages of vanadate enhances glucose tolerance and reduces hyperglycemia in streptozotocin-induced diabetic rats. Diabetes. 2001;50:2061-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Carpéné C, Visentin V, Morin N, Prévot D, Smih F, Rouet P, Jayat D, Fontana E, Lizcano JM. Characterization of semicarbazide-sensitive amine oxidase in human subcutaneous adipocytes and search for novel functions. Inflammopharmacology. 2003;11:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Baker G, Matveychuk D, MacKenzie EM, Holt A, Wang Y, Kar S. Attenuation of the effects of oxidative stress by the MAO-inhibiting antidepressant and carbonyl scavenger phenelzine. Chem Biol Interact. 2019;304:139-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Missaoui S, Abello V, Prévot D, Testar X, Carpéné C. Comparison of the insulin-like effects of vanadate, vanadyl, and tungstate in rodent and human fat cells. In: Metal Ions in Biology and Medecine. John Libbey Eurotext, Paris, 2008, 10, 776-781. Edited by Philippe Collery, Ivan Maymard, Theophile Thephanides, Lylia Khassanova, Thomas Collery. |
| 37. | Zhao Q, Chen D, Liu P, Wei T, Zhang F, Ding W. Oxidovanadium(IV) sulfate-induced glucose uptake in HepG2 cells through IR/Akt pathway and hydroxyl radicals. J Inorg Biochem. 2015;149:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Yraola F, García-Vicente S, Marti L, Albericio F, Zorzano A, Royo M. Understanding the mechanism of action of the novel SSAO substrate (C7NH10)6(V10O28).2H2O, a prodrug of peroxovanadate insulin mimetics. Chem Biol Drug Des. 2007;69:423-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Fiory F, Lombardi A, Miele C, Giudicelli J, Beguinot F, Van Obberghen E. Methylglyoxal impairs insulin signalling and insulin action on glucose-induced insulin secretion in the pancreatic beta cell line INS-1E. Diabetologia. 2011;54:2941-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 40. | Dallner OS, Chernogubova E, Brolinson KA, Bengtsson T. Beta3-adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation. Endocrinology. 2006;147:5730-5739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Mukaida S, Evans BA, Bengtsson T, Hutchinson DS, Sato M. Adrenoceptors promote glucose uptake into adipocytes and muscle by an insulin-independent signaling pathway involving mechanistic target of rapamycin complex 2. Pharmacol Res. 2017;116:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Olsen JM, Åslund A, Bokhari MH, Hutchinson DS, Bengtsson T. Acute -adrenoceptor mediated glucose clearance in brown adipose tissue; a distinct pathway independent of functional insulin signaling. Mol Metab. 2019;30:240-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Visentin V, Morin N, Fontana E, Prévot D, Boucher J, Castan I, Valet P, Grujic D, Carpéné C. Dual action of octopamine on glucose transport into adipocytes: inhibition via beta3-adrenoceptor activation and stimulation via oxidation by amine oxidases. J Pharmacol Exp Ther. 2001;299:96-104. [PubMed] |
| 44. | Schimmel RJ. Roles of alpha and beta adrenergic receptors in control of glucose oxidation in hamster epididymal adipocytes. Biochim Biophys Acta. 1976;428:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Irving E, Stoker AW. Vanadium Compounds as PTP Inhibitors. Molecules. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 70] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | Nachtman JP, Delor S, Brennan CE. Manganese neurotoxicity: effects of varying oxygen tension and EDTA on dopamine auto-oxidation. Neurotoxicology. 1987;8:249-253. [PubMed] |
| 47. | Pereira MJ, Carvalho E, Eriksson JW, Crans DC, Aureliano M. Effects of decavanadate and insulin enhancing vanadium compounds on glucose uptake in isolated rat adipocytes. J Inorg Biochem. 2009;103:1687-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Aureliano M. Decavanadate Toxicology and Pharmacological Activities: V10 or V1, Both or None? Oxid Med Cell Longev. 2016;2016:6103457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Acquier AB, Mori Sequeiros García M, Gorostizaga AB, Paz C, Mendez CF. Reactive oxygen species mediate dopamine-induced signaling in renal proximal tubule cells. FEBS Lett. 2013;587:3254-3260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Treviño S, Díaz A, Sánchez-Lara E, Sanchez-Gaytan BL, Perez-Aguilar JM, González-Vergara E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol Trace Elem Res. 2019;188:68-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 51. | Mulder AH, Tack CJ, Olthaar AJ, Smits P, Sweep FC, Bosch RR. Adrenergic receptor stimulation attenuates insulin-stimulated glucose uptake in 3T3-L1 adipocytes by inhibiting GLUT4 translocation. Am J Physiol Endocrinol Metab. 2005;289:E627-E633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Flechtner-Mors M, Jenkinson CP, Alt A, Biesalski HK, Adler G, Ditschuneit HH. Sympathetic regulation of glucose uptake by the alpha1-adrenoceptor in human obesity. Obes Res. 2004;12:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR. beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J Biol Chem. 1999;274:34795-34802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 54. | Mullins GR, Wang L, Raje V, Sherwood SG, Grande RC, Boroda S, Eaton JM, Blancquaert S, Roger PP, Leitinger N, Harris TE. Catecholamine-induced lipolysis causes mTOR complex dissociation and inhibits glucose uptake in adipocytes. Proc Natl Acad Sci. 111:17450-17455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 55. | Chernogubova E, Cannon B, Bengtsson T. Norepinephrine increases glucose transport in brown adipocytes via beta3-adrenoceptors through a cAMP, PKA, and PI3-kinase-dependent pathway stimulating conventional and novel PKCs. Endocrinology. 2004;145:269-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 56. | Tavernier G, Barbe P, Galitzky J, Berlan M, Caput D, Lafontan M, Langin D. Expression of beta3-adrenoceptors with low lipolytic action in human subcutaneous white adipocytes. J Lipid Res. 1996;37:87-97. [PubMed] |
