Published online Dec 15, 2020. doi: 10.4239/wjd.v11.i12.611
Peer-review started: August 10, 2020
First decision: September 21, 2020
Revised: September 28, 2020
Accepted: October 21, 2020
Article in press: October 21, 2020
Published online: December 15, 2020
Processing time: 124 Days and 21.1 Hours
Liraglutide is a glucagon-like peptide 1 receptor agonist analog that has been found to have a therapeutic effect in diabetes. In addition to its ability to treat diabetes, liraglutide has beneficial effects on the cardiovascular system and kidney as well as other beneficial effects, but its specific mechanism is not clear. In this study, a rat model of type 2 diabetes was established by administration of a high-sugar, high-fat diet combined with low-dose streptozotocin (STZ) to observe the effect of liraglutide on the kidneys of type 2 diabetes rats and the possible underlying mechanisms.
To explore whether liraglutide has a protective effect on type 2 diabetic rat kidneys and the underlying mechanisms.
Eight-week-old male Sprague-Dawley rats were randomly divided into a control group, model group, low-dose liraglutide group, and high-dose liraglutide group. Control rats were fed a standard diet, while model group and intervention group rats were fed high-sugar, high-fat feed for 1 mo and then intraperitoneally injected with 40 mg/kg STZ to induce type 2 diabetes. The low-dose and high-dose intervention groups received 100 µg/kg and 200 µg/kg liraglutide, respectively, once daily by subcutaneous injection. The control and model groups were given an equivalent volume of physiological saline for 8 wk. Pathological changes in renal tissues were observed by hematoxylin and eosin staining and periodic acid-Schiff staining, and GRP78 and caspase-12 expression was detected by Western blot and reverse transcription-polymerase chain reaction (RT-PCR).
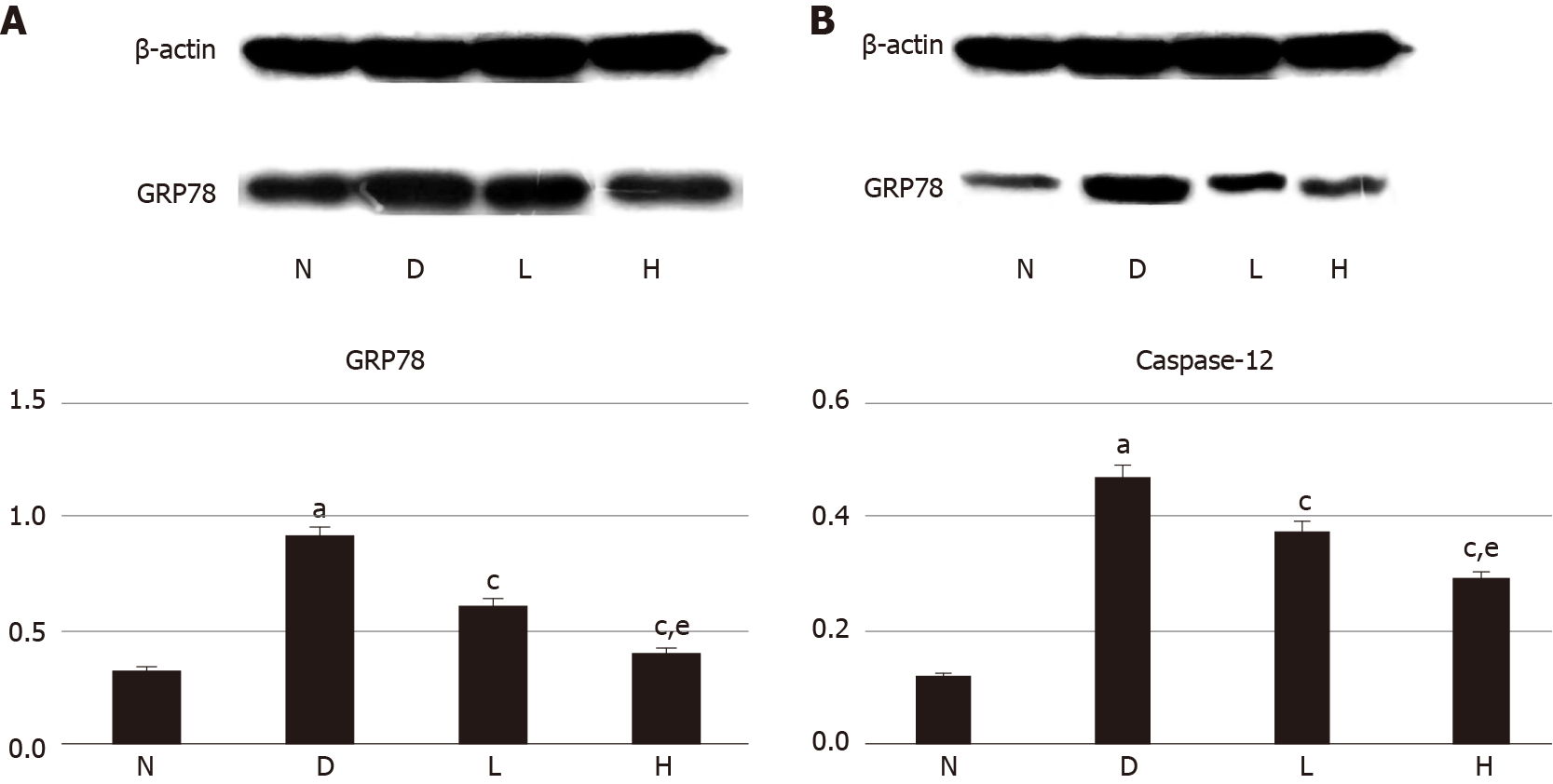
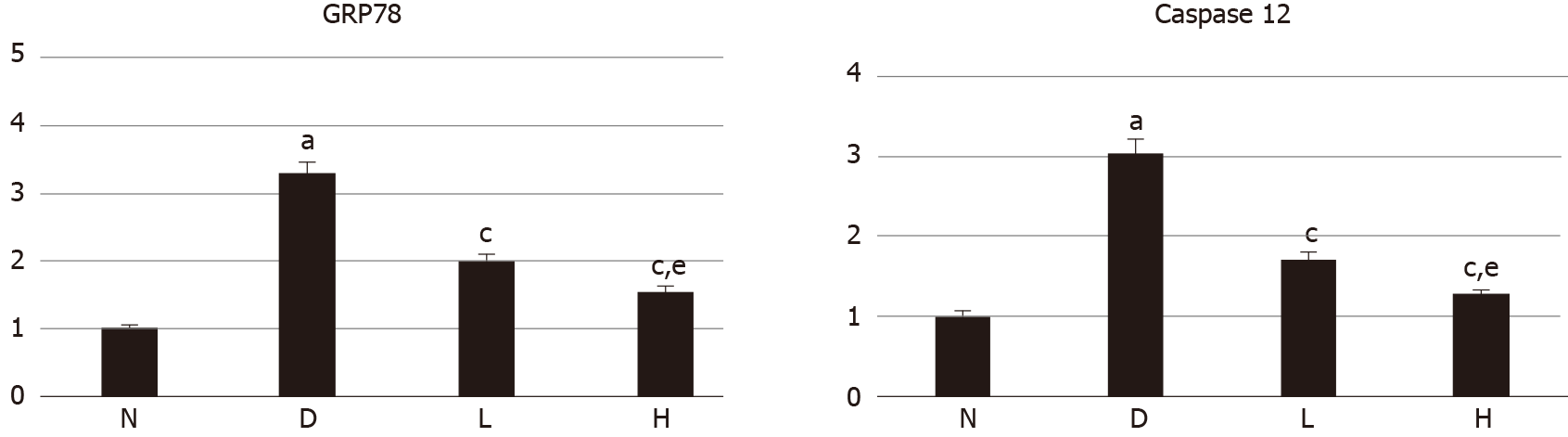
Western blot analysis showed that GRP78 and caspase-12 protein expression in kidney tissue was significantly higher in model rats than in normal rats and lower in the liraglutide-treated groups than in the model group, with a more significant decrease being observed in the high-dose group than in the low-dose group. RT-PCR showed that the mRNA expression of GRP78 and caspase-12 was higher in model rats than in control rats and lower in the liraglutide-treated groups than in the model group, with the high-dose group exhibiting a more significant decrease than the low-dose group.
Liraglutide may delay the progression of diabetic nephropathy by reducing endoplasmic reticulum stress and protect the kidneys in a dose-dependent manner.
Core Tip: This study explored whether liraglutide has a protective effect on type 2 diabetic rat kidneys and the underlying mechanisms using experimental animals. The conclusion is that liraglutide may delay the progression of diabetic nephropathy by reducing endoplasmic reticulum stress and protect the kidneys in a dose-dependent manner.
- Citation: Zhao XY, Yu TT, Liu S, Liu YJ, Liu JJ, Qin J. Effect of liraglutide on endoplasmic reticulum stress in the renal tissue of type 2 diabetic rats. World J Diabetes 2020; 11(12): 611-621
- URL: https://www.wjgnet.com/1948-9358/full/v11/i12/611.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i12.611
Overnutrition-induced metabolic disorders resulting from changes in lifestyle in modern society have become a threat to human health. In 2013, the International Diabetes Federation reported that 382 million adults worldwide were living with diabetes. In China, the number of adults with diabetes is 98.4 million[1], which accounts for more than a quarter of all patients; thus, China has the highest rate of diabetes worldwide. Diabetic nephropathy, which can progress to end-stage renal disease and has become the leading cause of death from diabetes, is a common microvascular complication of diabetes that seriously affects patient quality of life[2]. The kidneys are rich in endoplasmic reticulum structures, and an increasing number of studies have shown that endoplasmic reticulum stress (ERS) plays a unique role in the development of diabetic nephropathy[3].
Liraglutide is a glucagon-like peptide 1 (GLP-1) receptor agonist analog that has been found to have a therapeutic effect in diabetes. In addition to its ability to treat diabetes, liraglutide has beneficial effects on the cardiovascular system and kidney as well as other beneficial effects[4], but its specific mechanism is not clear. In this study, a rat model of type 2 diabetes was established by administration of a high-sugar, high-fat diet combined with low-dose streptozotocin (STZ) to observe the effect of liraglutide on the kidneys of type 2 diabetes rats and the possible underlying mechanisms.
The main reagents used in this study included streptozotocin (Sigma, United States), liraglutide (Noandande and Nord Company, Denmark), rabbit anti-GRP78 antibody, rabbit anti-caspase-12 antibody, and goat anti-rabbit secondary antibody (ABclonal). The analysis kits used in this study included the BCA kit, the RNA extraction kit (Takara, Japan), the reverse transcription kit (Takara, Japan), and the amplification kit. Polymerase chain reaction (PCR) primers (Takara, Japan) and glucose meters and test paper (Dongbao Pharmaceuticals) were also used.
Thirty male clean-grade Sprague-Dawley (SD) rats aged 8 wk and weighing 180-250 g were provided by Shanxi Medical University Animal Center (experimental animal license number: SCXK(Jin)2015-0001). Six of the SD rats were randomly selected for a control group and fed a regular diet. The remaining 24 rats were fed a high-sugar and high-fat diet. After 1 mo, the rats were fasted for 12 h and received an intraperitoneal injection of 40 mg/kg STZ[5], while the normal control group rats received an equivalent volume of citric acid-sodium citrate buffer. Three days later, blood was collected from the tail vein to measure blood sugar levels. A blood glucose value ≥ 16.7 mmol/L was considered to successfully establish the model. After the successful modeling, five rats died, all of which showed that the hair was erect and dry, the wheezing was obvious, the random blood glucose value was increased, and some blood glucose meters could not measure the blood glucose value. The cause of death might be high blood sugar leading to acute complications of diabetes. During the observation period after successful modeling, one rat was excluded from the group due to the blood glucose drop lower than the modeling standard. Thus, the modeling efficiency was about 75%.
A total of 30 rats were randomly selected as a normal control group. The remaining 24 were modeled with type 2 diabetes. The modelling rate was 75%. The successfully modeled 18 rats were randomly divided into three groups (6 rats per group): A model group (type 2 diabetic rats, given subcutaneous injection of normal saline, and compared with the other two groups injected with liraglutide), a low-dose liraglutide (100 µg/kg, subcutaneous injection, once daily, continuous administration for 8 wk) group[6], and a high-dose liraglutide (200 µg/kg, subcutaneous injection, once daily, continuous administration for 8 wk) group. The above three groups continued to be fed a high-sugar and high-fat diet for 1 wk, and the control group continued to be fed an ordinary diet for 1 wk. Drug intervention was performed after 1 wk. The control and model rats were given 100 µg/kg physiological saline once a day.
Every week, the weights of rats from each group were recorded, and the fasting blood sugar levels of rats from each group were measured with a blood glucose meter. Treatments were administered for 8 wk, and metabolic rat cages were used to collect 24-h urine from the four groups of rats. After the samples were centrifuged (3000 rpm/min, 15 min), the supernatant was collected and stored in a -80 °C freezer. After 12 h of fasting, chloral hydrate (10%, 0.3 mL/100 g)[7] was intraperitoneally administered the following day. Two milliliters of blood samples that were collected from each group were taken from the apex of the heart and centrifuged at 4 °C, and the supernatant was collected. The bilateral kidneys were removed, and some of the tissues were placed in 4% formaldehyde solution, embedded in paraffin, and sectioned. The remaining kidney tissues were stored in a -80 °C freezer.
Two milliliters of blood samples were taken from the apex of the heart, and serum was used for the test items. The extraction method was as follows: After collection, the blood was transferred into a clean and dry glass test tube, and immediately centrifuged at 2500 rpm/min for 2 min. The test tube was then placed in a 37 °C water bath for 4-5 min. Subsequently, a glass rod was used to peel the upper layer of fibrin to the side of the tube, and the test tube was centrifuged at 2500 rpm/min for 1-2 min to obtain the serum. No chemical reagents were added in the process of separating serum, which can keep the original composition of blood as much as possible and reduce interference to measurement items. After centrifugal separation of serum, an automatic biochemical analyzer was used to determine blood creatinine and blood urea nitrogen levels. The glucose oxidase membrane electrode method was used to measure blood glucose. The BCA kit was used to determine the 24 h urine protein content. Serum was collected from each group to detect fasting blood glucose, creatinine, and urea nitrogen levels. Twenty-four-hour urine samples were obtained from each group to measure 24-h urine protein levels.
Pieces of kidney tissue (approximately 30 mg) were homogenized with a mortar and pestle, and an appropriate amount of liquid nitrogen was added. The tissues were placed in 1.5 mL Eppendorf tubes after grinding, approximately 600 µL of RIPA lysis buffer was added, and the tissues were lysed on ice for 30 min. The samples were centrifuged at a low temperature (12000 rpm, 4 °C, 10 min), and the supernatant was collected and placed in a clean Eppendorf tube for protein extraction. The protein concentration was determined by the BCA method. The sample loading volume was calculated based on an amount of 60 µg of protein per well, 5 × loading buffer was added at a 4:1 ratio, the mixture was boiled at 100 °C for 5 min, and aliquots were obtained after boiling and stored in a -20 °C freezer. Sixty micrograms of protein was added to each well. After electrophoresis, the proteins were transferred to a polyvinylidene fluoride membrane. The membrane was blocked in 5% skimmed milk for 1 h and then incubated with rabbit GRP782 antibody and caspase-12 antibody diluted at 1:1000 in blocking solution overnight at 4 °C on a shaker. The membrane was washed with 1 × Tris buffered saline Tween (TBST) four times for 10 min each, incubated with goat anti-rabbit secondary antibody diluted at 1:5000 at room temperature for 1 h, washed with 1 × TBST four times for 10 min each, and subjected to grayscale scanning analysis on the Bio-ray system to calculate the A value.
According to the manufacturer’s instructions, RNA was extracted from kidney tissues from each group of rats with an RNA extraction kit (Takara, Japan). Then, a reverse transcription kit (Takara, Japan) was used to synthesize cDNA. The primer sequences were as follows: GRP78: forward, 5´-TCAGCCCACCGTAACAATCAAG-3´ and reverse, 5´-TCCAGTCAGATCAAATGTACCCAGA-3´; caspase-12: forward, 5´-CAATTCCGACAAACAGCTGAGTTTA-3´ and reverse, 5´-CATGGGCCA CTCCAACATTTAC-3´; and β-actin: forward, 5´-GGAGCCCC-3´ and reverse, 5´-GACTCATCGTACTCCTGCTTGCTG-3´. Two-step PCR was used for amplification. The PCR conditions were 40 cycles of 95 °C for 5 s and 60 °C for 30 s. After the reaction, the Ct value was recorded, and the relative expression level of the target gene was calculated based on the 2-ΔΔCt method.
Hematoxylin and eosin staining for observation of pathological changes in the kidneys: Six kidney specimens were prepared in each group. Paraffin sections were dewaxed, stained, washed with water, differentiated, rinsed, counterstained, and subjected to other treatments, and the pathological changes in the kidneys from each group were observed under a light microscope at a magnification of 40 ×.
Periodic acid-Schiff staining: After dewaxing, the sections of six cases in each group were oxidized with 0.5% periodic acid aqueous solution for 10 min, rinsed with free-flowing water for a few minutes, and then immersed in Schiff reagent for 30-40 min, with special attention paid to strict shading. The sectioned samples were rinsed with tap water for 10 min before incubation in hematoxylin solution for 30-60 s. After rinse with tap water for 5 min, the samples were differentiated in 1% hydrochloric acid solution for 3 s. Subsequently, the samples were dehydrated in anhydrous ethanol twice for 5 min each, and in xylene twice for 2 min each, and then a neutral gum was added dropwise to mount the slide. The histopathological changes of the samples were observed under a light microscope and images were acquired.
The experimental results were statistically analyzed with SPSS 19.0 statistical software, and the results are expressed as the mean ± SD. All data were tested for normality (Nonparametric Test). A paired-sample t-test was used for comparison before and after treatment. One-way analysis of variance followed by the post-hoc test was used for comparison between two groups. Homogeneity of variance test was used to test the homogeneity of variance. The Student-Newman-Keuls method was used to compare the difference between two groups if the variance was uniform. P < 0.05 indicated that the difference was statistically significant.
The rats in the control group were in good condition and active, and exhibited shiny hair. The litter of the control group animals was replaced once every 2-3 d. The rats in the model group were in poor condition, rarely moved, and exhibited a loss of hair, increased food and water intake, and increased urine output. The litter of the model group animals needed to be changed every day. The general condition of the rats in the liraglutide intervention groups was better than that of the rats in the model group, and the improvement in the high-dose liraglutide group was more obvious than that in the low-dose liraglutide group.
After 8 wk of intervention, the weight of the rats in the model group was significantly lower than that of the rats in the control group (P < 0.05), and the fasting blood glucose level of the rats in the model group was significantly higher than that of the rats in the control group (P < 0.05). The weight of the rats in the drug intervention groups was higher than that of the rats in the model group (P < 0.05), which was lower than that of the rats in the control group (P < 0.05). Fasting blood glucose levels were lower in the drug intervention groups than in the model group (P < 0.05), and the change was dose-dependent (Table 1).
| Group | n | Weight (g) (mean ± SD) | FBG (mmol/L) (mean ± SD) |
| Control group | 6 | 477.17 ± 23.82 | 4.58 ± 0.69 |
| Model group | 6 | 396.51 ± 9.15 | 22.57 ± 1.42 |
| Low-dose liraglutide group | 6 | 440.02 ± 34.22 | 19.43 ± 1.07 |
| High-dose liraglutide group | 6 | 434.67 ± 39.42 | 17.32 ± 1.02 |
| P value | < 0.05 | < 0.05 |
The blood creatinine, urea nitrogen, and 24-h urinary protein levels were higher in the model group than in the control group (P < 0.05). These indices were lower in the liraglutide-treated groups than in the model group (P < 0.05), and the levels were more significantly decreased in the high-dose liraglutide group than in the low-dose liraglutide group (P < 0.05; Table 2).
The expression of GRP78 and caspase-12 proteins in kidney tissues of the model group was significantly higher than that in kidney tissues of the normal control group (P < 0.05). GRP78 and caspase-12 protein levels in renal tissues were lower in the liraglutide intervention group than in the model group (P < 0.05), and the decrease observed in the high-dose liraglutide group was more significantly than that in the lose-dose liraglutide group (P < 0.05; Figure 1).
The mRNA expression levels of GRP78 and caspase-12 in the model group were higher than those in the control group (P < 0.05). The mRNA expression levels in the liraglutide-treated group were lower than those in the model group (P < 0.05), and the decrease in the high-dose group was more significant than that in the low-dose group (P < 0.05; Figure 2).
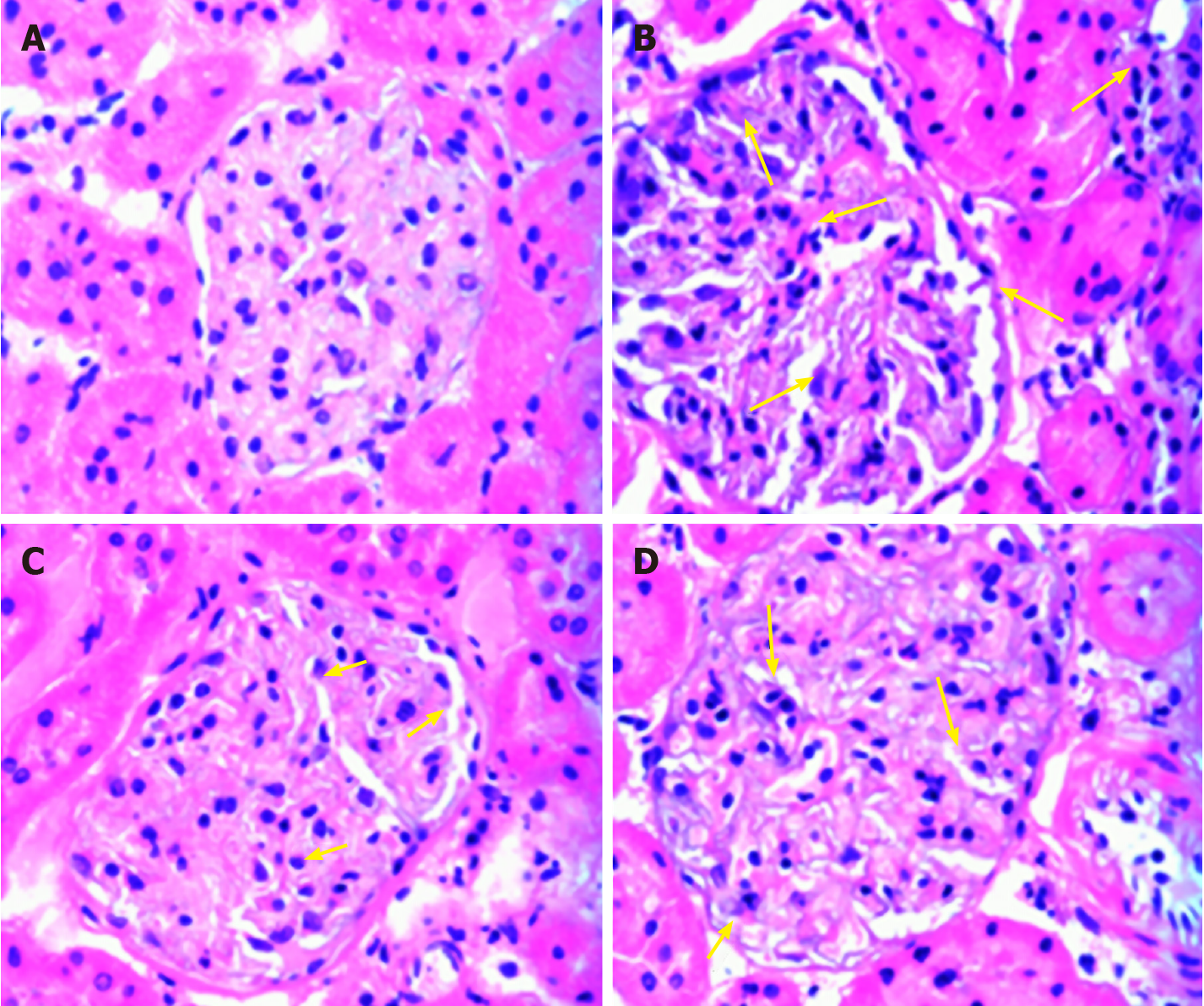
In the normal control group, the kidney glomeruli and tubules were clearly structured without obvious inflammatory cell infiltration and fibrosis; in the kidney of the model group, there was an increase in the volume of the glomeruli, the number of cells, and the extra mesangial matrix. The basement membrane thickened obviously. After the intervention of liraglutide, the glomerulus volume was slightly reduced compared with the model group, the infiltration of inflammatory cells in the interstitium was improved, and the glomerular basement membrane and mesangial proliferation were reduced; the above changes were more obvious in the high-dose group (Figure 3).
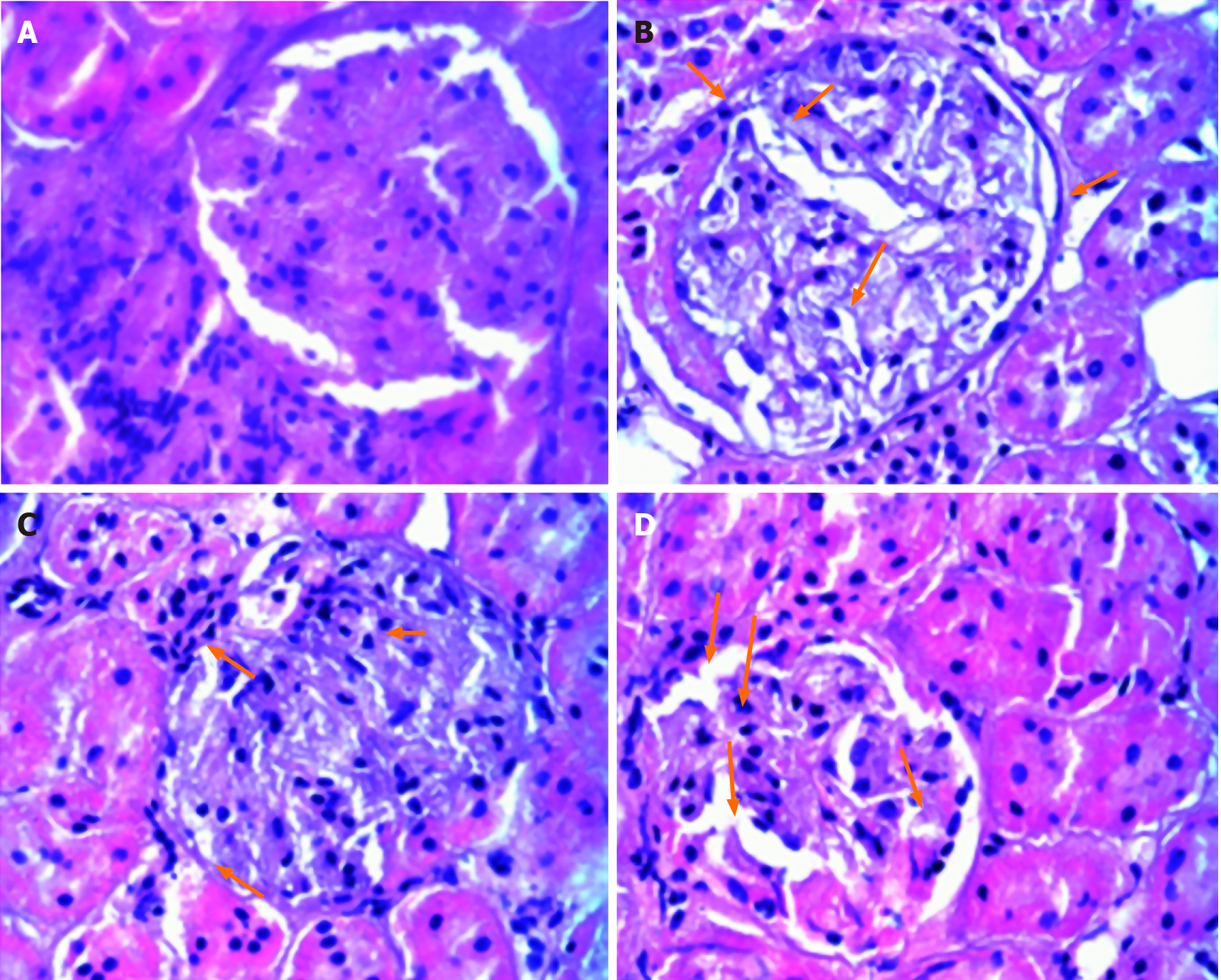
The structure of the kidney glomeruli and tubules in the normal control group was clear; the extra mesangial matrix in the kidney of the model group increased, the basement membrane thickened significantly. After the intervention of liraglutide, the glomerular basement membrane and mesangial proliferation of rats in the model group were reduced, and the above changes were more obvious in the high-dose group (Figure 4).
In the pathological section of the kidney, it was observed that the renal tubules in the model group had cystic expansion and the interstitial inflammatory cells infiltrated, which showed the early manifestations of renal interstitial fibrosis. Compared with the model group, the above changes in the liraglutide intervention group were alleviated. It can be judged that the use of liraglutide in the early stage of renal tubular interstitial fibrosis may achieve the reversal of renal interstitial fibrosis. However, because indicators such as platelet-derived growth factor and transforming growth factor-β have not been measured, there may be insufficient evidence for whether liraglutide can reverse the late stage of renal fibrosis.
In this study, we mainly found that liraglutide can reduce blood creatinine and urea nitrogen in type 2 diabetic rats, and reduce 24-h urine protein. Liraglutide has a therapeutic effect on diabetic nephropathy, but its specific mechanism was not clear in the past. We found through experimental results that this effect of liraglutide may be related to the reduction of ERS in the kidneys, so in the future, we can start by reducing the ERS to find new breakthroughs in the treatment of diabetic nephropathy.
The occurrence of diabetic nephropathy is related to ERS, but there are currently no reports about drugs for the treatment of diabetic nephropathy by reducing ERS. Related studies have shown that liraglutide can improve ERS and oxidative stress in in vivo experiments, and may regulate cell proliferation and apoptosis through autotrophy and ERS in vitro[8]. We found through research that liraglutide has a therapeutic effect on diabetic nephropathy, and this effect is related to reducing the stress of the renal endoplasmic reticulum. In previous studies, we found not only the effect of liraglutide in reducing ERS in the kidneys, but also similar findings in the liver of rats, but we have not further studied the molecular mechanism of this effect. It is only an indirect effect which may be related to self-addiction and needs further research. But this provides us with new ideas for the treatment of diabetes and diabetic nephropathy. In the future, we can find new ways to treat type 2 diabetes and diabetic nephropathy by reducing ERS.
The endoplasmic reticulum is the largest multifunctional organelle in the cell. It is composed of smooth endoplasmic reticulum and rough endoplasmic reticulum. Its most common functions include lipid and protein synthesis and the intracellular calcium balance regulation, and it is involved in protein folding and modification[9]. ERS is an important component of cellular stress. ERS results from various physiological and pathological factors that lead to dysfunction of the endoplasmic reticulum, causing unfolded proteins and misfolded proteins to accumulate in the endoplasmic reticulum. This process causes cell adaptation, damage, and even apoptosis[10]. During ERS, the morphology and structure of the endoplasmic reticulum are altered, and the relationships between the endoplasmic reticulum and other organelles are destroyed. ERS initiates the apoptotic pathway[11]. GRP78, which is mainly located in the endoplasmic reticulum, plays an important role in the process of ERS, and may promote the development of ERS, and it is the first protein identified as a molecular partner of the endoplasmic reticulum[12]. Caspase-12 is localized on the outer membrane of the endoplasmic reticulum and is activated only during ERS. Caspase-12 activation is associated with ERS-induced apoptosis[13]. We analyzed the expression of GRP78 and caspase-12 in the kidneys of rats from each group to evaluate the degree of ERS.
The kidney is rich in the endoplasmic reticulum, including glomerular podocytes and mesangial cells, as well as renal tubular epithelial cells. Because the endoplasmic reticulum is abundant in the kidney, this organ is highly vulnerable to ERS. Diabetic nephropathy is a serious complication of diabetes. In the pathogenesis of type 2 diabetes, glucose and lipid metabolism disorders, glucose-stimulated insulin secretion enhancement, and other factors can induce ERS[14]. In mice with diabetic nephropathy, RNA-dependent protein kinase-like endoplasmic reticulum kinase, GRP78, and CHOP levels were increased, and the kidneys exhibited severe glomerulosclerosis and tubulointerstitial fibrosis. In CHOP-/- mice, urinary albumin levels are reduced, and the pathological structure of the kidneys is alleviated[15]. These findings show that ERS is involved in the occurrence of diabetic nephropathy, possibly through caspase-12-induced podocyte and epithelial cell apoptosis, which disrupts kidney function[16]. In addition, studies have found that mammalian target of rapamycin complex 1 (mTORC1) acts in mammals as an energy receptor to maintain energy balance in the body. Excess nutrients can activate mTORC1, and excessive activation of the mTORC1 pathway causes ERS. Activation of mTORC1 itself can also destroy kidney podocytes and accelerate the occurrence of diabetic nephropathy[17]. Liraglutide is a GLP-1 receptor agonist analog that has been used to treat patients with type 2 diabetes in recent years and confirmed to have a hypoglycemic effect. In addition, it may exert cardiovascular benefits. Because liraglutide does not undergo renal metabolism[18], the dose does not need to be adjusted for patients with mild, moderate, and severe renal dysfunction. Studies indicate that liraglutide can increase insulin sensitivity and regulate lipid metabolism balance, but this effect is abolished in CHOP-/- mice[19], suggesting that liraglutide may reduce ERS and play another role that does not involve lowering blood sugar levels. In this study, a rat model of type 2 diabetes was established by administration of a high-sugar, high-fat diet in combination with a low dose of STZ. The model group rats were less aware, were less active, and exhibited more hair loss than the control group rats. In line with the clinical symptoms of "three more and one less", fasting blood glucose levels were significantly higher, and blood creatinine, urea nitrogen and 24-h urine protein levels were all higher in the model group than in the control group. Pathological examination showed destruction of kidney structure, which is in line with the typical characteristics of type 2 diabetes with nephropathy. After intervention with liraglutide, the general condition of the rats was improved. The blood creatinine, urea nitrogen, and 24-h urinary protein levels were lower in the intervention groups than in the model group. In the intervention groups, the alterations in kidney tissue structure were observed to be alleviated under the microscope. The improvements in the liraglutide-treated groups were obvious. These experimental results showed that in addition to lowering blood sugar and alleviating diabetes symptoms, liraglutide can also protect kidney tissues and therefore has a therapeutic effect in diabetic nephropathy. In addition, in this study, by comparing the expression of the ERS-related molecules GRP78 and caspase-12 in the kidneys of rats from each group, we found that the expression of GRP78 and caspase-12 in the kidneys of the model rats was higher than that in the kidneys of the control rats. After liraglutide intervention, the expression of the two molecules decreased, and the decrease in the high-dose liraglutide group was more significant than that in the low-dose liraglutide group. This indicates that the mechanism by which liraglutide alleviates diabetic nephropathy is likely related to a reduction in ERS.
Through our experimental results, we infer that liraglutide may have a therapeutic effect on diabetic nephropathy by reducing the stress level of the renal endoplasmic reticulum. But this study did not make further exploration of the mechanism, and this effect may only be an indirect effect.
In summary, this study shows that liraglutide may delay the progression of diabetic nephropathy by reducing ERS and that the role of liraglutide in protecting the kidney is dose-dependent. The abovementioned effects of liraglutide may provide new ideas for the treatment of diabetic nephropathy. However, there is no theoretical basis for whether liraglutide can reduce the incidence of diabetic neuropathy in patients with type 2 diabetes or whether the drug has a therapeutic effect in end-stage renal disease, and further research is still needed.
Liraglutide is beneficial to the kidneys in diabetics, but its exact role in the kidney is unclear.
To provide a new idea for the treatment of diabetes and diabetic nephropathy.
The study was to investigate the specific effects of liraglutide on the kidney.
To explore the effect of liraglutide on the kidney of rats with type 2 diabetes by establishing a rat model of type 2 diabetes
The mechanism of the treatment of diabetic nephropathy with liraglutide is probably related to the reduction of ER stress.
This study indicated that liraglutide may delay the progression of diabetic nephropathy by reducing endoplasmic reticulum stress, and the effect of liraglutide on renal protection is dose-dependent.
The role of liraglutide may provide new ideas for the treatment of diabetic nephropathy.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Tanbouly D, Turkmen K S-Editor: Chen XF L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Tuttle KR, Bakris GL, Bilous RW, Chiang JL, de Boer IH, Goldstein-Fuchs J, Hirsch IB, Kalantar-Zadeh K, Narva AS, Navaneethan SD, Neumiller JJ, Patel UD, Ratner RE, Whaley-Connell AT, Molitch ME. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 416] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 2. | Ioannou K. Diabetic nephropathy: is it always there? Hormones. 16:351-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Shao DC, Xue H, Lu LM. [The role of endoplasmic reticulum stress in diabetic nephropathy]. Sheng Li Ke Xue Jin Zhan. 2015;46:111-115. [PubMed] |
| 4. | Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hänselmann A, Nilsson B, Møller JE, Hjort J, Rasmussen J, Boesgaard TW, Schou M, Videbaek L, Gustafsson I, Flyvbjerg A, Wiggers H, Tarnow L. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 399] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 5. | Zhang M, Lv XY, Li J, Xu ZG, Chen L. The characterization of high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Exp Diabetes Res. 2008;2008:704045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 412] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 6. | Shi XY. Experimental Zoology of Modern Medicine. Beijing: People's Military Medical Publishing House, 2000: 1-620. |
| 7. | Gu XQ. Notes on Pharmaceutical Preparations. Beijing: People's Medical Publishing House, 1983: 1-1048. |
| 8. | Demirtas L, Guclu A, Erdur FM, Akbas EM, Ozcicek A, Onk D, Turkmen K. Apoptosis, autophagy & endoplasmic reticulum stress in diabetes mellitus. Indian J Med Res. 2016;144:515-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 9. | Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 1188] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 10. | Ariyasu D, Yoshida H, Hasegawa Y. Endoplasmic Reticulum (ER) Stress and Endocrine Disorders. Int J Mol Sci. 2017;18:382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 11. | Iurlaro R, Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283:2640-2652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 795] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 12. | Zhao Y, Yan Y, Zhao Z, Li S, Yin J. The dynamic changes of endoplasmic reticulum stress pathway markers GRP78 and CHOP in the hippocampus of diabetic mice. Brain Res Bull. 2015;111:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Xie RJ, Han B, Yang T, Yang Q. Activation of Caspase12, a key molecule in endoplasmic reticulum stress related apoptosis pathway, induces apoptosis of hepatocytes in rats with hepatic fibrosis. Shijie Huaren Xiaohua Zazhi. 2016;24:2470-2477. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Liu M, Hodish I, Haataja L, Lara-Lemus R, Rajpal G, Wright J, Arvan P. Proinsulin misfolding and diabetes: mutant INS gene-induced diabetes of youth. Trends Endocrinol Metab. 2010;21:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 15. | Lakshmanan AP, Thandavarayan RA, Palaniyandi SS, Sari FR, Meilei H, Giridharan VV, Soetikno V, Suzuki K, Kodama M, Watanabe K. Modulation of AT-1R/CHOP-JNK-Caspase12 pathway by olmesartan treatment attenuates ER stress-induced renal apoptosis in streptozotocin-induced diabetic mice. Eur J Pharm Sci. 2011;44:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Cao YP, Hao YM, Liu QJ, Wang J, Li H, Duan HJ. [The relationship between endoplasmic reticulum stress and its particular apoptosis way caspase-12 and apoptosis in renal cortex of diabetic rats]. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2011;27:236-240. [PubMed] |
| 17. | Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121:2181-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 456] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 18. | Imamura S, Hirai K, Hirai A. The glucagon-like peptide-1 receptor agonist, liraglutide, attenuates the progression of overt diabetic nephropathy in type 2 diabetic patients. Tohoku J Exp Med. 2013;231:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Wang P, Guo XY, Gao Y, Liu J, Shan XJ, Zhang R, Ge XC, Feng ZB, Li GF. Effects of liraglutide on ATF4/CHOP pathway of pancreatic beta cells in rats fed with high-fat diet. Chongqing Yixue. 2017;46:3755-3758. [DOI] [Full Text] |