Published online Nov 15, 2020. doi: 10.4239/wjd.v11.i11.481
Peer-review started: June 29, 2020
First decision: July 30, 2020
Revised: August 16, 2020
Accepted: October 5, 2020
Article in press: October 5, 2020
Published online: November 15, 2020
Processing time: 136 Days and 20.4 Hours
The coronavirus disease 2019 (COVID-19) outbreak that occurred in late 2019 has posed a huge threat to the health of all humans, especially for individuals who already have diabetes mellitus (DM). DM is one of the most serious diseases that affect human health, with high morbidity and rates of complications. Medical scientists worldwide have been working to control blood sugar levels and the complications associated with sugar level alterations, with an aim to reduce the adverse consequences of acute and chronic complications caused by DM. Patients with DM face great challenges during the pandemic owing to not only changes in the allocation of medical resources but also their abnormal autoimmune status, which reduces their resistance to infections. This increases the difficulty in treatment and the risk of mortality. This review presents, from an epidemiological viewpoint, information on the susceptibility of patients with DM to COVID-19 and the related treatment plans and strategies used in this population.
Core Tip: Diabetes is one of the most common chronic diseases worldwide. Since November 2019, coronavirus disease 2019 has severely affected the routine care of a large number of patients with diabetes, hypertension, or coronary heart disease worldwide. Poor control of blood glucose may lead to secondary infection and acute complications, and the infection may also worsen the blood glucose. Therefore, it is necessary to manage diabetic patients during this period.
- Citation: Lou XQ, Wang DW, Wang JF, Du B. New thoughts on the diagnosis and treatment of patients with diabetes mellitus in relation to coronavirus disease. World J Diabetes 2020; 11(11): 481-488
- URL: https://www.wjgnet.com/1948-9358/full/v11/i11/481.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i11.481
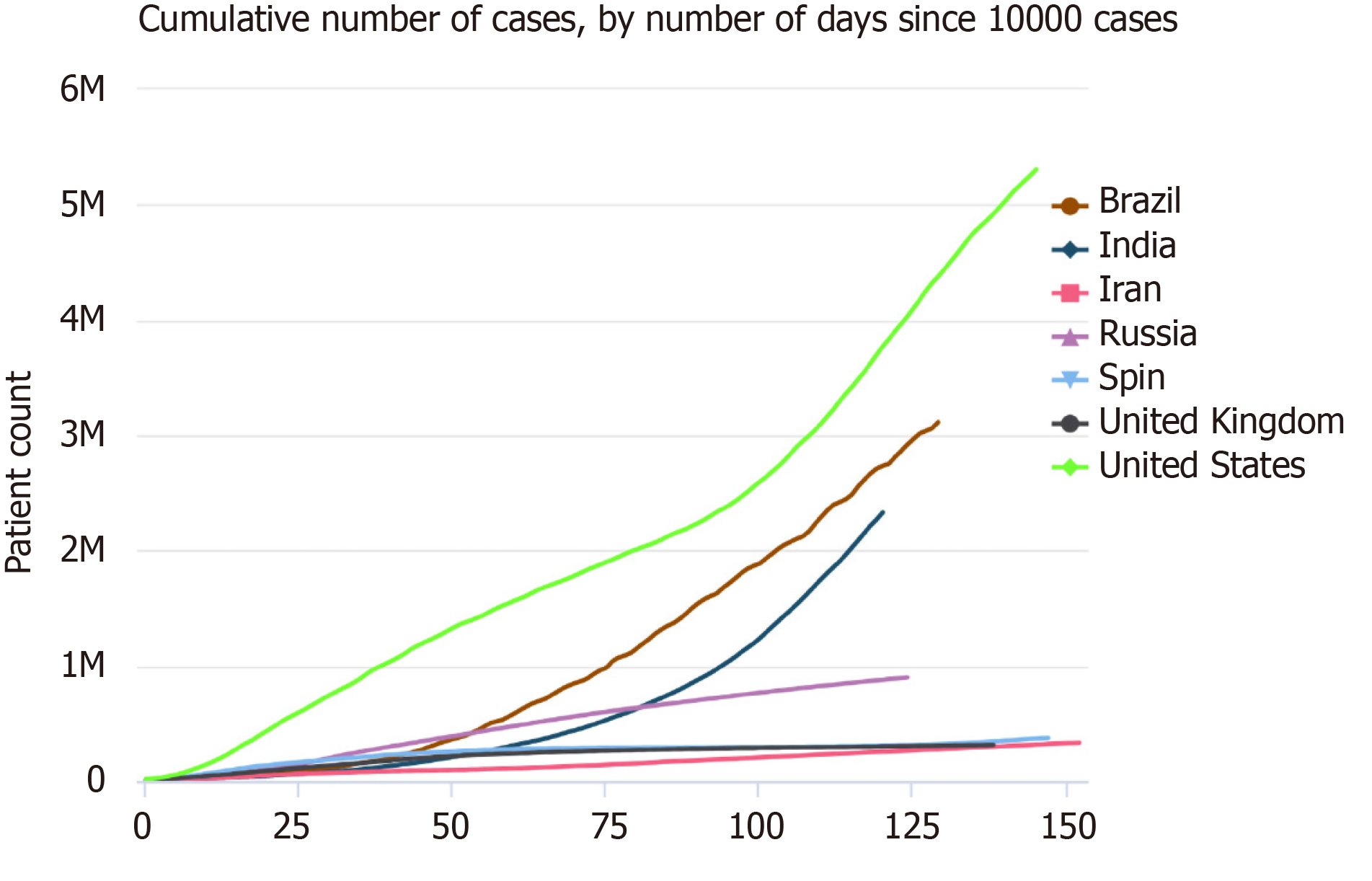
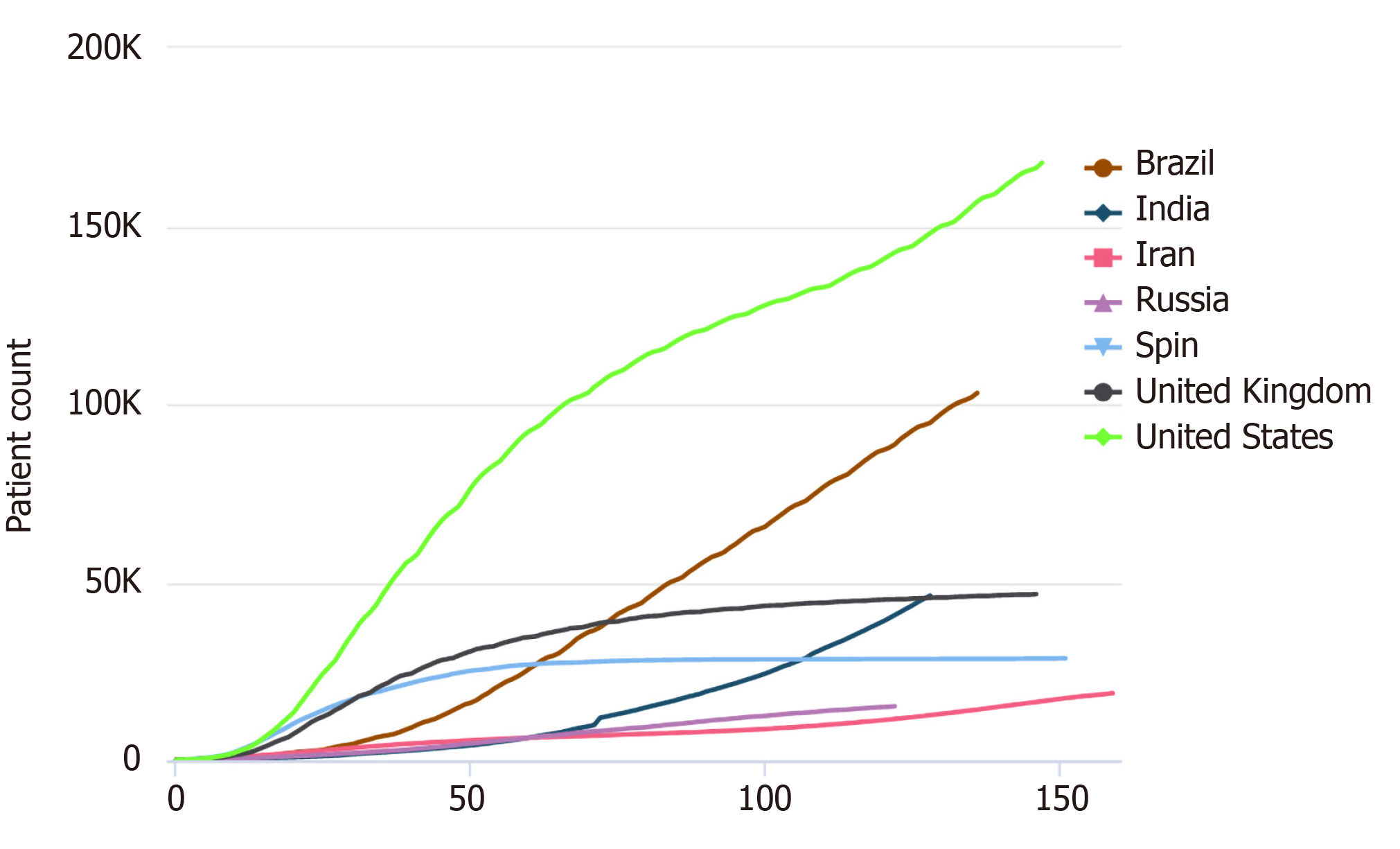
Diabetes mellitus (DM) is one of the most common chronic diseases worldwide. The latest epidemiological studies show that the prevalence rate of DM in Chinese adults is 12.8%; however, this value is not considered to represent the actual prevalence as a large number of individuals are in the pre-DM stage or have not been diagnosed[1]. According to the Global Burden of Disease report published in 2015, the prevalence of DM increased from approximately 333 million to approximately 435 million in 10 years[2], and it is expected to affect 642 million individuals by 2040[3]. Since November 2019, coronavirus disease 2019 (COVID-19) has severely affected the routine care of many patients with DM, hypertension, or coronary heart disease worldwide. According to data from the Chinese Centers for Disease Control and Prevention, there were 89507 cases of COVID-19 in the Chinese mainland and 20553873 cases globally as of August 12, 2020. We were stunned by the real-time statistics from Worldometers, which also show the severity of the current pandemic (Figures 1 and 2; source: https://www.worldometers.info/coronavirus/worldwide-graphs/). Poor control of blood glucose levels may lead to secondary infections and acute complications, and such infections may consequently worsen the blood glucose level. Owing to the delays in medical treatment and blood glucose level monitoring in the current situation, the blood glucose level could fluctuate, and complications may occur or be aggravated. According to statistics from Wuhan, COVID-19 mainly affects male and elderly patients with concurrent pulmonary disease, arterial hypertension, and DM[4]; further, approximately 12%-22% of patients with COVID-19 have DM. Therefore, it is necessary to appropriately manage patients with DM during this period.
During the COVID-19 epidemic, most countries and regions have adopted home isolation, an effective anti-epidemic measure to prevent and control viral transmission. However, it delays the treatment of patients with DM to some extent, especially those who are newly diagnosed with DM. At the same time, the majority of medical resources in most general hospitals in severe epidemic areas have been allocated to support fever clinics and isolation wards; this strategy may reduce the number of patient visits with endocrinologists.
The risk of anxiety and depression in patients with DM is approximately twice as high as that in healthy individuals[5,6]. Owing to the COVID-19 pandemic, tens of thousands of patients have fallen ill and died in many countries, especially elderly patients with chronic diseases; this contributes to increased negative emotions among patients with DM. Anxiety and depression are major risk factors for the aggravation of DM and early emergence of complications[7]. Therefore, the emotional stress caused by the COVID-19 pandemic should be considered seriously.
It is generally believed that patients with DM have a higher incidence of infections than the general population. A study showed that DM was the main risk factor for premature mortality and that in addition to cardiovascular diseases and cancer, infectious diseases contributed significantly to the reduction in life expectancy among patients with DM[8,9]. Clinical studies from Canada and the Netherlands showed that DM increased the susceptibility to lower respiratory tract, urinary tract, skin (bacterial), and mucosal infections[10,11]. Two other studies reported that DM is an independent risk factor for infection-related mortality[12,13]. In response to the COVID-19 outbreak, epidemiological statistics from Wuhan, China revealed that the prevalence rate of DM in patients with COVID-19 pneumonia was 19%[14]. In a later report, the most prevalent comorbidity was hypertension (16.9%), followed by DM (8.2%)[15]. Therefore, we speculate that patients with DM are also susceptible to COVID-19.
There is no clear epidemiological evidence on how the immune responses change in patients with DM. Most studies have revealed that in patients with DM, the function of phagocytic cells, especially polymorphonuclear leukocytes and neutrophils, is impaired in a high glucose state. It has been reported that adhesion and chemotactic function are altered in these patients; as antibacterial activity is impaired, bacterial phagocytosis and killing after stimulation are also reduced[16]. Another study confirmed that T-cell regulatory function is decreased and that the IL-2 signaling pathway is impaired in patients with type 2 DM[17]. An earlier study showed that compared with normal levels, high glucose and high blood sugar levels promote the adhesion of leukocytes to endothelial cells by upregulating cell surface adhesion proteins, which may be related to the activation of NF-kB[18].
Patients with DM should strengthen their awareness of self-control strategies to prevent infection and make self-adjustments to their diets at home based on online medical treatments. At the same time, they should achieve reasonable nutrition collocation and perform moderate exercise to prevent overweight. Patients with DM should also monitor and control their blood glucose levels under the guidance of an endocrinologist and prevent hyperglycemia or hypoglycemia. They should also avoid contact with crowds while traveling and wear masks and gloves rationally and then change them in time.
The treatment of DM requires not only the clinician's mastery of the disease to formulate an appropriate treatment plan but also long-term self-management by the patient. This includes self-monitoring of blood glucose levels and appropriate adjustment of the treatment plan based on the levels obtained. Particularly, during the COVID-19 epidemic, it is necessary to strengthen self-management, which can improve diet control and regularity of exercise to a certain extent. Improving the understanding of DM-related knowledge will help patients scientifically adjust their diet, reasonably distribute calories, and meet the individual requirements of exercise frequency and intensity.
During the COVID-19 epidemic, it is better to obtain medical treatment online, including via telephone consultation services, to reduce traveling and prevent cross-infection in hospitals. In special circumstances, patients could seek medical advice and be treated through the admission system in time.
Owing to the absolute lack of insulin secretion, patients with type 1 DM rely on the injection of exogenous insulin for controlling their blood glucose levels. Therefore, the use of insulin cannot be interrupted during the COVID-19 pandemic. It is suggested that patients reserve a sufficient number of insulin doses to cope with this situation. For patients already diagnosed with COVID-19, it is necessary to strengthen blood glucose level monitoring, reasonably regulate diet, and appropriately adjust the insulin treatment plan. Treatment with an insulin pump or basal insulin combined with prandial insulin is also recommended.
Numerous antidiabetic medicines are currently available for DM treatment and appropriate adjustments of oral glucose-lowering drugs are important. Patients with DM who have well-controlled blood glucose and glycosylated hemoglobin levels may continue their original regimens to lower blood glucose levels. They should pay attention toward monitoring their blood glucose levels regularly. In case of a diagnosis of COVID-19, adjustment of the insulin treatment plan and strengthening of blood glucose level monitoring are recommended. Good control of the blood glucose level is beneficial for recovery from inflammation and the improvement of immunity.
As for the decision regarding whether to continue oral drug treatment during COVID-19, it should be made according to the patient's blood glucose level and the mechanism of action of the oral drug. To the best of our knowledge, human angiotensin-converting enzyme 2 (ACE2) is the receptor used by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to invade target cells. The current view of researchers is that the potential impact of commonly used antidiabetic drugs on the clinical effects of COVID-19 pneumonia may be related to whether they affect ACE2[19].
Metformin is a traditional first-line drug for patients with type 2 DM. The main pharmacological effects of metformin are associated with 5′-AMP-activated protein kinase (AMPK). Some scholars have clarified the potential role of AMPK in regulating the expression and stability of ACE2[20]. In theory, metformin may increase the stability of ACE2 in the respiratory tract, thereby promoting the development of SARS-CoV-2 infection. In contrast, metformin plays a crucial role in blood glucose control and immune regulation which may have a beneficial effect on patients' outcomes. Some scholars also believe that metformin could not only prevent the entry of SARS-CoV-2 but also induce the activation of ACE2 through the AMPK signaling pathway, thus preventing harmful sequelae[21,22]. A retrospective analysis reported a downward trend in mortality among patients with COVID-19 and DM treated with metformin[23]. Penlioglou et al[24] have reported the potential positive effects of metformin and pioglitazone on COVID-19-induced liver injury. Therefore, there is no reason to discontinue metformin in patients with COVID-19 and DM. However, in patients with severe pneumonia, metformin is not recommended because of the risk of hypoxia and respiratory failure; metformin may cause accumulation of lactic acid and aggravate metabolic disorders. Conversely, metformin may increase the symptoms of nausea and diarrhea; therefore, metformin use is not recommended in such patients.
Acarbose is an alpha-glucosidase inhibitor. The main mechanism of action of acarbose is to delay the absorption of carbohydrates in the intestinal tract and the absorption of glucose from carbohydrate sources. It mainly reduces postprandial blood glucose levels and prevents the fluctuation of blood glucose levels. In fact, there is no conclusive evidence that acarbose increases the incidence of COVID-19 in patients with DM. In patients infected with SARS-CoV-2, acarbose may cause gastrointestinal discomfort, which may limit its use. If patients with COVID-19 have poor appetite, slow gastrointestinal motility, or even diarrhea, acarbose use is not recommended. However, for patients with mild symptoms, acarbose is a good choice.
Sulfonylureas and non-sulfonylurea insulin secretagogues are traditional glucose-lowering agents that have clear antidiabetic effects and are widely used in patients with type 2 DM. However, during the COVID-19 pandemic, patients with mild infection adhering to a regular diet, no gastrointestinal discomfort, and less hypoglycemia can continue to use sulfonylureas. Patients who would continue with oral medication should adjust the dose appropriately with careful monitoring of blood sugar levels. However, for patients with unstable vital signs, severe gastrointestinal reactions, an irregular diet, and even frequent hypoglycemia, it is not recommended to continue using these drugs.
Thiazolidinediones, which are known to be insulin sensitizers, increase the sensitivity of the skeletal muscle, liver, and adipose tissue to insulin, thus improving the utilization of glucose by cells to lower the blood glucose level; this makes them a good choice for patients with type 2 DM and severe insulin resistance. Can pioglitazone be potentially useful in treating patients with COVID-19? Carboni et al[25] stressed that pioglitazone can be administered to patients who are receiving statins as this combination can reduce the production of C-reactive protein and other laboratory markers of inflammation[26]. In fact, there is no evidence that patients with type 2 DM and COVID-19 should stop using thiazolidinediones.
Dipeptidyl peptidase 4 (DPP-4) inhibitors are dipeptidase-peptidase inhibitors. They lower the blood glucose level by suppressing the inactivation of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulin secretion peptide (GIP). This promotes insulin release from pancreatic beta cells by increasing the levels of endogenous GLP-1 and GIP and inhibiting the secretion of pancreatic glucagon from islet alpha cells. At present, it is uncertain whether inhibiting or regulating DPP-4 would be the most appropriate strategy for patients with COVID-19. However, DPP4 may be a potential target for preventing and reducing the risk of COVID-19 infection, and the progression of acute respiratory complications would be prolonged in patients with type 2 DM with COVID-19 receiving DPP-4 inhibitors. Although patients with type 2 DM are exposed to low-level chronic inflammation, which can lead to an abnormal immune response[1], whether DPP-4 plays a potential role in COVID-19 development remains unclear. Therefore, we cannot assume that DPP-4 inhibitors reduce the risk of acute respiratory complications in patients with type 2 DM and COVID-19; therefore, we need to distinguish between DPP-4 inhibition and DPP-4 regulation until future studies show different results[27-29]. A meta-analysis showed that DPP-4 inhibitor treatment did not significantly increase the risk of upper respiratory tract infection, and the risk of respiratory tract infection associated with DPP-4 inhibitor treatment was comparable to that associated with placebo or active control treatment[30].
Sodium-dependent glucose transporter 2 (SGLT-2) inhibitors inhibit the reabsorption of glucose in the kidneys, allowing excess glucose to be excreted via urine and lowering blood sugar levels. SGLT-2 inhibitors are a new class of antidiabetic medicines, and because of their specific target distribution in the kidney, they have a minimal impact on other tissues and organs. Further, they have a good effect in patients with obesity and insulin-resistant type 2 DM, and the associated risk of hypoglycemia is very low. SGLT-2 inhibitors are an ideal option for patients with type 2 DM during the COVID-19 pandemic. Numerous clinical trials have shown that these drugs can provide benefits related to the heart and kidneys in addition to glycemic control[31]. However, whether SGLT-2 inhibitors could provide vital organ protection among patients with COVID-19 remains unclear. A phase 3, multi-national, double-blind, placebo-controlled randomized trial has been started (DARE-19; clinical trial number NCT04350593). However, if patients with COVID-19 have a low caloric intake, gastrointestinal absorption disorders, insufficient blood volume, ketoacidosis, or infections in the urinary tract and reproductive system, suspension of the use of such drugs is recommended. A researcher raised some concerns that the DARE-19 trial seems to be an extremely risky proposition and that the treatment of comorbidities at the cost of losing track of viral infection treatment would be futile[32]. Discontinuing the use of SGLT-2 inhibitors and switching to insulin treatment would be a better choice in such cases.
GLP-1 is an incretin hormone secreted in the gut which stimulates the secretion of insulin and promote glucose metabolism by acting on the GLP-1 receptor. GLP-1 receptor agonists can not only promote insulin secretion and reduce blood sugar, but also repair the function of islet cells. As they play a glucose-dependent role in lowering blood glucose levels, the risk of hypoglycemia is very low. Further, they are highly effective hypoglycemic agents with good safety and play an important role in weight loss. Therefore, GLP-1 receptor agonists are an ideal choice for patients with type 2 DM, especially those with obesity. Although ACE inhibitors, angiotensin 2 receptor blockers, SGLT-2 inhibitors, GLP-1 receptor agonists, pioglitazone, and probably insulin seem to increase the number of ACE 2 receptors on the cells, which are used by SARS-CoV-2 for entry, no evidence shows that the use of these agents might be harmful in terms of contracting or worsening COVID-19[33]. Patients with COVID-19 should pay attention to the gastrointestinal side effects of GLP-1 receptor agonists, such as delayed gastric emptying, abdominal distension, and diarrhea. Therefore, patients with intestinal infections need to stop using GLP-1 receptor agonists and use insulin instead.
During the COVID-19 pandemic, individualized treatment, a rational diet, and systematic and strict glucose level monitoring should be advised for patients with DM to control their blood glucose levels and reduce the adverse outcomes of antidiabetic medicines.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haidara MA S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Ma RCW. Epidemiology of diabetes and diabetic complications in China. Diabetologia. 2018;61:1249-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 349] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 2. | Ingelfinger JR, Jarcho JA. Increase in the Incidence of Diabetes and Its Implications. N Engl J Med. 2017;376:1473-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Gao HX, Regier EE, Close KL. International Diabetes Federation World Diabetes Congress 2015. J Diabetes. 2016;8:300-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6657] [Article Influence: 1331.4] [Reference Citation Analysis (0)] |
| 5. | Furuya M, Hayashino Y, Tsujii S, Ishii H, Fukuhara S. Comparative validity of the WHO-5 Well-Being Index and two-question instrument for screening depressive symptoms in patients with type 2 diabetes. Acta Diabetol. 2013;50:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Gois CJ, Ferro AC, Santos AL, Sousa FP, Ouakinin SR, do Carmo I, Barbosa AF. Psychological adjustment to diabetes mellitus: highlighting self-integration and self-regulation. Acta Diabetol. 2012;49 Suppl 1:S33-S40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Musselman DL, Betan E, Larsen H, Phillips LS. Relationship of depression to diabetes types 1 and 2: epidemiology, biology, and treatment. Biol Psychiatry. 2003;54:317-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 392] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 8. | Knapp S. Diabetes and infection: is there a link?--A mini-review. Gerontology. 2013;59:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (1)] |
| 9. | Rao Kondapally Seshasai S, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup PH, Mukamal KJ, Gillum RF, Holme I, Njølstad I, Fletcher A, Nilsson P, Lewington S, Collins R, Gudnason V, Thompson SG, Sattar N, Selvin E, Hu FB, Danesh J; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2142] [Cited by in RCA: 2021] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 10. | Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 562] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 11. | Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, Rutten GE. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 687] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 12. | Bertoni AG, Saydah S, Brancati FL. Diabetes and the risk of infection-related mortality in the U.S. Diabetes Care. 2001;24:1044-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Yende S, van der Poll T, Lee M, Huang DT, Newman AB, Kong L, Kellum JA, Harris TB, Bauer D, Satterfield S, Angus DC; GenIMS and Health ABC study. The influence of pre-existing diabetes mellitus on the host immune response and outcome of pneumonia: analysis of two multicentre cohort studies. Thorax. 2010;65:870-877. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18194] [Article Influence: 3638.8] [Reference Citation Analysis (0)] |
| 15. | Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX, China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1711] [Cited by in RCA: 2188] [Article Influence: 437.6] [Reference Citation Analysis (0)] |
| 16. | Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14:29-34. [PubMed] [DOI] [Full Text] |
| 17. | Sheikh V, Zamani A, Mahabadi-Ashtiyani E, Tarokhian H, Borzouei S, Alahgholi-Hajibehzad M. Decreased regulatory function of CD4+CD25+CD45RA+ T cells and impaired IL-2 signalling pathway in patients with type 2 diabetes mellitus. Scand J Immunol. 2018;88:e12711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Morigi M, Angioletti S, Imberti B, Donadelli R, Micheletti G, Figliuzzi M, Remuzzi A, Zoja C, Remuzzi G. Leukocyte-endothelial interaction is augmented by high glucose concentrations and hyperglycemia in a NF-kB-dependent fashion. J Clin Invest. 1998;101:1905-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 319] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 19. | Ursini F, Ciaffi J, Landini MP, Meliconi R. COVID-19 and diabetes: Is metformin a friend or foe? Diabetes Res Clin Pract. 2020;164:108167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Zhang J, Dong J, Martin M, He M, Gongol B, Marin TL, Chen L, Shi X, Yin Y, Shang F, Wu Y, Huang HY, Zhang J, Zhang Y, Kang J, Moya EA, Huang HD, Powell FL, Chen Z, Thistlethwaite PA, Yuan ZY, Shyy JY. AMP-activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am J Respir Crit Care Med. 2018;198:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 21. | Ursini F, Russo E, Pellino G, D'Angelo S, Chiaravalloti A, De Sarro G, Manfredini R, De Giorgio R. Metformin and Autoimmunity: A "New Deal" of an Old Drug. Front Immunol. 2018;9:1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 22. | Sharma S, Ray A, Sadasivam B. Metformin in COVID-19: A possible role beyond diabetes. Diabetes Res Clin Pract. 2020;164:108183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 145] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 23. | Luo P, Qiu L, Liu Y, Liu XL, Zheng JL, Xue HY, Liu WH, Liu D, Li J. Metformin Treatment Was Associated with Decreased Mortality in COVID-19 Patients with Diabetes in a Retrospective Analysis. Am J Trop Med Hyg. 2020;103:69-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 24. | Penlioglou T, Papachristou S, Papanas N. COVID-19 and Diabetes Mellitus: May Old Anti-diabetic Agents Become the New Philosopher's Stone? Diabetes Ther. 2020;11:1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Carboni E, Carta AR, Carboni E. Can pioglitazone be potentially useful therapeutically in treating patients with COVID-19? Med Hypotheses. 2020;140:109776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 26. | Forst T, Wilhelm B, Pfützner A, Fuchs W, Lehmann U, Schaper F, Weber M, Müller J, Konrad T, Hanefeld M. Investigation of the vascular and pleiotropic effects of atorvastatin and pioglitazone in a population at high cardiovascular risk. Diab Vasc Dis Res. 2008;5:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Iacobellis G. COVID-19 and diabetes: Can DPP4 inhibition play a role? Diabetes Res Clin Pract. 2020;162:108125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 28. | Morin N. Response to COVID-19 and diabetes: Can DPP4 inhibition play a role? - GLP-1 might play one too. Diabetes Res Clin Pract. 2020;164:108160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Kokic Males V. Letter to the editor in response to the article "COVID-19 and diabetes: Can DPP4 inhibition play a role?". Diabetes Res Clin Pract. 2020;163:108163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Yang W, Cai X, Han X, Ji L. DPP-4 inhibitors and risk of infections: a meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2016;32:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Minze MG, Will KJ, Terrell BT, Black RL, Irons BK. Benefits of SGLT2 Inhibitors Beyond Glycemic Control - A Focus on Metabolic, Cardiovascular and Renal Outcomes. Curr Diabetes Rev. 2018;14:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Chatterjee S. SGLT-2 inhibitors for COVID-19 - A miracle waiting to happen or just another beat around the bush? Prim Care Diabetes. 2020;14:564-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Ceriello A, Standl E, Catrinoiu D, Itzhak B, Lalic NM, Rahelic D, Schnell O, Škrha J, Valensi P; Diabetes and Cardiovascular Disease (D&CVD) EASD Study Group. Issues of Cardiovascular Risk Management in People With Diabetes in the COVID-19 Era. Diabetes Care. 2020;43:1427-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |