Published online Dec 15, 2020. doi: 10.4239/wjd.v11.i12.596
Peer-review started: July 14, 2020
First decision: September 21, 2020
Revised: September 28, 2020
Accepted: October 13, 2020
Article in press: November 9, 2020
Published online: December 15, 2020
Processing time: 152 Days and 0.5 Hours
Modern guidelines recommend sodium-glucose cotransporter-2 (SGLT2) inhibitors as the preferred antihyperglycemic agents for patients with type 2 diabetes and chronic kidney disease. However, the mechanisms underlying the renal protective effect of SGLT2 inhibitors are not fully understood.
To estimate the effect of the SGLT2 inhibitor, empagliflozin (EMPA), on the structure of podocytes and nephrin expression in glomeruli in db/db diabetic mice.
We treated 8-wk-old male db/db mice with EMPA (10 mg/kg/d) or vehicle for 8 wk. Age-matched male db/+ mice were included as non-diabetic controls. Parameters of body composition, glycemic and lipid control, and plasma concentrations of leptin, insulin and glucagon were assessed. We evaluated renal hypertrophy as kidney weight adjusted to lean mass, renal function as plasma levels of creatinine, and albuminuria as the urinary albumin-to-creatinine ratio (UACR). Renal structures were studied by light and transmission electron microscopy with a focus on mesangial volume and podocyte structure, respectively. Glomerular nephrin and transforming growth factor beta (TGF-β) were assessed by immunohistochemistry.
Severe obesity and hyperglycemia developed in db/db mice prior to the start of the experiment; increased plasma concentrations of fructosamine, glycated albumin, cholesterol, leptin, and insulin, and elevated UACR were detected. Mesangial expansion, glomerular basement membrane thickening, and increased area of TGF-β staining in glomeruli were revealed in vehicle-treated mice. Podocytopathy was manifested by effacement of foot processes; nephrin-positive areas in glomeruli were reduced. EMPA decreased the levels of glucose, fructosamine and glycated albumin, UACR, kidney hypertrophy, mesangial expansion, glomerular basement membrane thickening, and glomerular TGF-β staining, alleviated podocytopathy and restored glomerular staining of nephrin.
These data indicate that EMPA attenuates podocytopathy in experimental diabetic kidney disease. The anti-albuminuric effect of EMPA could be attributed to mitigation of podocyte injury and enhancement of nephrin expression.
Core Tip: In the present study, we assessed the influence of the sodium-glucose cotransporter-2 (SGLT2) inhibitor empagliflozin (EMPA) on glomerular structure, with a focus on podocytes, and glomerular staining of nephrin in db/db mice, a model of type 2 diabetic nephropathy. We treated 8-wk-old mice with EMPA (10 mg/kg/d) or vehicle for 8 wk. The results demonstrated that EMPA attenuates podocytopathy and enhances glomerular nephrin staining. These effects were accompanied by mitigation of renal hypertrophy and glomerular transforming growth factor beta expression and a decrease in albuminuria. These results contribute to the understanding of the renal protective effect of SGLT2 inhibitors in diabetes.
- Citation: Klimontov VV, Korbut AI, Taskaeva IS, Bgatova NP, Dashkin MV, Orlov NB, Khotskina AS, Zavyalov EL, Klein T. Empagliflozin alleviates podocytopathy and enhances glomerular nephrin expression in db/db diabetic mice. World J Diabetes 2020; 11(12): 596-610
- URL: https://www.wjgnet.com/1948-9358/full/v11/i12/596.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i12.596
Kidney disease is a significant contributor to global morbidity and mortality. In 2017, 697.5 million cases of chronic kidney disease (CKD) were registered; the global prevalence was 9.1%. CKD is considered to be the cause of 35.8 million disability-adjusted life-years, a third of them are associated with diabetes[1]. Since 2000, an increase in the incidence of diabetes-associated end-stage renal disease (ESRD) has been reported in many countries and populations around the globe, including Australia, Bosnia and Herzegovina, Malaysia, Mexico, the Philippines, the Republic of Korea, Russia, Scotland, Singapore, and Taiwan[2]. In the United States, 746557 cases of ESRD were observed in 2017[3]. In patients with diabetes the CKD stage is associated with lower scores in health-related quality of life[4]. Consequently, the development of new approaches for renal protection remains a priority challenge.
The inhibitors of sodium-glucose cotransporter-2 (SGLT2) have opened up new prospects for the prevention of diabetes-related CKD. In randomized clinical trials, SGLT2 inhibitors [empagliflozin (EMPA), canagliflozin and dapagliflozin specifically] ameliorated albuminuria and prevented the decline in renal function in participants with type 2 diabetes (T2D)[5-10]. In particular, in the EMPA-REG OUTCOME trial, EMPA treatment reduced the risk of macroalbuminuria, the decline in renal function, the induction of renal replacement therapy and renal death[5,10]. SGLT2 inhibitors are recommended as preferred add-on antihyperglycemic agents for patients with T2D and CKD by the American Diabetes Association and the European Association for the Study of Diabetes[11,12].
The mechanisms underlyingthe renal protective effect of SGLT2 inhibitors are not fully understood. Among other effects, diminishing intraglomerular pressure and hyperfiltration, suppression of inflammation and fibrogenic pathways, protection against ischemic kidney damage, elevation of glucagon-like peptide-1 and glucagon levels, have been widely discussed[13-15]. Improving tubular metabolism and oxy-genation is considered another mechanism of the protective effect[16]. In glomeruli, podocytes may be the cornerstone of the effect of these drugs. Recently it was revealed that the SGLT2 molecule is expressed in podocytes and is upregulated by the albumin load both in vivo and in vitro[17].
Diabetic kidney disease (DKD) is characterized by podocyte damage depicted by the effacement of foot processes (FPs) and disruption of the slit diaphragm, resulting in elevated permeability for albumin[15]. The effacement of FPs is considered to be a result of actin cytoskeleton disruption. Normally, the cortical actin network binds with proteins of the slit diaphragm, such as nephrin and podocin. Nephrin is considered an adhesion protein and is expressed by mature podocytes[18]. However, the intracellular domain of nephrin acts as a signaling molecule[15]. Mutation-caused abnormalities of the nephrin gene result in congenital nephrotic syndrome of the Finnish type[18]. The increased urinary excretion of nephrin is associated with the albuminuric pattern of CKD in patients with T2D[19]. Since the slit diaphragm provides the most selective barrier for albumin[20], it can be assumed that the anti-albuminuric action of SGLT2 inhibitors could be mediated by an effect on podocytes. Indeed, in the model of fructose- and streptozotocin-induced diabetes in rats, dapagliflozin enhanced glomerular nephrin expression and mitigated histological signs of diabetic ne-phropathy[21].
The aim of this study was to estimate the influence of the SGLT2 inhibitor EMPA on glomerular structural changes, with a special focus on podocytes, and glomerular staining of nephrin in db/db mice, a model of T2D.
The experiment was carried out on SPF male db/db mice (BKS.Cg-Dock7m+/+Leprdb/J, stock No. 000642, The Jackson Laboratory, United States). These animals are characterized by knock-down of the leptin receptor, which causes diabetes due to impaired satiety, elevated food consumption, and insulin resistance in hepatic, adipose and muscle tissue[22-24]. The db/db mice usually become polyphagic and obese at 4 wk of age and exhibit elevated blood glucose between the fourth and eighth wk. These mice demonstrate shifts in the production of pancreatic and gastrointestinal hormones, and adipokines, which are similar to those in human T2D[25].
The study was approved by the Animal Ethics Committee of the IC&G SB RAS (Permission 21, April 1, 2014).
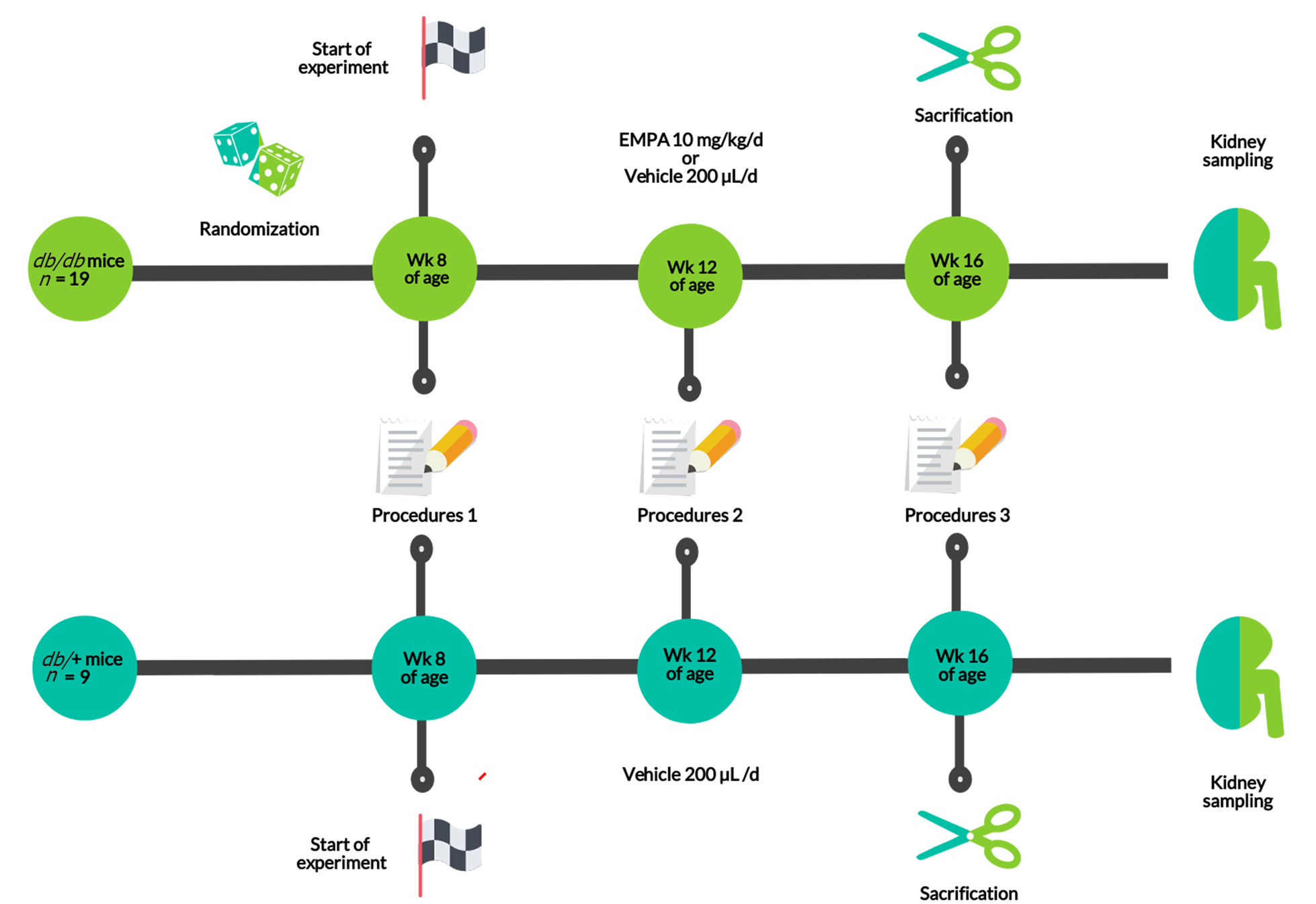
The experiment was a comparative placebo-controlled study in three parallel groups (Figure 1). At 8 wk of age, db/db diabetic mice were randomized to either EMPA or vehicle (EMPA group, Placebo group). Age-matched heterozygous db/+ mice were included as non-diabetic controls.
EMPA (Boehringer Ingelheim, Germany) was given at a daily dose of 10 mg/kg, in accordance with previous investigations[26,27]. The agent was suspended in 200 µL of saline and administered intragastrically. The db/db mice, randomized to vehicle, received 200 µL of saline intragastrically. The duration of the experiment was 8 wk.
Mice were housed in OptiMICE cages (Animal Care Systems, United States), 2-3 animals/cage. Food (Ssniff, Germany) and water was provided ad libitum. A 24h cycle of light and dark, temperature of 23±2°C and relative humidity of 45%±10% were maintained.
Body weight and parameters of body composition were estimated every 4 wk. A magnetic resonance imaging (MRI) Body Composition Analyzer (Echo Medical Systems, United States) was used for this procedure. We sampled 150-200 μL of heparinized blood from the retro-orbital sinus with an interval of 4 wk. In addition, urine samples were collected in Petri dishes, which were placed under the animal until spontaneous urination occurred. The obtained samples were frozen (–80 ºC).
At 16 wk of age (8th wk of the experiment), the animals were decapitated following administration of isoflurane and kidney tissue samples were obtained. Renal structure was estimated by light microscopy, immunohistochemistry (IHC) and transmission electronic microscopy (TEM).
The plasma levels of glucose, total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol, triglycerides, creatinine, and uric acid were measured using an AU480 Chemical Analyzer (Beckman Coulter, United States). The plasma concentrations of glycated proteins (fructosamine and glycated albumin) were assessed by ELISA (Mouse FTA ELISA kit and Mouse GAL ELISA kit, MyBioSource Inc., United States). The levels of leptin, insulin and glucagon were assessed by a bead array assay (BioRad, United States). Urinary concentrations of albumin and creatinine were determined by ELISA (Mouse Albumin ELISA kit and Mouse Creatinine kit, Cristal Chem, United States). Albuminuria was expressed as the urinary albumin-to-creatinine ratio (UACR).
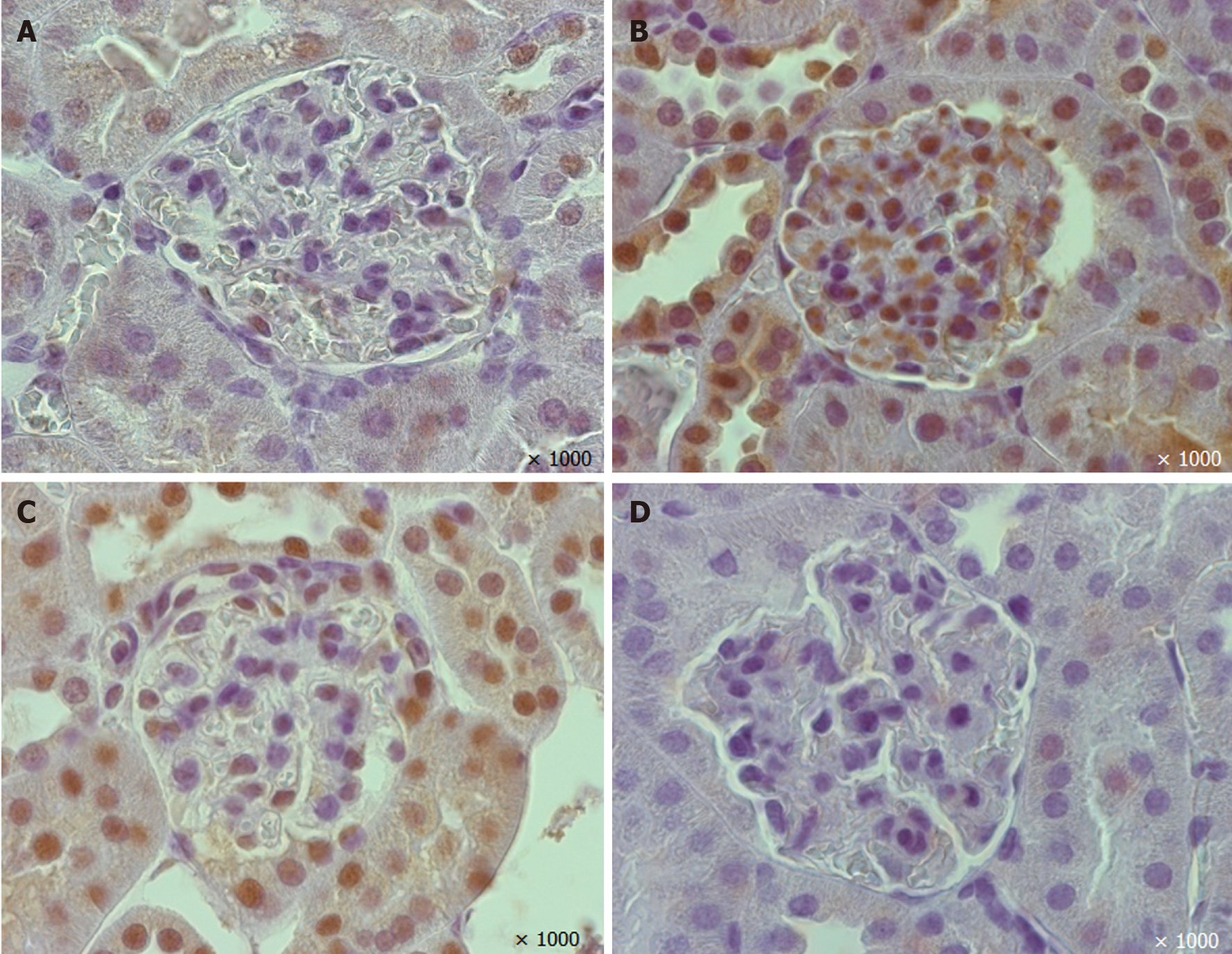
Mayer`s hematoxylin and eosin (H&E) stained 5-µm paraffin-embedded renal tissue sections were observed by light microscopy. For IHC, formalin-fixed, paraffin-embedded 5-µm sections were prepared. Primary antibody to nephrin (1:100, ab58968, Abcam, United Kingdom) or transforming growth factor beta (TGF-β) (1:100, ab66043, Abcam, United Kingdom) were used with the corresponding secondary antibodies (1/2000; Goat Anti-Rabbit IgG H&L+HRP antibody, ab205718, Abcam, United Kingdom). PBS-Tween 20 solution was used for the secondary antibody control.
A morphometric analysis was performed using the Image J platform (https://imagej.net, United States). At least ten glomeruli were estimated on each H&E and IHC slide. The cells under lattice sites of a 5-μm grid were counted and fractional mesangial and capillary volume, volumetric density (VV) of nephrin-positive and TGF-β positive areas in glomeruli were assessed.
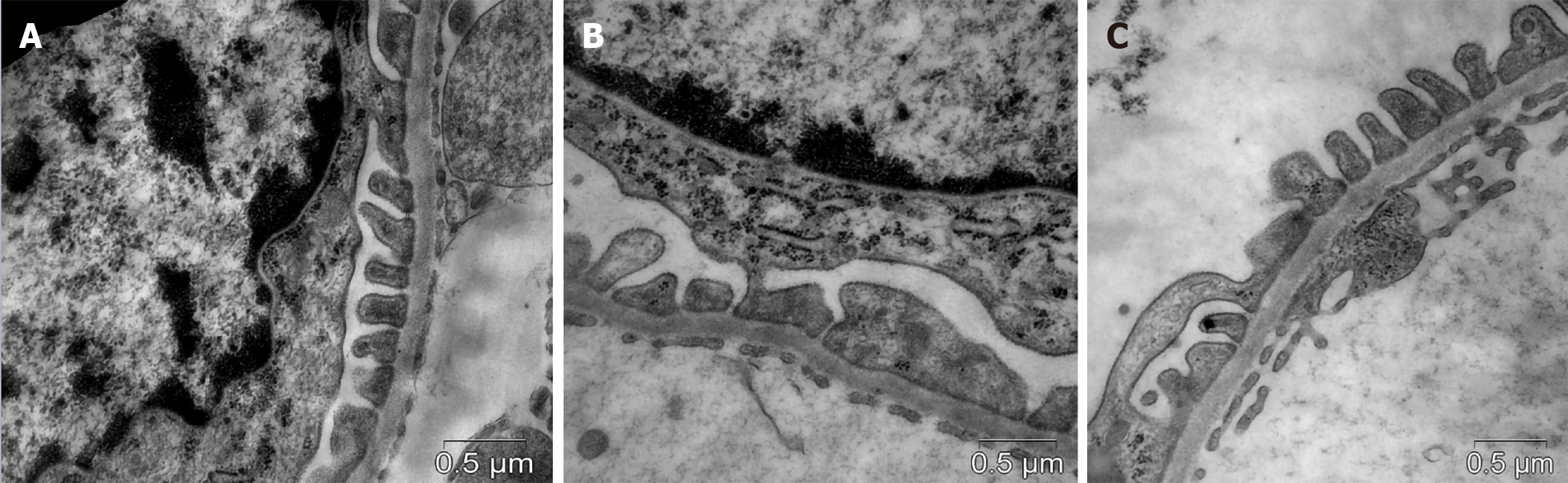
For TEM, paraformaldehyde-OsO4–fixed samples of renal cortex were embedded in Epon-812. The uranyl acetate and lead citrate contrasted thin sections (70-100 nm) were investigated using a JEM-1400 microscope (JEOL, Japan). In the ultrastructural images, the GBM width, the mean width and numerical density (NA) of podocyte FPs were estimated. The GBM was assessedat magnification ×100000. Magnification ×40000 was used for the FP width, whereas the measurement of NA was performed at magnification ×15000.
Statistical analysis was carried out using STATISTICA 12.0 software (Dell, United States). The non-parametric Mann-Whitney U-test, the ANOVA-χ2 test, and Wilcoxon signed rank test were used to assess the differences between two independent groups, three and two dependent groups, respectively. The non-parametric Spearman rank test was used to determine the correlations between variables. We considered the differences as significant at a Pvalue below 0.05.
At the age of 8 wk, i.e. at the start of experiment, all diabetic db/db mice showed a substantial elevation in body weight and fat mass when compared to non-diabetic controls (Table 1). These shifts in the placebo group remained stable throughout the experiment. A slight reduction in body weight was observed in control animals (P = 0.01). In contrast, in EMPA-treated mice, both body weight and fat mass demonstrated a further increase (P = 0.008 and P = 0.03, respectively).
| Parameter | Age in wk | Group | ||
| Non-diabetic db/+ mice (n = 9) | db/db diabetic mice | |||
| Vehicle (n = 10) | EMPA (n = 9) | |||
| Body weight (g) | 8 | 26.8 (25.0-31.1) | 37.1 (32.1–41.6)b | 37.4 (28.5–41.5)b |
| 12 | 26.2 (23.5–28.2) | 37.5 (35.8–40.5)b | 46.2 (38.8–53.0)b,c | |
| 16 | 24.8 (21.6–27.7)e | 37.8 (34.3–40.4)b | 52.6 (42.5–62.2)b,c,f | |
| Fat mass (g) | 8 | 3.1 (1.9–4.1) | 18.3 (16.2–22.5)a | 18.4 (13.5–20.6)b |
| 12 | 2.7 (1.9–3.3) | 19.5 (17.0–22.7)b | 25.3 (21.7–30.9)b,c | |
| 16 | 2.7 (2.1–4.5) | 19.6 (17.4–22.7)b | 28.0 (22.2–35.3)a,d,e | |
| Fat mass (%) | 8 | 11.4 (8.5–16.1) | 50.6 (46.6–55.3)b | 49.3 (46.8–50.0)b |
| 12 | 10.7 (8.1–12.4) | 52.5 (45.2–57.0)b | 55.5 (51.5–58.3)b | |
| 16 | 11.7 (7.6–13.2) | 55.0 (42.5–62.8)b | 53.4 (49.1–56.7)b | |
High glucose levels were observed in db/db mice at the start of the experiment (P < 0.000001, Table 2). Both fructosamine and glycated albumin concentrations were increased also (P < 0.000001 and P = 0.00001, respectively). The vehicle-treated db/db mice demonstrated a further elevation of these parameters during the experiment (P = 0.005). In the EMPA group, all glycemic parameters improved significantly (all P = 0.02), although normal values were not reached.
| Parameter | Age in wk | Group | ||
| Non-diabetic db/+ mice (n = 9) | Diabetic db/db mice | |||
| Vehicle (n = 10) | EMPA (n = 9) | |||
| Glucose (mmoL/L) | 8 | 9.50 (5.10–11.4) | 28.5 (16.8–40.1)b | 23.1 (15.3–35.4)b |
| 12 | 9.20 (6.10–9.50) | 30.4 (22.7–48.0)b | 16.3 (13.6–20.7)b,d,e | |
| 16 | 9.50 (8.50–12.2) | 32.7 (22.5–53.1)b | 16.1 (9.9–23.6)b,d,e | |
| Fructosamine (µmoL/L) | 8 | 237 (217–249) | 456 (424–511)b | 480 (425–579)b |
| 16 | 239 (222–296) | 622 (524–672)b,f | 468 (341–491)b,c,e | |
| Glycated albumin (µmoL/L) | 8 | 107 (103–127) | 227 (206–239)b | 235 (217–261)b |
| 16 | 117 (109–133) | 283 (252–349)b,f | 210 (166–245)b,c,e | |
| Total cholesterol (mmoL/L) | 8 | 2.76 (1.80–3.96) | 2.72 (1.48–4.86) | 2.76 (1.60–4.05) |
| 16 | 2.37 (1.98–2.97) | 3.57 (1.68–4.86)e | 4.05 (2.10–5.22)e | |
| HDL-cholesterol (mmoL/L) | 8 | 1.17 (0.50–1.62) | 1.20 (0.51–2.01) | 1.40 (0.93–1.89) |
| 16 | 1.26 (0.45–1.80) | 1.50 (0.48–2.19) | 2.31 (1.05–2.88)e | |
| LDL-cholesterol (mmoL/L) | 8 | 1.32 (0.81–2.10) | 1.02 (0.84–2.72) | 1.07 (0.84–2.60) |
| 16 | 0.81(0.75–1.11)f | 0.81 (0.72–1.14) | 1.05 (0.60–1.41) | |
| Triglycerides (mmoL/L) | 8 | 1.74 (0.90–2.70) | 1.14 (0.51–2.30) | 1.25 (0.87–2.30) |
| 16 | 1.35 (0.81–2.58) | 1.79 (1.17–3.54)e | 1.77 (1.29–3.87)e | |
| Creatinine (µmoL/L) | 8 | 67.0 (47.0–78.0) | 76.4 (70.2–84.2) | 70.5 (54.0–82.2) |
| 12 | 68.6 (48.5–74.5) | 76.5 (64.5–100.5)a | 74.7 (46.2–85.8)a | |
| 16 | 61.8 (54.0–71.7) | 75.6 (63.9–89.4)a | 81.3 (55.8–91.2)a | |
| Uric acid (µmoL/L) | 8 | 273 (104–420) | 173 (143–341) | 281 (165–507) |
| 16 | 235 (159–271) | 170 (143–187) | 204 (179-242) | |
There was an increase in plasma total cholesterol levels throughout the experiment in mice treated with placebo (+31.3%, P = 0.04) or EMPA (+46.7%, P = 0.03). In the EMPA group, a marked elevation of HDL-cholesterol was observed (+65%, P = 0.04). However, we recorded no significant changes in the plasma LDL-cholesterol concentrations. There was an increase in the levels of plasma triglycerides in both diabetic groups throughout the experiment (placebo: +57%, P = 0.048; EMPA: +41.6%, P = 0.046).
Diabetic mice had extremely high levels of leptin (Table 3). In the vehicle group, the median level of this hormone exceeded the control by 29.4-fold at wk 8 (P = 0.00005) and by 23.7-fold at wk 16 (P = 0.00002). In EMPA-treated mice a further increase in concentrations of leptin was observed at wk 16 (+46.0%, P = 0.04). A marked increase in insulin levels was revealed in vehicle-treated db/db mice at the start (P = 0.0003) and at the end of the experiment (P = 0.04). Glucagon levels did not significantly change. Treatment with EMPA did not significantly change the levels of insulin and glucagon.
| Hormone | Age in wk | Group | ||
| Non-diabetic db/+ mice (n = 9) | Diabetic db/db mice | |||
| Vehicle (n = 10) | EMPA (n = 9) | |||
| Leptin (ng/mL) | 8 | 3.3 (1.4–6.54) | 97.1 (53.2–114.4)b | 93.4 (80.8–133.1)b |
| 16 | 3.8 (1.5–6.3) | 90.0 (21.2–151.4)b | 136.4 (53.0–171.2)b,c,d | |
| Insulin (ng/mL) | 8 | 5.6 (3.4–18.0) | 25.6 (11.5–45.2)b | 21.2 (10.8–40.8)b |
| 16 | 10.0 (2.1–22.2) | 22.1 (9.8–33.2)a | 20.8 (6.0–56.7)a | |
| Glucagon (ng/mL) | 8 | 370 (250–2670) | 660 (190–2760) | 450 (290–2180) |
| 16 | 370 (150–2120) | 605 (260–2960) | 390 (240–860) | |
Diabetic db/db mice had slightly increased plasma creatinine levels at wk 12 and 16 compared to db/+ heterozygotes (P <0.01, Table 2). No deviations were observed in creatinine levels at wk 16 when compared to wk 8 in all the experimental groups.
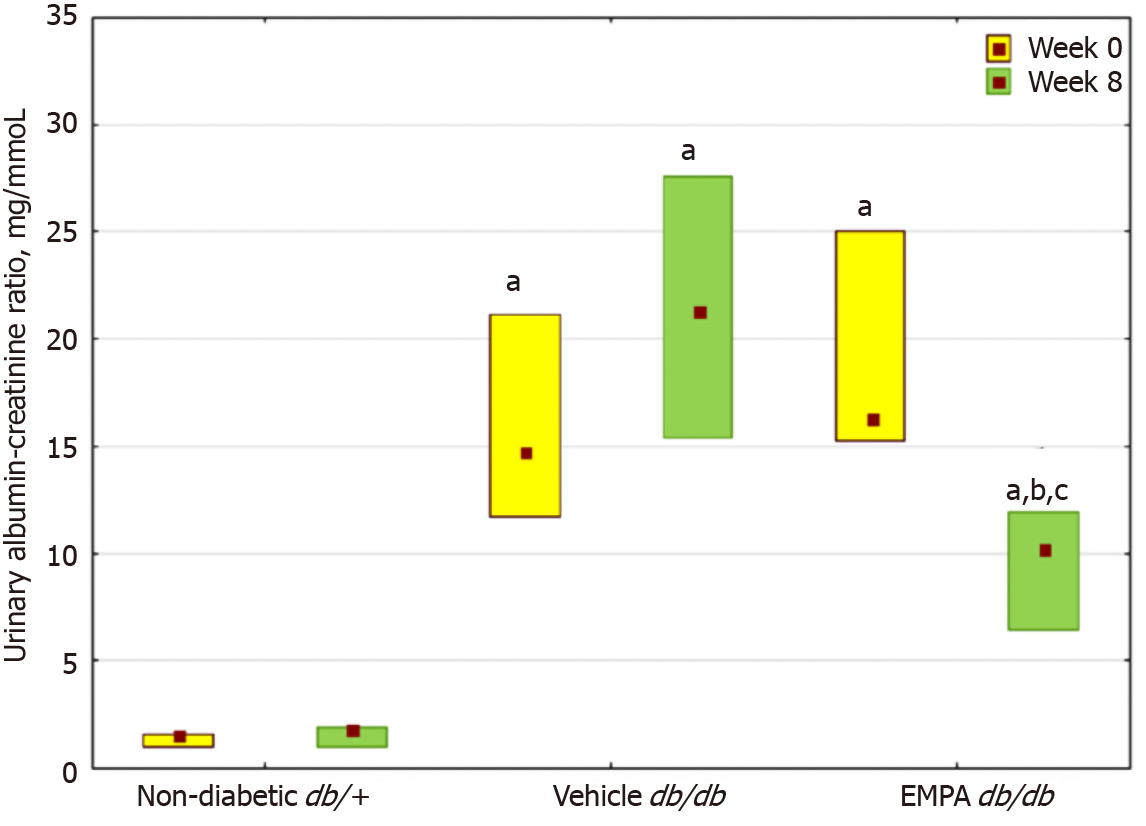
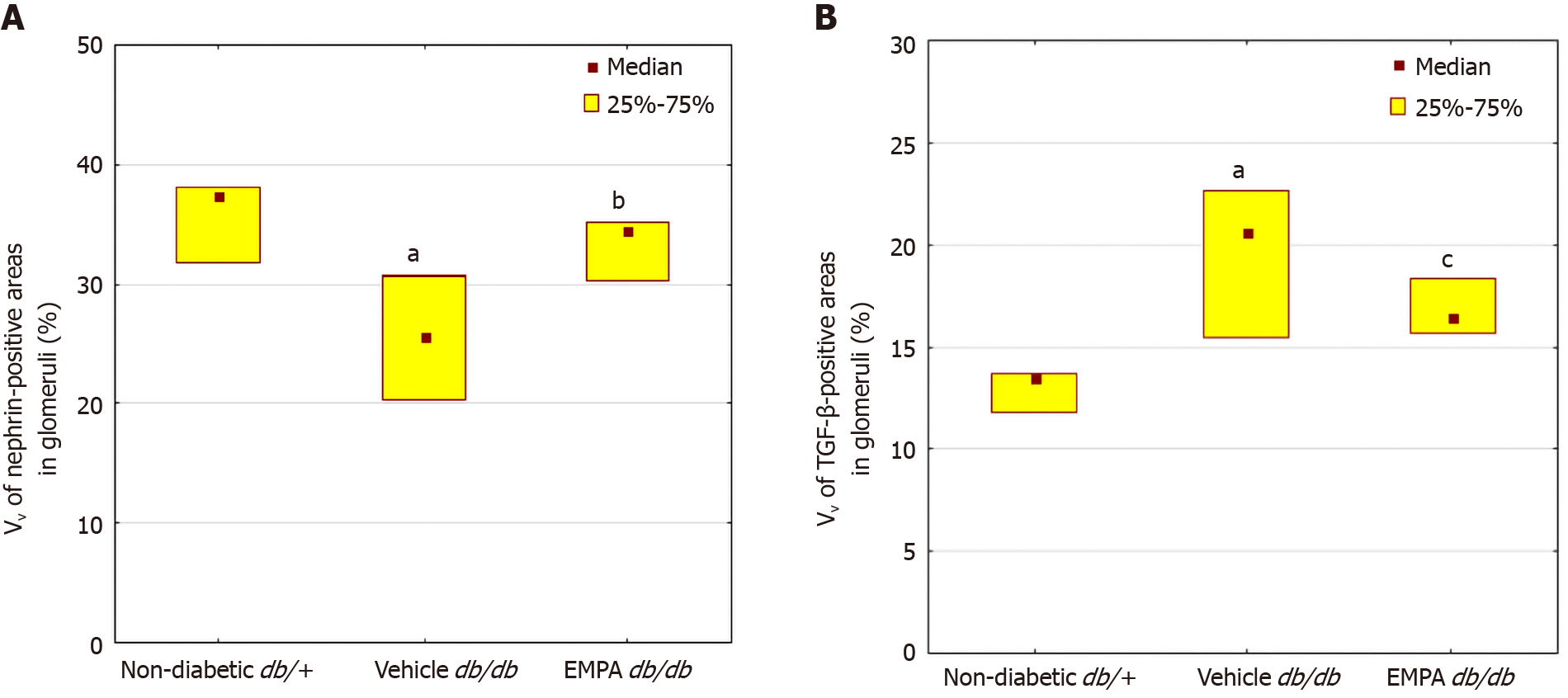
An elevation in the UACR was noted in db/db mice at 8 wk of age (Figure 2). In the vehicle group, a further increase in the UACR was observed after 8 wk. In contrast, EMPA treatment significantly decreased the UACR.
We observed glomerular hypertrophy, mesangial expansion and GBM thickening, which matched structural signs of diabetic nephropathy, in the vehicle-treated diabetic mice. Table 4 demonstrates the differences in mesangial fractional volume and GBM width between the groups. A significant reduction in mesangial volume and GBM width was revealed in the EMPA group (P = 0.0008 and P = 0.02). At the end of the experiment, these parameters were similar in the control and EMPA groups. There was no difference in the volume of glomerular capillaries between control and diabetic animals.
| Parameter | Group | ||
| Non-diabetic db/+ mice (n = 9) | Diabetic db/db mice | ||
| Vehicle (n = 10) | EMPA (n = 9) | ||
| Mesangium, fractional volume (%) | 14.4 (9.8–18.5) | 38.6 (34.5–42.7)a | 25.5 (20.4–35.8)d |
| Capillaries, fractional volume (%) | 31.3 (28.9–38.7) | 34.6 (30.9–36.1) | 33.3 (29.5–37.0) |
| GBM, mean width (nm) | 135 (116–157) | 163 (135–203)a | 139 (116–225)b |
| Podocyte FPs, mean width (nm) | 220 (191–242) | 372 (299–426)a | 203 (162–317)c |
| Podocyte FPs (NA, nm–1) | 3.39 (3.00–3.79) | 2.73 (1.92–3.51)a | 3.31 (2.32–4.18)b |
Diabetic db/db mice showed effacement of FPs as a manifestation of podocytopathy (Figure 3). In vehicle-treated animals, the mean width of FPs was 1.7-fold greaterand NA of FPs was 1.5-fold lower compared with controls (Table 4). In the group of actively treated animals, the width and NA of podocyte FPs were similar to those in db/+ controls.
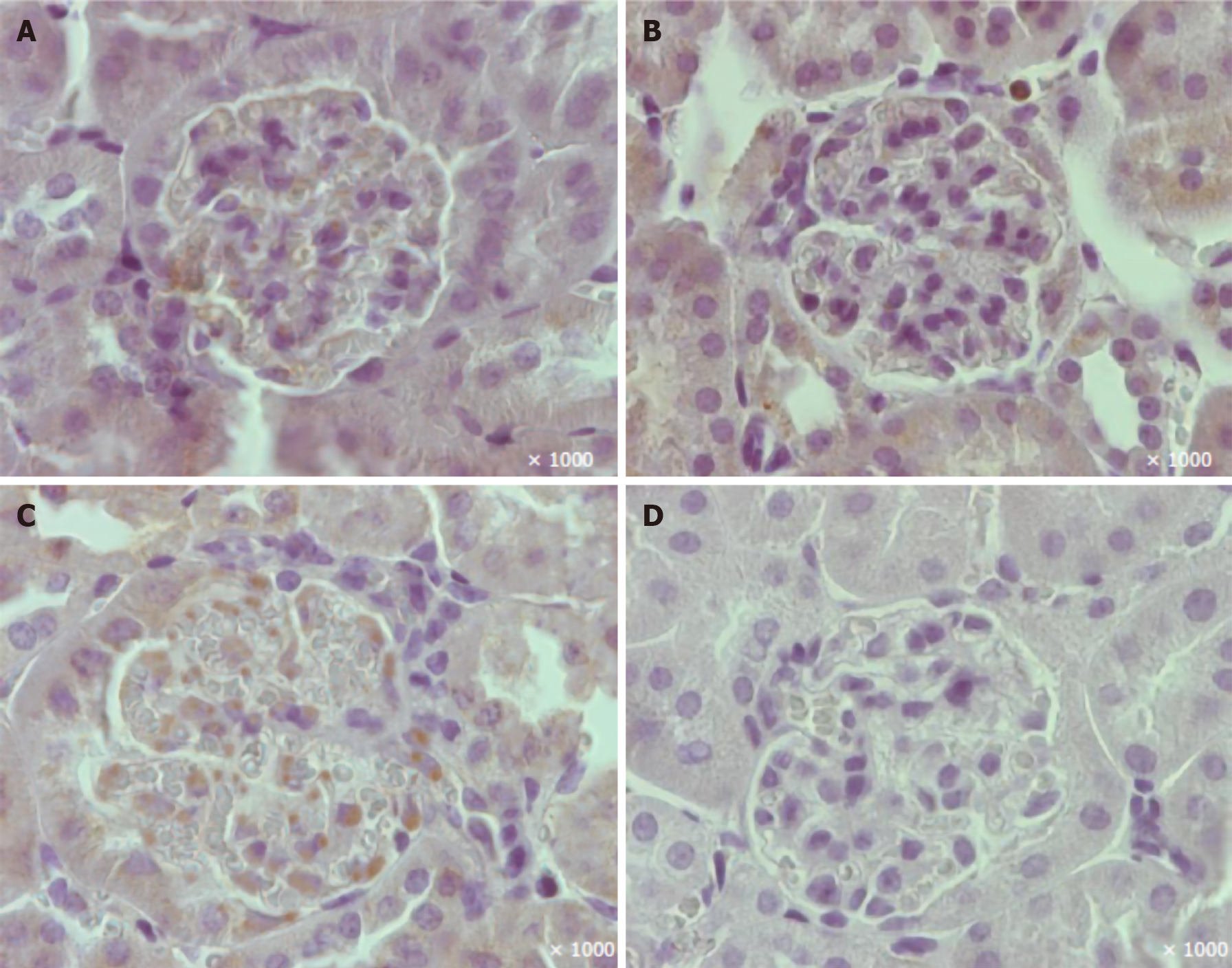
A reduction in the percentage of glomerular nephrin-positive areas was observed in vehicle-treated mice (Figures 4 and 6). In the EMPA group, staining for glomerular nephrin was markedly increased.
The mice treated with vehicle demonstrated larger TGF-β positive areas in the glomeruli than non-diabetic mice (P=0.00001, Figures 5 and 6). EMPA decreased TGF-β staining (P = 0.0004).
Fructosamine and glycated albumin demonstrated positive correlations with mesangial volume, mean width of GBM and FPs, and TGF-β staining; the correlations with NA of podocyte FPs and nephrin-positive areas were negative (Table 5). Plasma creatinine correlated positively with mesangial volume, and demonstrated a negative correlation with nephrin-positive areas in glomeruli. The UACR levels were positively correlated with mesangial volume, GBM width, width of podocyte FPs and TGF-β-staining. The UACR was negatively correlated with nephrin staining.
| Parameter | Fructosamine | Glycated albumin | Plasma creatinine | UACR |
| Mesangial volume, VV | r = 0.69 | r = 0.67 | r = 0.57 | r =0.81 |
| P = 0.0002 | P = 0.0004 | P = 0.004 | P = 0.000002 | |
| Capillary volume, VV | r = 0.13 | r = 0.11 | r = 0.30 | r = 0.05 |
| P > 0.05 | P > 0.05 | P > 0.05 | P > 0.05 | |
| GBM width | r = 0.69 | r = 0.69 | r = 0.21 | r = 0.53 |
| P = 0.0008 | P = 0.007 | P > 0.05 | P = 0.02 | |
| Podocyte FPs, width | r = 0.62 | r = 0.63 | r = –0.15 | r = 0.50 |
| P = 0.003 | P = 0.003 | P > 0.05 | P = 0.03 | |
| Podocyte FPs, NA | r = –0.57 | r = -0.58 | r = 0.04 | r = –0.25 |
| P = 0.008 | P = 0.007 | P > 0.05 | P > 0.05 | |
| Nephrin-positive areas in glomeruli, VV | r = –0.68 | r = –0.65 | r = –0.51 | r = –0.76 |
| P = 0.006 | P = 0.009 | P = 0.04 | P = 0.001 | |
| TGF-β positive areas in glomeruli, VV | r = 0.65 | r = 0.65 | r = 0.09 | r = 0.67 |
| P = 0.003 | P = 0.002 | P >0.05 | P = 0.001 |
In this study, we tested the hypothesis that the anti-albuminuric activity of EMPA could be mediated by its effect on podocyte integrity. We chose db/db mice as a model of T2D. In these animals, we documented obesity as an increase in the fat mass verified by MRI. The observed hyperglycemia, hypercholesterolemia, hypertriglyceridemia, hyperinsulinemia and hyperleptinemia characterized the metabolic profile of T2D. The structural signs of DKD in db/db mice were accompanied by a marked increase in urinary albumin excretion and a slight increase in plasma creatinine. These findings are in accordance with data from prior investigations which demonstrated that structural signs of DKD in db/db mice appeared at 8 wk and were retained at 12 and 16 wk of age[28], whereas a reduction in creatinine clearance occurred later, at the age of 15 wk[29]. In our study, we used male db/db mice in the experiment; in a previous study, EMPA also demonstrated anti-albuminuric and anti-fibrotic effects in the kidneys of female db/db mice[30].
In our model of T2D, EMPA prevented the elevation of albuminuria and preserved podocyte morphology. Specifically, EMPA alleviated the effacement of FPs, a principal sign of podocytopathy. It was previously shown that ipragliflozin, another SGLT2 inhibitor, preserved podocyte integrity in db/db diabetic mice[31]. Dapagliflozin also limited podocyte injury in a protein-overload model of proteinuric nondiabetic nephropathy[17]. In uninephrectomized db/db mice, treatment with metformin, ramipril, and EMPA increased a density of podocyte filtration slit [32]. In these studies, as well as in our work, the alleviation of podocytopathy was accompanied by a decrease in the protein excretion.
As the podocyte FPs form the most refined layer of the glomerular filter, changes in their molecular and structural organization could lead to increased permeability to albumin. In this study, we found a reduction in glomerular nephrin-positive staining in db/db mice. The data are in agreement with those from previous studies which demonstrated down-regulation of glomerular nephrin expression in patients with T2D[33,34]. Traditionally, nephrin is considered a structural component of the slit diaphragm. At present, the cytoplasmic domain of the nephrin molecule is presumed to be involved in the development of glomerular diseases[35]. Nephrin regulates podocyte cytoskeleton organization, and nephrin down-regulation may alter the actin structure, leading to the effacement of FPs and slit diaphragm breakdown[15]. In our study, an enlargement of nephrin-positive areas under EMPA treatment was associated with a reduction in albuminuria. These results provided further support for the notion that the anti-albuminuric effect of EMPA could be mediated, at least partially, via enhancement of nephrin expression.
We observed a reduction in glomerular TGF-β-positive areas in EMPA-treated mice. Overexpression of TGF-β is considered to be a cornerstone among molecular mechanisms of diabetic glomerulosclerosis. In addition, TGF-β1 enhances the permeability of the glomerular barrier and decreases tubular reabsorption of albumin[36]. It was found that the TGF-β signaling pathway impairs nephrin expression in podocytes[37,38]. Therefore, a decrease in TGF-β expression could also contribute to the anti-albuminuric effect of EMPA.
It is still a matter of debate whether SGLT2 inhibitors exert their renal protective activity in a glucose-independent manner. In our study, the severity of podocytopathy correlated with hyperglycemia. In EMPA-treated mice, we observed a reduction in plasma fructosamine and glycated albumin levels, regarded as intermediate-term indicators of glycemic control[39], accompanied by a decrease in plasma glucose. Although normoglycemia was not achieved under EMPA treatment, the podocyte morphology improved significantly. The results give further support to the notion that the non-glycemic effects of SGLT2 inhibitors could be important for their protective effect on the kidneys. Further evidence of this assumption was obtained recently in the DAPA-CKD randomized clinical trial. In this trial, dapagliflozin demonstrated a protective effect on renal function in both diabetic and non-diabetic CKD[40].
The changes in the body weight should be taken into account when interpreting the effects of SGLT2 inhibitors. In our study, EMPA contributed to the additional increase in body weight and fat mass, while the opposite pattern was observed in clinical studies[41]. The increase in the body weight was observed under administration of EMPA[26,42], dapagliflozin[43] and ipragliflozin[44] in experiments similar to ours. However, no increase in food consumption was documented during EMPA treatment[26,42]. The mechanism of EMPA-induced increase in rodent body weight requires further research. Interestingly, we found a marked increase in plasma cholesterol levels in the EMPA group, but the change was attributed to HDL-cholesterol elevation. Thus, it is unlikely that the shifts in the fat mass or lipid metabolism could contribute to the renal protective activity of EMPA.
In this study, we demonstrated that EMPA restores the structure of podocyte FPs in diabetes. It was postulated that the changes in FP morphology may be the result of podocyte cytoskeletal reconfiguration[45]. In cultured podocytes, the SGLT2 inhibitor dapagliflozin limited cytoskeletal remodeling induced by albumin load[17]. Thus, podocytes could be considered direct targets of SGLT2 inhibitors. We have shown recently that EMPA reactivated autophagic flux in the podocytes of db/db mice[46]; the promotion of autophagy could be an explanation of the renal protective effect of SGLT2 inhibitors[46,47].
The results of this study indicate that EMPA attenuates podocytopathy in db/db mice, a model of T2D nephropathy. The anti-albuminuric effect of EMPA could be attributed to the mitigation of podocyte injury and enhanced nephrin expression.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors have opened up new prospects for the prevention of chronic kidney disease (CKD). Modern guidelines recommend SGLT2 inhibitors as the preferred antihyperglycemic agents for patients with type 2 diabetes (T2D) and CKD. In the empagliflozin (EMPA)-REG OUTCOME trial, the SGLT2 inhibitor EMPA prevented increased albuminuria and progression of renal function decline in T2D patients.
The exact mechanisms of the renal effect of SGLT2 inhibitors are not fully understood. Since diabetic kidney disease is accompanied by podocyte injury and this injury results in albuminuria, it can be speculated that the anti-albuminuric action of SGLT2 inhibitors could be mediated via podocytes. The influence of SGLT2 inhibitors on podocyte structure and function remains to be clarified.
In this study, we estimated the effect of EMPA on glomerular structural changes, with a special focus on podocytes, and glomerular nephrin staining, in db/db mice, a model of T2D. Thereby, we assessed the hypothesis that the anti-albuminuric effect of EMPA is linked with the preservation of podocyte integrity in diabetic kidney disease.
We treated 8-wk-old db/db male mice with EMPA (10 mg/kg/d) or vehicle for 8 wk. Heterozygous db/+ mice were included as non-diabetic controls. Body weight and body composition were assessed every 4 wk. The plasma levels of glucose, glycated proteins, lipids, creatinine, leptin, insulin, glucagon, and albuminuria were monitored. Renal structure was studied by light and transmission electron microscopy. Glomerular nephrin and transforming growth factor beta (TGF-β) were assessed by immunohistochemistry. The fractional mesangial and capillary volume, glomerular basement membrane and podocyte foot processes width, numerical density of podocyte foot processes, as well as volumetric density of nephrin-positive and TGF-β-positive areas in glomeruli were quantified.
Throughout the experiment, diabetic mice showed a dramatic elevation in the body weight and fat mass. The observed increase in serum levels of glucose, glycated proteins, cholesterol, triglycerides, leptin and insulin characterized the metabolic profile of T2D. In db/db mice, EMPA mitigated renal hypertrophy, decreased mesangial fractional volume, the width of glomerular basement membrane, and glomerular TGF-β staining. EMPA-treated db/db mice demonstrated fewer signs of podocyte foot process effacement and restored glomerular staining of nephrin. These effects were correlated with a reduction of albuminuria. The improvement in podocyte integrity was observed even though normoglycemia was not achieved.
This is the first study to quantitatively describe the effects of EMPA on podocyte structure and glomerular staining of nephrin in a model of T2D. The data indicate that EMPA attenuates podocytopathy in experimental diabetic kidney disease. The anti-albuminuric effect of EMPA could be attributed to mitigation of podocyte injury and enhancement of nephrin expression. The protective effect of EMPA on the kidneys is realized even in condition of suboptimal glycemic control.
Uncovering the molecular pathways which are important for the effect of SGLT2 inhibitors on podocyte homeostasis is a challenge for further research.
Light and electron microscopy was carried out at the Joint Access Center for Microscopy of Biological Objects of IC&G SB RAS (Novosibirsk, Russia). We are sincerely thankful to Irina Ischenko, Maria Borisova, Sergey Bayborodin, Taisiya Aleshina, and Victor Rosin for their facilitation with animal procedures, tissue processing, and microscopy.
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Association for the Study of Diabetes, No. 200190.
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Saha S S-Editor: Fan JR L-Editor: Webster JR P-Editor: Ma YJ
| 1. | GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3928] [Cited by in RCA: 3788] [Article Influence: 757.6] [Reference Citation Analysis (0)] |
| 2. | Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 944] [Article Influence: 157.3] [Reference Citation Analysis (1)] |
| 3. | Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, Gu H, Hirth RA, Hutton D, Jin Y, Kapke A, Kurtz V, Li Y, McCullough K, Modi Z, Morgenstern H, Mukhopadhyay P, Pearson J, Pisoni R, Repeck K, Schaubel DE, Shamraj R, Steffick D, Turf M, Woodside KJ, Xiang J, Yin M, Zhang X, Shahinian V. US Renal Data System 2019 Annual Data Report: Epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75:A6-A7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 552] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 4. | Zimbudzi E, Lo C, Ranasinha S, Gallagher M, Fulcher G, Kerr PG, Russell G, Teede H, Usherwood T, Walker R, Zoungas S. Predictors of health-related quality of life in patients with co-morbid diabetes and chronic kidney disease. PLoS One. 2016;11:e0168491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, Johansen OE, Woerle HJ, Broedl UC, Zinman B; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2303] [Cited by in RCA: 2535] [Article Influence: 281.7] [Reference Citation Analysis (0)] |
| 6. | Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 7. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3976] [Article Influence: 662.7] [Reference Citation Analysis (0)] |
| 8. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4247] [Article Influence: 707.8] [Reference Citation Analysis (0)] |
| 9. | Lin YH, Huang YY, Hsieh SH, Sun JH, Chen ST, Lin CH. Renal and glucose-lowering effects of empagliflozin and dapagliflozin in different chronic kidney disease stages. Front Endocrinol (Lausanne). 2019;10:820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Butler J, Zannad F, Fitchett D, Zinman B, Koitka-Weber A, von Eynatten M, Zwiener I, George J, Brueckmann M, Cheung AK, Wanner C. Empagliflozin improves kidney outcomes in patients with or without heart failure. Circ Heart Fail. 2019;12:e005875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461-2498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 797] [Article Influence: 113.9] [Reference Citation Analysis (0)] |
| 12. | Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, D'Alessio DA, Davies MJ. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63:221-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 342] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 13. | Alicic RZ, Neumiller JJ, Johnson EJ, Dieter B, Tuttle KR. Sodium-glucose cotransporter 2 inhibition and diabetic kidney disease. Diabetes. 2019;68:248-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 14. | Davidson JA. SGLT2 inhibitors in patients with type 2 diabetes and renal disease: overview of current evidence. Postgrad Med. 2019;131:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Lin JS, Susztak K. Podocytes: the Weakest Link in Diabetic Kidney Disease? Curr Diab Rep. 2016;16:45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 16. | Yu SM, Leventhal JS, Cravedi P. Totally tubular, dude: rethinking DKD pathogenesis in the wake of SGLT2i data. J Nephrol. 2020;:. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Cassis P, Locatelli M, Cerullo D, Corna D, Buelli S, Zanchi C, Villa S, Morigi M, Remuzzi G, Benigni A, Zoja C. SGLT2 inhibitor dapagliflozin limits podocyte damage in proteinuric nondiabetic nephropathy. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 18. | Garg P. A Review of podocyte biology. Am J Nephrol. 2018;47 Suppl 1:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 19. | Korbut AI, Klimontov VV, Vinogradov IV, Romanov VV. Risk factors and urinary biomarkers of non-albuminuric and albuminuric chronic kidney disease in patients with type 2 diabetes. World J Diabetes. 2019;10:517-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Tojo A, Kinugasa S. Mechanisms of glomerular albumin filtration and tubular reabsorption. Int J Nephrol. 2012;2012:481520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Oraby MA, El-Yamany MF, Safar MM, Assaf N, Ghoneim HA. Dapagliflozin attenuates early markers of diabetic nephropathy in fructose-streptozotocin-induced diabetes in rats. Biomed Pharmacother. 2019;109:910-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Harris RB. Direct and indirect effects of leptin on adipocyte metabolism. Biochim Biophys Acta. 2014;1842:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 203] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 23. | Flak JN, Myers MG Jr. Minireview: CNS mechanisms of leptin action. Mol Endocrinol. 2016;30:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 128] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Hussain Z, Khan JA. Food intake regulation by leptin: Mechanisms mediating gluconeogenesis and energy expenditure. Asian Pac J Trop Med. 2017;10:940-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Korbut AI, Klimontov VV, Orlov NB, Khotskina AS, Zav'yalov EL. Relationships between body composition and plasma levels of pancreatic, gut, and adipose tissue hormones in db/db mice, a model of type 2 diabetes mellitus. Bull Exp Biol Med. 2019;167:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 26. | Gallo LA, Ward MS, Fotheringham AK, Zhuang A, Borg DJ, Flemming NB, Harvie BM, Kinneally TL, Yeh SM, McCarthy DA, Koepsell H, Vallon V, Pollock C, Panchapakesan U, Forbes JM. Once daily administration of the SGLT2 inhibitor, empagliflozin, attenuates markers of renal fibrosis without improving albuminuria in diabetic db/db mice. Sci Rep. 2016;6:26428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 27. | Ojima A, Matsui T, Nishino Y, Nakamura N, Yamagishi S. Empagliflozin, an inhibitor of sodium-glucose cotransporter 2 exerts anti-inflammatory and antifibrotic effects on experimental diabetic nephropathy partly by suppressing AGEs-receptor axis. Horm Metab Res. 2015;47:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 28. | Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol. 2003;284:F1138-F1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 383] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 29. | Cohen MP, Clements RS, Hud E, Cohen JA, Ziyadeh FN. Evolution of renal function abnormalities in the db/db mouse that parallels the development of human diabetic nephropathy. Exp Nephrol. 1996;4:166-171. [PubMed] [DOI] [Full Text] |
| 30. | Aroor AR, Das NA, Carpenter AJ, Habibi J, Jia G, Ramirez-Perez FI, Martinez-Lemus L, Manrique-Acevedo CM, Hayden MR, Duta C, Nistala R, Mayoux E, Padilla J, Chandrasekar B, DeMarco VG. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc Diabetol. 2018;17:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 125] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 31. | Kamezaki M, Kusaba T, Komaki K, Fushimura Y, Watanabe N, Ikeda K, Kitani T, Yamashita N, Uehara M, Kirita Y, Shiotsu Y, Sakai R, Fukuda T, Yamazaki M, Fukui M, Matoba S, Tamagaki K. Comprehensive renoprotective effects of ipragliflozin on early diabetic nephropathy in mice. Sci Rep. 2018;8:4029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Motrapu M, Świderska MK, Mesas I, Marschner JA, Lei Y, Martinez Valenzuela L, Fu J, Lee K, Angelotti ML, Antonelli G, Romagnani P, Anders HJ, Anguiano L. Drug testing for residual progression of diabetic kidney disease in mice beyond therapy with metformin, ramipril, and empagliflozin. J Am Soc Nephrol. 2020;31:1729-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Langham RG, Kelly DJ, Cox AJ, Thomson NM, Holthöfer H, Zaoui P, Pinel N, Cordonnier DJ, Gilbert RE. Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: effects of angiotensin converting enzyme inhibition. Diabetologia. 2002;45:1572-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 161] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Toyoda M, Suzuki D, Umezono T, Uehara G, Maruyama M, Honma M, Sakai T, Sakai H. Expression of human nephrin mRNA in diabetic nephropathy. Nephrol Dial Transplant. 2004;19:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Denhez B, Geraldes P. Regulation of nephrin phosphorylation in diabetes and chronic kidney injury. Adv Exp Med Biol. 2017;966:149-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Chang AS, Hathaway CK, Smithies O, Kakoki M. Transforming growth factor-β1 and diabetic nephropathy. Am J Physiol Renal Physiol. 2016;310:F689-F696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 37. | Huang H, You Y, Lin X, Tang C, Gu X, Huang M, Qin Y, Tan J, Huang F. Inhibition of TRPC6 signal pathway alleviates podocyte injury induced by TGF-β1. Cell Physiol Biochem. 2017;41:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Wang XB, Zhu H, Song W, Su JH. Gremlin regulates podocyte apoptosis via transforming growth factor-β (TGF-β) pathway in diabetic nephropathy. Med Sci Monit. 2018;24:183-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 39. | Desouza CV, Holcomb RG, Rosenstock J, Frias JP, Hsia SH, Klein EJ, Zhou R, Kohzuma T, Fonseca VA. Results of a study comparing glycated albumin to other glycemic indices. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1586] [Cited by in RCA: 3127] [Article Influence: 625.4] [Reference Citation Analysis (1)] |
| 41. | Neeland IJ, McGuire DK, Chilton R, Crowe S, Lund SS, Woerle HJ, Broedl UC, Johansen OE. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13:119-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 42. | Lin B, Koibuchi N, Hasegawa Y, Sueta D, Toyama K, Uekawa K, Ma M, Nakagawa T, Kusaka H, Kim-Mitsuyama S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc Diabetol. 2014;13:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 312] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 43. | Terami N, Ogawa D, Tachibana H, Hatanaka T, Wada J, Nakatsuka A, Eguchi J, Horiguchi CS, Nishii N, Yamada H, Takei K, Makino H. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One. 2014;9:e100777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 262] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 44. | Takasu T, Takakura S. Protective effect of ipragliflozin on pancreatic islet cells in obese type 2 diabetic db/db mice. Biol Pharm Bull. 2018;41:761-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Falkenberg CV, Azeloglu EU, Stothers M, Deerinck TJ, Chen Y, He JC, Ellisman MH, Hone JC, Iyengar R, Loew LM. Fragility of foot process morphology in kidney podocytes arises from chaotic spatial propagation of cytoskeletal instability. PLoS Comput Biol. 2017;13:e1005433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Korbut AI, Taskaeva IS, Bgatova NP, Muraleva NA, Orlov NB, Dashkin MV, Khotskina AS, Zavyalov EL, Konenkov VI, Klein T, Klimontov VV. SGLT2 inhibitor empagliflozin and DPP4 inhibitor linagliptin reactivate glomerular autophagy in db/db mice, a model of type 2 diabetes. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 47. | Packer M. Role of impaired nutrient and oxygen deprivation signaling and deficient autophagic flux in diabetic CKD development: implications for understanding the effects of sodium-glucose cotransporter 2-inhibitors. J Am Soc Nephrol. 2020;31:907-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |