Published online Dec 15, 2020. doi: 10.4239/wjd.v11.i12.572
Peer-review started: July 9, 2020
First decision: October 6, 2020
Revised: October 20, 2020
Accepted: November 11, 2020
Article in press: November 11, 2020
Published online: December 15, 2020
Processing time: 156 Days and 11.7 Hours
SX-fraction (SXF) is a bioactive glycoprotein with anti-diabetic and hypoglycemic activities that have been documented in several reports. We have reviewed those studies herein and also explored the possible mechanism of its hypoglycemic activity. The early animal studies of SXF using diabetic mice showed the significant reduction in the three diabetic parameters, serum glucose, insulin, and triglyceride, suggesting its anti-diabetic activity. The limited clinical studies also showed that SXF led to the significant reduction in the fasting blood glucose levels of type 2 diabetic patients within 2 wk or a month, suggesting its hypoglycemic activity. To explore the hypoglycemic mechanism of SXF, its possible effects on the insulin signal transduction pathway was examined in vitro. Particularly, activities of insulin receptor, insulin receptor substrate 1, and protein kinase B, which are essential elements playing a key regulatory role in the signal pathway, were studied using skeletal muscle L6 cells. The status of these three parameters were examined under a high glucose (35 mmol/L) milieu with SXF and assessed using the enzyme-linked immunosorbent assay. Such studies revealed that all three parameters (insulin receptor, insulin receptor substrate 1, and protein kinase B) were inactivated by high glucose, indicating a disruption of the signal pathway. However, such an inactivation was reversed or re-activated by SXF to successfully carry out the sequential signaling events. In fact, a measurement of glucose uptake in cells showed that SXF did increase a glucose uptake while high glucose decreased it. Therefore, SXF has anti-diabetic and hypoglycemic activities through activation of the insulin signal pathway and appears to be a safe, natural agent for lowering the serum glucose levels in type 2 diabetic patents and improving their diabetic conditions.
Core Tip: We have been searching for a natural agent capable of controlling diabetic conditions in patients with type 2 diabetes for years. We then came across “SX-fraction (SXF)”, isolated from maitake mushroom (Grifola frondorsa), with anti-diabetic and hypoglycemic activities. In this short review, we chronologically described several key studies of SXF such as animal studies using diabetic mice (to demonstrate anti-diabetic activity), limited clinical studies on volunteer patients with type 2 diabetes (to show hypoglycemic activity), and in vitro study using skeletal muscle cells (to elucidate the hypoglycemic mechanism). After all, SXF appears to be a natural, safe, effective agent for treatment of patients with type 2 diabetes, although more (clinical) studies are yet required for confirmation.
- Citation: Konno S. SX-fraction: Promise for novel treatment of type 2 diabetes. World J Diabetes 2020; 11(12): 572-583
- URL: https://www.wjgnet.com/1948-9358/full/v11/i12/572.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i12.572
Diabetes mellitus, or simply diabetes, is a metabolic ailment of steady high blood glucose condition (hyperglycemia) that affected over 34.2 million Americans (10.5% of the United States population) in 2018[1]. Those include 26.9 million diagnosed cases and 7.3 million undiagnosed cases and among diagnosed people, men (14 million) had a slightly higher prevalence than women (12.8 million)[1]. This is a chronic disease with high blood glucose level, which can cause various adverse clinical complications, including retinopathy, neuropathy, and nephropathy. If left untreated, it could then lead to blindness, renal failure, amputation, coma, and even death[2,3]. It is not a simple physiological disorder and requires medical attention.
The two types of diabetes, type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes[4], are commonly represented. Type 1 diabetes is primarily attributed to insulin deficiency, representing < 20% of all cases of diabetes[5], whereas type 2 diabetes has a > 80% incidence rate, involving multiple factors such as insufficient insulin secretion, peripheral insulin resistance (in muscle and adipose tissue), and excessive hepatic glucose production[5,6]. Hence, type 2 diabetes with such complex etiologies seems to be more prevalent than type 1 diabetes. Regarding treatment, type 1 diabetes due primarily to insulin deficiency (from dysfunction of pancreatic β-cells) can be better managed through insulin injection than type 2 diabetes. On the other hand, insulin deficiency is not the primary problem in many type 2 diabetic patients but peripheral insulin resistance is rather a major factor for their hyperglycemic conditions, making it more difficult to treat[6]. Because of such insulin resistance, for instance, sulfonylurea derivatives[7] that are currently used in oral therapy to stimulate insulin secretion (from pancreatic β-cells) have often failed to show the expected efficacy and outcomes. Other pharmaceuticals, such as troglitazone[8] and metformin[9,10], have been also used to enhance peripheral insulin sensitivity. Although these drugs led to some improvements in a glycemic control, several adverse effects also drew attention. Metformin was found to have potential adverse effects such as lactic acidosis due to renal impairment, sepsis, septic shock, cardiac arrest, or liver failure[9]. Similarly, troglitazone has been taken off the United States market, due to severe hepatoxicity[8].
Hence, there are yet no specific and effective regimens for treatment of type 2 diabetes even after all these years. At least, we now understand that more effective modalities rely primarily on how to overcome insulin resistance. In fact, it is urgently and strongly demanded to implement specific therapeutic modalities or find safer and more effective agents to abolish insulin resistance, eventually alleviating complicated diabetic conditions.
We have been seeking for some natural products/agents with hypoglycemic activity and came across a mushroom extract known as “SX-fraction (SXF)”, isolated from maitake mushroom (Grifola frondosa). SXF with an approximately 20000 Da molecular weight is a water-soluble glycoprotein and a number of scientific and medical studies have been performed on SXF. Those include diabetic mice and limited clinical studies of type 2 diabetic patients, which demonstrated hypoglycemic and anti-diabetic activities of SXF[11-14]. We chronologically described those studies herein and also explored the possible hypoglycemic mechanism of SXF, which had not been yet fully understood. Our working hypothesis was that SXF might stimulate or activate the dysfunctional insulin signal transduction pathway[15]. It is rather an oversimplified scheme depicting that SXF might primarily act on the signal pathway, which carries out a cascade of biochemical events and eventually leads to the increase in glucose uptake by insulin responsive cells. We then focused on the possible status changes in the three key diabetic parameters, such as insulin receptor (IR)[16], insulin receptor substrate 1 (IRS-1)[17], and protein kinase B (Akt)[18]. More details are described later on.
Before we start discussing the SXF studies, it is worthwhile mentioning a little more about “maitake mushroom” because it is where SXF originally comes from. This mushroom is not just another mushroom people are familiar with. It is an edible, tasty, medicinal mushroom, which has been available by cultivation since the mid-1980s. This mushroom is often used in cooking and also believed to provide us with certain health benefits, which have been claimed in anecdotes and folklore. Nevertheless, a number of scientific and medical studies have been extensively performed on “Maitake D-fraction (PDF)”[19], a bioactive extract from maitake mushroom, in the past 35 years. Such studies then revealed or postulated a number of physiological benefits including immunostimulatory, anticancer/antitumor, and antiviral effects[20-23], and potential treatments of hypertension, hypercholesterolemia, obesity etc.[24-26]. Particularly, it is significant that its antiviral activity on human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) has been confirmed by the United States National Cancer Institute in 1992[23]. In addition, apoptosis-inducing activity of PDF has been also demonstrated in human prostate cancer cells in vitro[27,28]. Most importantly, as far as the safety of PDF is concerned, the United States Food and Drug Administration has exempted PDF from a phase I toxicology study and also approved it for the investigational new drug application allowing a phase II pilot study on those patients with advanced breast and prostate cancers[29]. Moreover, a randomized clinical study of PDF on healthy subjects (non-cancerous participants) reported no apparent adverse effects on any participants[30]. Thus, these findings support the safety of PDF without any potential side effects, granting its use in cancer patients as well as normal healthy people.
Similar to PDF, we now have SXF with potential anti-diabetic and hypoglycemic activities, which has been also isolated from maitake mushroom. Accordingly, we have reviewed the available data from the early and recent SXF studies, providing with essential/valuable information on SXF in terms of its safety and potential efficacy on patients with type 2 diabetes.
Regarding the terms used for “SXF” in this review, it should be advised that SXF currently available today has been developed and formulated several times in the past. Initially, it was called a whole maitake powder containing SXF component, followed by a crude SXF (20%-feed), a formulated SXF, maitake powder caplet, and current SXF. To avoid any confusions with these names, we purposely and nearly exclusively used the term, SXF, throughout this review. No matter how its name has been changed, the key active ingredient responsible for its anti-diabetic and hypoglycemic activities was “SXF” presented in all former products. Hence, although you may find these different names in the literatures, the outcomes of such studies were indeed attributed to SXF after all.
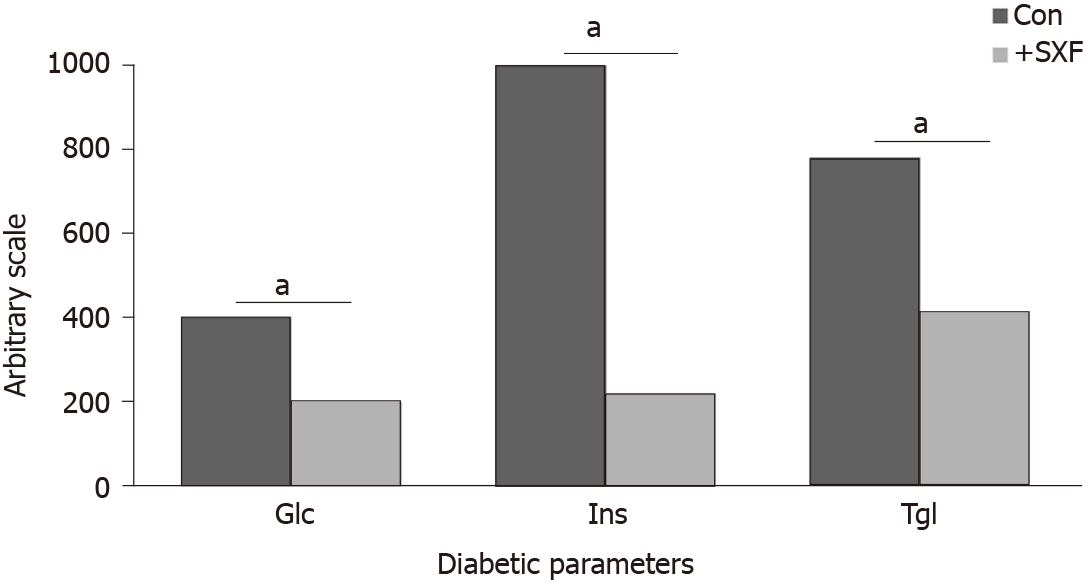
First of all, SXF and other similar materials/products used in all studies described herein were primarily provided by the manufacturer (Mushroom Wisdom, Inc., East Rutherford, NJ, United States). In this study[11], diabetic mice were equally divided into the two groups: the experimental group received both normal feed and crude SXF (20%-feed) daily, while the control (sham) group consumed only normal feed. After 8 wk, compared to the control group, the experimental group showed significant improvements in the three diabetic parameters, blood glucose (Glc), insulin (Ins), and triglyceride (Tgl) levels. The Glc, Ins, and Tgl levels were 400 mg/dL, 1200 µU/mL, and 780 mg/dL, respectively, in the control group, whereas those were 200 mg/mL, 220 µU/mL, and 410 mg/dL, respectively, in the experimental group (Figure 1). Thus, such a significant decrease in these diabetic parameters with SXF indicates its anti-diabetic effect in diabetic mice.
The study was continued with a “feed-switch” of crude SXF supply between the two former groups. The former control group now received crude SXF while the former experimental (with SXF) group served as the control. This “switch” continued for 4 wk to eliminate the possibility of body weight that could affect the outcomes because changes in body weights were actually seen during the previous (8-wk) study period. It became clear even only 1 wk later that all three parameters were significantly improved or lowered with SXF supplement, whereas they sharply elevated once SXF was deprived. However, no changes in body weights were detected this time (4-wk period). For instance, Glc of the former control group decreased from 400 mg/dL to 155 (with SXF) but that of the former experimental group increased from 180 to 365 (without SXF). All these changes in parameters are summarized in Table 1. Therefore, this study confirmed that SXF has indeed improved all diabetic parameters, supporting the notion that SXF is a potential anti-diabetic agent.
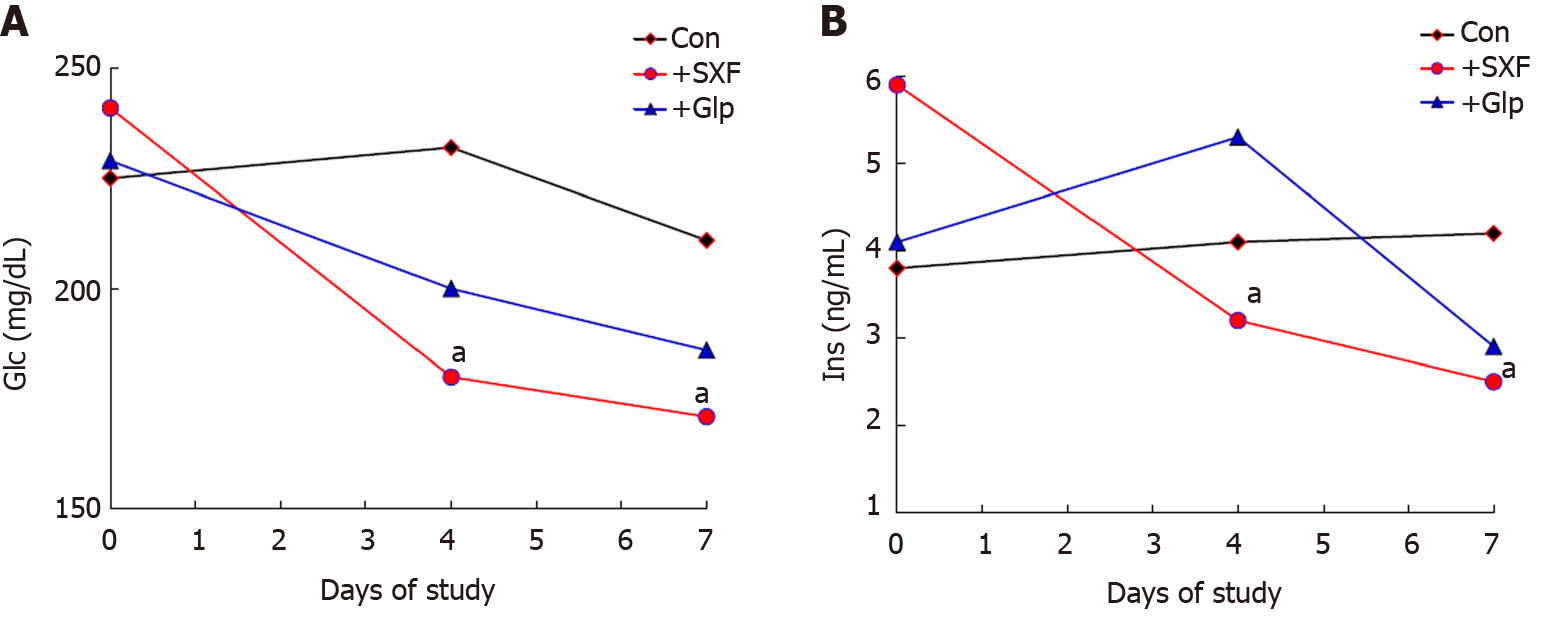
In the next study[12], as SXF has a direct/primary impact on diabetic conditions (in mice), whether it could be as effective as (or better than) an anti-diabetic drug clinically used was then assessed. Glipizide (Glp), one of oral medications, was chosen to compare with formulated SXF. Diabetic mice were divided into the three groups as the control, the SXF, and the Glp groups. Specified amounts of SXF or Glp were separately given to mice daily for 7 d (1 wk), and the two diabetic parameters, Glc and Ins, were measured at Days 0, 4, and 7. As shown in Figure 2A, the Glc levels in the SXF group were significantly (P < 0.05) lower than those of the control group at Days 4 and 7. Although those of the Glp group also declined, the difference was not statistically significant (P > 0.06). Similarly, the Ins levels at Days 4 and 7 were also significantly (P < 0.05) lowered in the SXF group, while those values of the Glp were not significantly lowered (Figure 2B). Thus, these results show that SXF is capable of effectively and significantly lowering the Glc and Ins levels in mice within a week, improving their diabetic conditions. This finding also demonstrates that “SXF” is practically the key ingredient with anti-diabetic activity because the two different forms of SXF, formulated SXF (used in this study) and crude SXF (in the early studies above), yet showed the consistent results. Moreover, this study also indicates that SXF appears to have a better efficacy than an oral anti-diabetic drug (Glp) tested.
Taken together, these animal studies show that SXF does have anti-diabetic activity, capable of directly lowering the Glc, Ins, and Tgl levels, as evidenced by the fact that a withdrawal of SXF immediately raised those parameters back to the diabetic state within a week. Additionally, SXF appears to more effectively work on diabetic conditions (in mice) than Glp, by adequately controlling glucose/insulin metabolism without any side effects. Although the potential (long-term) side effects of Glp have been rarely documented at present, such a possibility cannot be utterly ruled out. This further assures that SXF is safer and more effective (than a drug) on ameliorating diabetic conditions presumably in type 2 diabetic patients. Nonetheless, as more tests/studies are required for confirming the actual efficacy of SXF on those patients, especially more clinical studies should be actively conducted.
Encouraged by the early animal studies described above, some clinical case/studies were then conducted to assess the possible hypoglycemic effect of SXF on several volunteers with type 2 diabetes (25 to 75 years old).
The first case study[13] presents a complete glycemic control with SXF in a newly diagnosed male patient with type 2 diabetes. He had the initial fasting blood glucose (FBG) value of 248 mg/dL (the normal FBG range of 55-115 mg/dL) with the glycosylated hemoglobin A1c (HbA1c) level of 11.5% (the normal HbA1c range of 5%-7.5%). No diabetes-related complications such as retinopathy, neuropathy etc. had yet developed. He immediately received oral glyburide (2.5 mg)[7] daily and his FBG level declined to approximately 180 mg/dL over the next 2 d. He then started also taking a SXF tablet daily with glyburide and his FBG dramatically declined to approximately 100 mg/dL in a couple of days and remained at nearly 80-90 mg/dL for the next 3 mo. At the same time, his HbA1c also went down to normal 5.2% (from initial 11.5%). Glyburide was next cut down to a half (1.25 mg) with daily SXF, and his FBG levels remained similarly at 80-90 mg/dL for another 2 mo. Eventually, he was kept on daily SXF only while glyburide was completely withdrawn; however, his FBG levels yet remained at approximately 90 mg/dL and his HbA1c also remained at normal 5.6% over 6 mo. This patient currently remains normoglycemic over 20 years only with a daily SXF intake since his initial diagnosis. His chronological improvements with SXF are summarized in Table 2.
| Diagnosis/Prognosis | FBG (mg/dL) | HbA1c (%) |
| Initial diagnosis | 248 | 11.5 |
| 3rd month prognosis (with 2.5 mg glyburide and SXF) | 80-90 | 5.2 |
| 5th month prognosis (with 1.25 mg glyburide and SXF) | 80-90 | N/D |
| 9th month prognosis (with SXF only) | Approximately 90 | 5.6 |
Another case study included 7 volunteers with type 2 diabetes who received a SXF tablet three times a day for 4 wk. Their FBS levels were measured at a beginning of a SXF trial (Day 0), 2nd wk (Day 14), and 4th wk (Day 28). What percent (%) of FBG declined with SXF (if any) was also calculated by the differences of FBG values between Day 0 and Day 28. We found that all 7 patients demonstrated the significant decreases in their FBG levels, with over 30% (30%-63%) decline, under a SXF regimen in 2 to 4 wk (Table 3). Actually, we found that the apparent improvements or decreases in the FBG levels were seen in 2 wk after the first SXF intake, although those values yet kept gradually declining until 4 wk. Additionally, no participants have presented any palpable ailments associated with SXF during this trial, further confirming its safety in human use without adverse effects. Therefore, these results imply that SXF has a hypoglycemic activity, capable of effectively lowering the FBG levels of type 2 patients.
| Patients | Age (yr) | Sex | FBG (mg/dL) Before SXFa | After SXFa | % of FBGdeclined with SXF |
| A | 44 | M | Approximately 260 | 90-100 | Approximately 63 |
| B | 75 | F | Approximately 200 | 110-130 | Approximately 40 |
| C | 25 | F | 150-180 | 110-120 | Approximately 30 |
| D | 37 | M | 180-200 | 120-140 | Approximately 32 |
| E | 64 | F | Approximately 220 | 130-150 | Approximately 37 |
| F | 41 | M | Approximately 210 | 100-110 | Approximately 50 |
| G | 53 | F | 170-190 | 100-110 | Approximately 42 |
Overall, theses limited clinical studies demonstrated the hypoglycemic effect of SXF on actual type 2 diabetic patients. Once patients started a SXF regimen, its hypoglycemic effect could be seen within 2 wk to a month. Moreover, SXF can be taken safely with medications without any contraindications since all participated patients were also taking some oral medications with few adverse effects during this SXF trial. Interestingly, we were able to follow up the two patients, the first case patient and one of 7 additional patients, and they are still normoglycemic today (2020) even without taking SXF. Unfortunately, although we have lost a contact with the rest of 6 patients, we are confident that they are doing well as long as they keep taking daily SXF. We then wonder if SXF might have cured diabetes in those two patients after they took SXF daily over 10 years. Is it possible that a long term (over 10 years) intake of SXF may cure diabetes? As we have only the two valid cases, we could speculate but are yet unable to draw an affirmative conclusion at this point. Additional studies and evidences are undoubtedly required for confirmation. However, these findings are indeed noteworthy and will give us a hope and promise of SXF.
Animal and clinical studies of SXF[11-14] tend to support the anti-diabetic and hypoglycemic effects of SXF for prevention and/or treatment of type 2 diabetes. However, it is yet important to understand or elucidate how it actually works, i.e., the hypoglycemic mechanism of SXF, which is not fully understood at present. In fact, although the primary cause of developing type 2 diabetes is due to “insulin resistance” of insulin responsive cells[6], why or how they become insulin resistant remains unknown. Nevertheless, the problem would be substantially solved if insulin resistance were somehow reversed to “insulin response”. We then hypothesized that such insulin resistance could be primarily due to a dysfunction or inactivation of the insulin signal transduction pathway. If SXF were somehow capable of activating this pathway to be functional, insulin resistance might be abolished, becoming insulin responsive/sensitive.
Hence, our study focused on examining if SXF would modulate activities of the three key parameters that play a significant role in the signal pathway, i.e., IR, IRS-1, and Akt[16-18]. Activation of these parameters requires “phosphorylation” or they must be phosphorylated to become active and functional[16]. For such activation, specifically the tyrosine (y) residues in both IR and IRS-1 but the serine (s) residues in Akt should be more or highly phosphorylated[16-18]. Their activation is essentially required for carrying out a cascade of biochemical events in the signal pathway, leading to the increased glucose uptake by cells that eventually lowers the blood glucose level[15]. We thus tested if SXF would be able to actually activate the (dysfunctional) signal pathway.
In this study[31], rat skeletal muscle L6 cells (American Type Culture Collection; Manassas, VA, United States) were used as our in vitro model. They were cultured in Roswell Park Memorial Institute 1640 medium containing 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 mg/mL). We first examined if a high concentration (hyperglycemia) of Glc would affect activities of IR, IRS-1, and Akt in differentiated L6 cells (myotubes). Cells were exposed to high Glc (35 mmol/L) for 24 h and then cultured with SXF (300 µg/mL) or insulin (INS; 100 nmol/L) for 15 min. INS was used as a positive control. After 15 min, all cells were harvested, lysed, and subjected to enzyme-linked immunosorbent assay for IR, IRS-1, and Akt separately.
Enzyme-linked immunosorbent assay was performed following the vender’s protocol (Invitrogen, Carlsbad, CA, United States). Briefly, 40 µg of cell lysates obtained from all cells involved in experiments was introduced to the 96 -well plate coated with antibodies [anti-IR(y), -IRS-1(y), or -Akt(s)] and incubated at room temperature for 24 h to complete the antigen-antibody binding. After removing cell lysates, antibody detection solution was then added to the plate, which was incubated at room temperature for 1 h. After discarding solution, anti-rabbit IgG-HRP conjugate was next added to the plate, followed by 30-min incubation. Chromogen reagent was added to the plate that was incubated for another 30 min. Stop solution was then added to end the colorimetric reaction, and the intensities of (yellow-colored) products were measured at 450 nm in a microplate reader. The phosphorylation levels of IR(y), IRS-1(y), or Akt(s) were assessed relative to that of the respective standard that was run simultaneously. The results are interpreted as – the higher phosphorylation level, the greater activity.
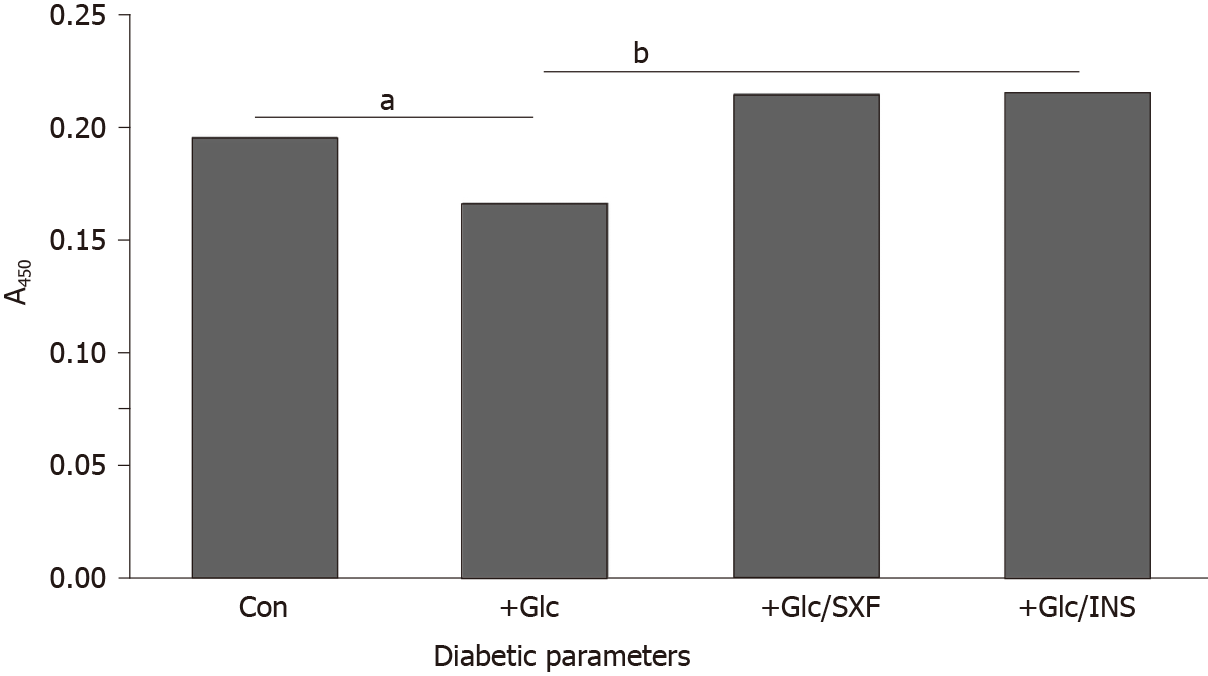
Figure 3 shows that the phosphorylation level of IR(y) in control cells was reduced by approximately 15% with 24 -h Glc (35 mmol/L) exposure, representing the loss of IR activity. However, SXF has elevated the reduced IR level to approximately 10% and approximately 29% higher than controls and Glc-suppressed level, respectively. This elevated IR(y) level with SXF was rather similar to that with INS exposure. Thus, these results indicate that SXF may re-activate Glc-suppressed/inactivated IR to convey the signal to carry on the subsequent events.
A significance of IR should be emphasized again. The IR is a heterotetrameric glycoprotein consisting of two extracellular α-subunits with approximately 135000 Da and two transmembrane β-subunits with 95000 Da linked by disulfide bonds to form a β-α-α-β structure[32,33]. Particularly, the β-subunit of the IR is an insulin-sensitive tyrosine kinase (TK), which can autophosphorylate itself and also phosphorylate tyrosine (y) residues of intracellular substrates such as IRS-1[33]. This activation (via autophosphorylation) of IR is substantially required for the IR to be functional and respond to insulin[33,34]. Activation of IR then triggers activation of downstream molecules such as IRS-1, Akt, and other molecules, to carry on a cascade of signaling events and fulfill the insulin-mediated biological response – i.e. the insulin signal transduction[34,35]. Hence, activation of IR (by insulin) is the most critical step in the insulin signal pathway. However, a marked decrease in TK activity resulted from IR dysfunction has been often found in the livers of type 2 diabetics, and similarly a decreased TK activity was seen in skeletal muscle, adipose tissue, and red blood cells of other patients[33,36]. It thus appears that a significant decrease in TK activity of IR, likely due to an inability of autophosphorylating β-subunits, is a common pheno-menon in most of type 2 diabetics.
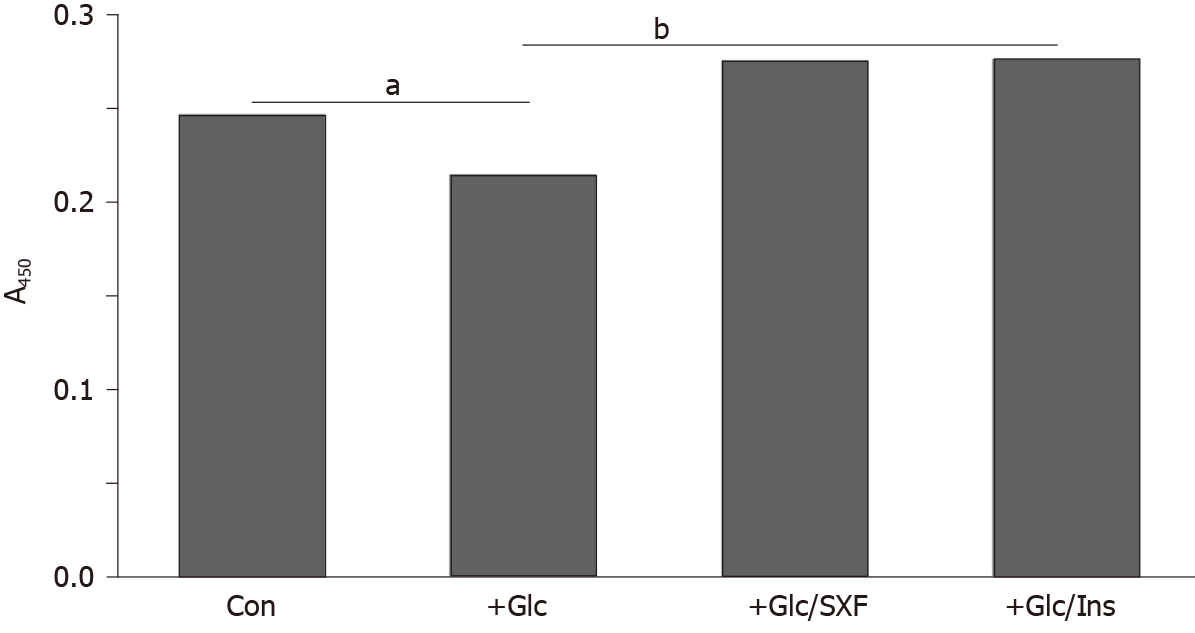
Figure 4 shows that high Glc led to an approximately 12% decrease in IRS-1(y) phosphorylation (compared to controls), suggesting inactivation of IRS-1. SXF was yet capable of increasing the reduced IRS-1(y) level by approximately 11% higher than controls or approximately 20% higher than Glc-suppressed one. This SXF-elevated IRS-1 (y) level was also similarly seen in that with INS. As the increase in (Glc-suppressed) IRS-1(y) phosphorylation with SXF indicates activation of IRS-1, it is feasible that the subsequent signaling events would be carried out routinely.
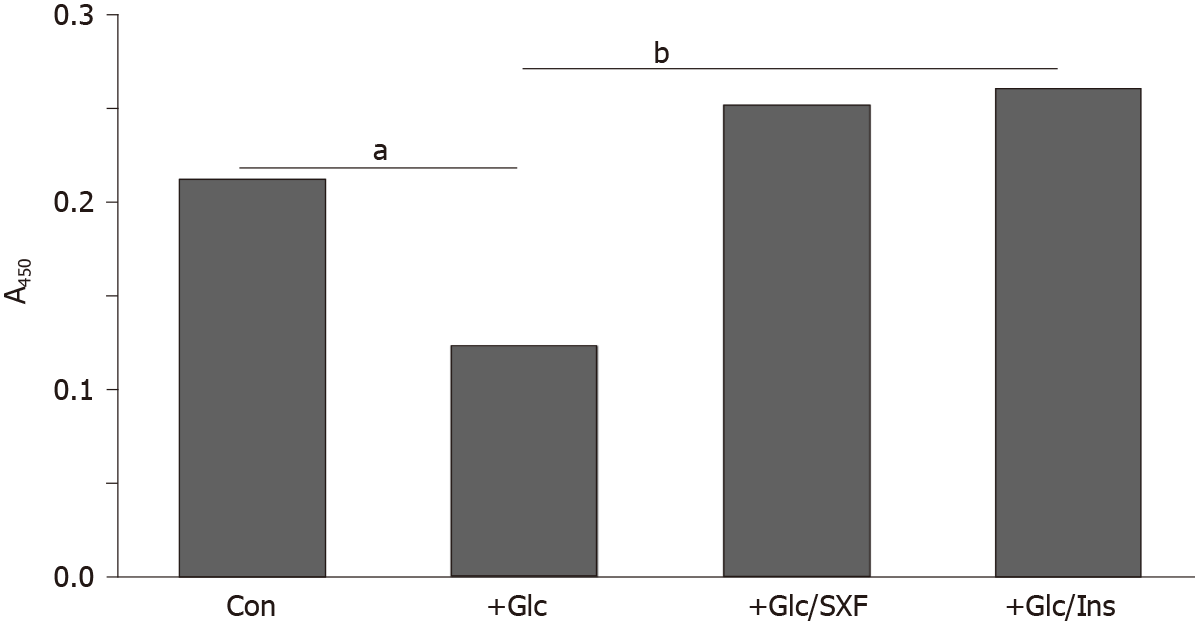
Figure 5 shows an approximately 42% decrease in the Akt(s) phosphorylation level in Glc-treated cells (compared to controls), indicating inactivation of Akt. However, SXF was capable of preventing this Akt inactivation, elevating its decreased level by approximately 2-fold or approximately 18% higher than controls. Similarly, INS also elevated the Glc-suppressed Akt(s) level by approximately 2.1-fold or approximately 22% greater than controls.
This finding points out that Akt plays a critical role in the progress of the successive signaling events. Activation of Akt is succeeded by activated phosphatidylinositol 3-kinase, mediated through activation of IRS-1[15,18,34]. This Akt activation not only allows the signal pathway to carry on but also leads to its last event of glucose uptake[18]. The study here showed that high Glc certainly inactivated Akt but SXF was yet capable of significantly (re)activating it (Figure 4). Thus, it is apparent that the signal pathway was successfully carried on with activated Akt (through phosphatidylinositol 3-kinase activation).
Taken together, although high Glc indeed inactivates IR, shutting down the rest of the signal pathway, SXF is capable of first activating IR, followed by sequential activation of IRS-1 and Akt, to carry on the signaling cascade. This will eventually lead to the translocation of glucose transport-4 (GLUT4)[18,37] to promote glucose uptake.
To confirm if the SXF-activated signal pathway will ultimately facilitate glucose uptake (by insulin-responsive cells), such a study was also performed following the method previously described with minor modifications[38]. Differentiated L6 cells were seeded in the 6-well plate and treated with high Glc (35 mmol/L) for 24 h. After discarding Glc, all wells were washed thoroughly with Krebs-Ringer-Phosphate buffer to remove residual Glc. Cells were briefly cultured with SXF (300 µg/mL) or INS (100 nmol/L) for 15 min. INS was used as a positive control capable of facilitating glucose uptake. To initiate glucose uptake, a radioactive ligand, 2-deoxy-D-[1-3H]-glucose (
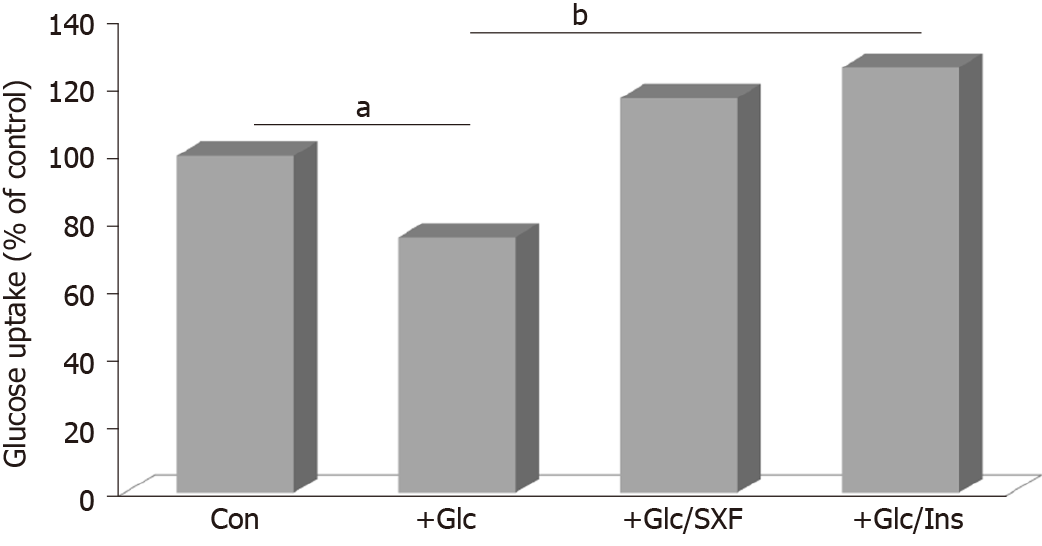
Figure 6 shows that glucose uptake was reduced by approximately 24% with high Glc (compared to controls) but SXF prevented or reversed its reduction, increasing it by approximately 1.5-fold greater than Glc-reduced one or approximately 17% higher than controls. Certainly, INS exhibited the best stimulatory effects of approximately 1.7-fold and approximately 26% higher glucose uptake than Glc-reduced one and controls, respectively. Overall, these results indicate that high Glc can reduce or inhibit glucose uptake (due to an incompletion of the signal pathway), but such a reduction could be reversed or overcome with SXF, subsequently increasing/facilitating glucose uptake (as a successful completion of the signal pathway).
As seen here, the increased glucose uptake can thus indicate the completion of the signal pathway. We observed that glucose uptake was substantially reduced with high Glc, resulting in the incomplete signaling event, whereas SXF was capable of facilitating glucose uptake even under high Glc, indicating the successful, complete signaling event. Actually, such an increased glucose uptake (with SXF) would be closely associated with GLUT4 translocation to the plasma membrane and its localization is known to be essential and required for active glucose uptake[18,37,39]. Although the induced GLUT4 translocation has not been examined in this study, such a study is currently in progress.
Taken all together, one of the important findings here is that high Glc concentration does disrupt the insulin signal pathway by inactivating the key regulatory elements such as IR, IRS-1, and Akt. It implies that the persistently high blood glucose level would cause the signal pathway to become dysfunctional and insulin insen-sitive/resistant, leading subsequently to diabetic manifestation. Another important finding is that SXF is capable of sequentially activating those key regulators to successfully carry out the signal pathway, ultimately facilitating glucose uptake. This will result in lowering the blood glucose level, exhibiting the hypoglycemic effect of SXF. In brief, the signal pathway impaired with high Glc (becoming insulin resistant) could be yet (re)activated by SXF, promoting glucose uptake. Therefore, it is plausible that activation of the signal pathway with SXF may principally account for its underlying hypoglycemic mechanism. Further confirmation yet requires for additional studies.
In the meantime, the key question is to promptly find “what agents” would specifically stimulate or (re)activate the (dysfunctional) IR to trigger and successfully carry out the entire insulin signal pathway. In other words, we must actively pursue the finding of the effective means and/or agents/substances capable of activating IR(y) to execute the signal pathway for a better glucose control (in type 2 patients). At least, SXF appears to be such a promising candidate at present.
Animal studies and limited clinical studies support the anti-diabetic and hypoglycemic effects of SX-fraction on diabetic mice and type 2 diabetic patients. The possible hypoglycemic mechanism of SX-fraction seems to be primarily associated with activation of the insulin signal pathway. Particularly, activation of insulin receptor with SX-fraction is indeed the most critical step that triggers a cascade of signaling events. Meanwhile, more organized clinical studies on SX-fraction should be actively and widely conducted to validate the safety and efficacy of SX-fraction as a potential hypoglycemic agent for patients with type 2 diabetes. Such studies are thus warranted.
I thank Donna Noonan for a generous gift of SX-fraction and other related materials.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang D S-Editor: Zhang L L-Editor: A P-Editor: Ma YJ
| 1. | Centers for Diabetes Control and Prevention. National Diabetes Statistics Report 2020. Available from: https://www.cdc.gov/diabetes/library/features/diabetes-stat-report.html. |
| 2. | Alper J. Biomedicine. New insights into type 2 diabetes. Science. 2000;289:37-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Wagman AS, Nuss JM. Current therapies and emerging targets for the treatment of diabetes. Curr Pharm Des. 2001;7:417-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35:S11-S63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 969] [Cited by in RCA: 1365] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 5. | Caro JF. Effects of glyburide on carbohydrate metabolism and insulin action in the liver. Am J Med. 1990;89:17S-25S; discussion 51S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Groop LC. Insulin resistance: the fundamental trigger of type 2 diabetes. Diabetes Obes Metab. 1999;1 Suppl 1:S1-S7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Groop LC. Sulfonylureas in NIDDM. Diabetes Care. 1992;15:737-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 279] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. N Engl J Med. 1994;331:1188-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 635] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15:755-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 406] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995;333:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 937] [Cited by in RCA: 879] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 11. | Kubo K, Aoki H, Nanba H. Anti-diabetic activity present in the fruit body of Grifola frondosa (Maitake). I. Biol Pharm Bull. 1994;17:1106-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Manohar V, Talpur NA, Echard BW, Lieberman S, Preuss HG. Effects of a water-soluble extract of maitake mushroom on circulating glucose/insulin concentrations in KK mice. Diabetes Obes Metab. 2002;4:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Konno S, Tortorelis DG, Fullerton SA, Samadi AA, Hettiarachchi J, Tazaki H. A possible hypoglycaemic effect of maitake mushroom on Type 2 diabetic patients. Diabet Med. 2001;18:1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Preuss HG, Echard B, Bagchi D, Perricone NV, Zhuang C. Enhanced insulin-hypoglycemic activity in rats consuming a specific glycoprotein extracted from maitake mushroom. Mol Cell Biochem. 2007;306:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3583] [Cited by in RCA: 3651] [Article Influence: 152.1] [Reference Citation Analysis (0)] |
| 16. | Youngren JF. Regulation of insulin receptor function. Cell Mol Life Sci. 2007;64:873-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 125] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296:E581-E591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 395] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 18. | Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372-31378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 974] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 19. | Mizuno T, Zhuang C. Maitake, Grifola frondosa: Pharmacological effects. Food Rev Int. 1995;11:135-149. [RCA] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Adachi K, Nanba H, Kuroda H. Potentiation of host-mediated antitumor activity in mice by beta-glucan obtained from Grifola frondosa (maitake). Chem Pharm Bull (Tokyo). 1987;35:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Hishida I, Nanba H, Kuroda H. Antitumor activity exhibited by orally administered extract from fruit body of Grifola frondosa (maitake). Chem Pharm Bull (Tokyo). 1988;36:1819-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Gu CQ, Li J, Chao FH. Inhibition of hepatitis B virus by D-fraction from Grifola frondosa: synergistic effect of combination with interferon-alpha in HepG2 2.2.15. Antiviral Res. 2006;72:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | National Cancer Institute, Developmental Therapeutics Program. In-vitro anti-HIV drug screening results (National Science Council F195001). January, 1992. |
| 24. | Adachi K, Nanba H, Otsuka M, Kuroda H. Blood pressure-lowering activity present in the fruit body of Grifola frondosa (maitake). I. Chem Pharm Bull (Tokyo). 1988;36:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Kubo K, Nanba H. The effect of maitake mushrooms on liver and serum lipids. Altern Ther Health Med. 1996;2:62-66. [PubMed] |
| 26. | Nakai R, Masui H, Horio H, Ohtsuru M. Effect of maitake (Grifola frondosa) water extract on inhibition of adipocyte conversion of C3H10T1/2B2C1 cells. J Nutr Sci Vitaminol (Tokyo). 1999;45:385-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Fullerton SA, Samadi AA, Tortorelis DG, Choudhury MS, Mallouh C, Tazaki H, Konno S. Induction of apoptosis in human prostatic cancer cells with beta-glucan (Maitake mushroom polysaccharide). Mol Urol. 2000;4:7-13. [PubMed] |
| 28. | Konno S. Synergistic potentiation of D-fraction with vitamin C as possible alternative approach for cancer therapy. Int J Gen Med. 2009;2:91-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Maitake Products, Inc. D-fraction obtained IND for clinical study (corporate publication). Paramus, NJ, February, 1998. |
| 30. | Glauco S, Jano F, Paolo G, Konno S. Safety of maitake D-fraction in healthy subjects: Assessment of common hematologic parameters. Altern Complement Ther. 2004;10:228-230. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Konno S, Alexander B, Zade J, Choudhury M. Possible hypoglycemic action of SX-fraction targeting insulin signal transduction pathway. Int J Gen Med. 2013;6:181-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Jacobs S, Hazum E, Shechter Y, Cuatrecasas P. Insulin receptor: covalent labeling and identification of subunits. Proc Natl Acad Sci. 1979;76:4918-4921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 176] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Qureshi SA, Ding V, Li Z, Szalkowski D, Biazzo-Ashnault DE, Xie D, Saperstein R, Brady E, Huskey S, Shen X, Liu K, Xu L, Salituro GM, Heck JV, Moller DE, Jones AB, Zhang BB. Activation of insulin signal transduction pathway and anti-diabetic activity of small molecule insulin receptor activators. J Biol Chem. 2000;275:36590-36595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol. 2018;19:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 481] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 35. | Kasuga M. Structure and function of the insulin receptor-a personal perspective. Proc Jpn Acad Ser B Phys Biol Sci. 2019;95:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Arner P, Pollare T, Lithell H, Livingston JN. Defective insulin receptor tyrosine kinase in human skeletal muscle in obesity and type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1987;30:437-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Zorzano A, Palacín M, Gumà A. Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol Scand. 2005;183:43-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Prabhakar PK, Doble M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine. 2009;16:1119-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 39. | Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 887] [Article Influence: 73.9] [Reference Citation Analysis (0)] |