| 57. | Jost MM, Jost P, Klein J, Klein HH. The beta3-adrenergic agonist CL316,243 inhibits insulin signaling but not glucose uptake in primary human adipocytes. Exp Clin Endocrinol Diabetes. 2005;113:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Liu X, Pérusse F, Bukowiecki LJ. Chronic norepinephrine infusion stimulates glucose uptake in white and brown adipose tissues. Am J Physiol. 1994;266:R914-R920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Giordano A, Frontini A, Murano I, Tonello C, Marino MA, Carruba MO, Nisoli E, Cinti S. Regional-dependent increase of sympathetic innervation in rat white adipose tissue during prolonged fasting. J Histochem Cytochem. 2005;53:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 60. | Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 763] [Cited by in RCA: 842] [Article Influence: 60.1] [Reference Citation Analysis (0)] |
| 61. | Fischer K, Ruiz HH, Jhun K, Finan B, Oberlin DJ, van der Heide V, Kalinovich AV, Petrovic N, Wolf Y, Clemmensen C, Shin AC, Divanovic S, Brombacher F, Glasmacher E, Keipert S, Jastroch M, Nagler J, Schramm KW, Medrikova D, Collden G, Woods SC, Herzig S, Homann D, Jung S, Nedergaard J, Cannon B, Tschöp MH, Müller TD, Buettner C. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med. 2017;23:623-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 273] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 62. | Reilly SM, Hung CW, Ahmadian M, Zhao P, Keinan O, Gomez AV, DeLuca JH, Dadpey B, Lu D, Zaid J, Poirier B, Peng X, Yu RT, Downes M, Liddle C, Evans RM, Murphy AN, Saltiel AR. Catecholamines suppress fatty acid re-esterification and increase oxidation in white adipocytes via STAT3. Nat Metab. 2020;2:620-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 63. | Hašková P, Kovaríková P, Koubková L, Vávrová A, Macková E, Simunek T. Iron chelation with salicylaldehyde isonicotinoyl hydrazone protects against catecholamine autoxidation and cardiotoxicity. Free Radic Biol Med. 2011;50:537-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 64. | Pizzinat N, Marti L, Remaury A, Leger F, Langin D, Lafontan M, Carpéné C, Parini A. High expression of monoamine oxidases in human white adipose tissue: evidence for their involvement in noradrenaline clearance. Biochem Pharmacol. 1999;58:1735-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Scior T, Guevara-Garcia JA, Do QT, Bernard P, Laufer S. Why Antidiabetic Vanadium Complexes are Not in the Pipeline of "Big Pharma" Drug Research? Curr Med Chem. 2016;23:2874-2891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 66. | Samira M, Mounira T, Kamel K, Yacoubi MT, Ben Rhouma K, Sakly M, Tebourbi O. Hepatotoxicity of vanadyl sulfate in nondiabetic and streptozotocin-induced diabetic rats. Can J Physiol Pharmacol. 2018;96:1076-1083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Schugar RC, Shih DM, Warrier M, Helsley RN, Burrows A, Ferguson D, Brown AL, Gromovsky AD, Heine M, Chatterjee A, Li L, Li XS, Wang Z, Willard B, Meng Y, Kim H, Che N, Pan C, Lee RG, Crooke RM, Graham MJ, Morton RE, Langefeld CD, Das SK, Rudel LL, Zein N, McCullough AJ, Dasarathy S, Tang WHW, Erokwu BO, Flask CA, Laakso M, Civelek M, Naga Prasad SV, Heeren J, Lusis AJ, Hazen SL, Brown JM. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017;19:2451-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 68. | Lent-Schochet D, McLaughlin M, Ramakrishnan N, Jialal I. Exploratory metabolomics of metabolic syndrome: A status report. World J Diabetes. 2019;10:23-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 69. | Ghasemi F, Rezvani AR, Ghasemi K, Graiff C. Glycine and metformin as new counter ions for mono and dinuclear vanadium(V)-dipicolinic acid complexes based on the insulin-enhancing anions: Synthesis, spectroscopic characterization and crystal structure. J Molec Struct. 2018;1154:319-326. [RCA] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Treviño S, Sánchez-Lara E, Sarmiento-Ortega VE, Sánchez-Lombardo I, Flores-Hernández JÁ, Pérez-Benítez A, Brambila-Colombres E, González-Vergara E. Hypoglycemic, lipid-lowering and metabolic regulation activities of metforminium decavanadate (H2Metf)3 [V10O28]·8H2O using hypercaloric-induced carbohydrate and lipid deregulation in Wistar rats as biological model. J Inorg Biochem. 2015;147:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Treviño S, Velázquez-Vázquez D, Sánchez-Lara E, Diaz-Fonseca A, Flores-Hernandez JÁ, Pérez-Benítez A, Brambila-Colombres E, González-Vergara E. Metforminium Decavanadate as a Potential Metallopharmaceutical Drug for the Treatment of Diabetes Mellitus. Oxid Med Cell Longev. 2016;2016:6058705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |