Published online Oct 15, 2020. doi: 10.4239/wjd.v11.i10.468
Peer-review started: April 19, 2020
First decision: May 20, 2020
Revised: May 25, 2020
Accepted: August 25, 2020
Article in press: August 25, 2020
Published online: October 15, 2020
Processing time: 177 Days and 18.5 Hours
Coronavirus disease 2019 (COVID-19) is an emerging infectious disease that has spread rapidly around the world. Previous studies have indicated that COVID-19 patients with diabetes are prone to having poor clinical outcomes.
To systematically evaluate the prevalence of diabetes among COVID-19 patients in China and its impact on clinical outcomes, including ICU admission, progression to severe cases, or death.
We searched studies published in PubMed, Web of Science, and EMBASE from December 1, 2019 to March 31, 2020 to identify relevant observational study that investigated the prevalence of diabetes among COVID-19 patients or its impact on clinical outcomes. We used a random-effects or fixed-effects model to estimate the pooled prevalence of diabetes and risk ratio (RR) and its 95% confidence interval (CI) of diabetes on outcomes. Funnel plots were used to evaluate the publication bias and the heterogeneity was evaluated by I2 statistic.
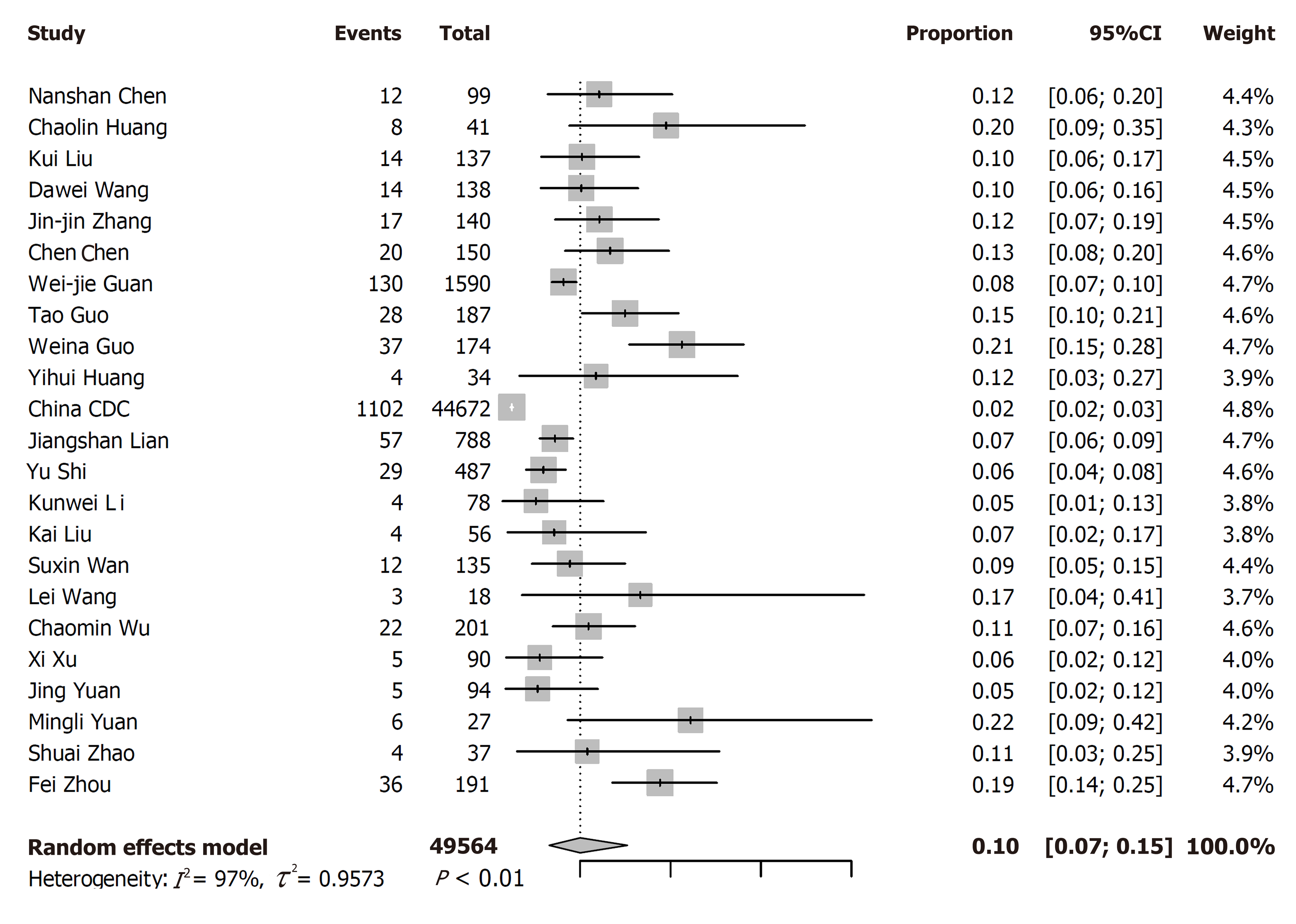
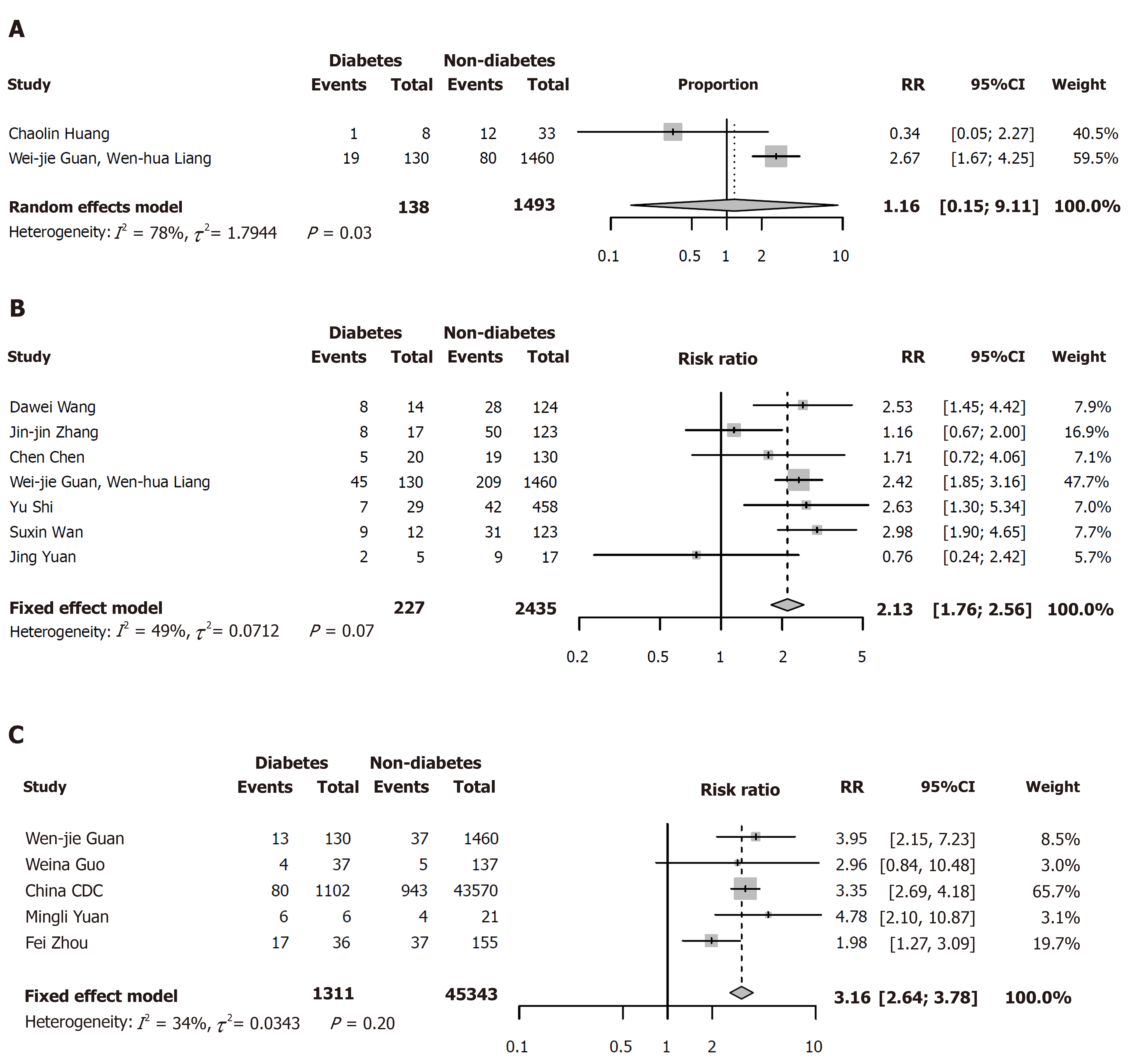
Twenty-three eligible articles including 49564 COVID-19 patients (1573 with and 47991 without diabetes) were finally included. The pooled prevalence of diabetes was 10% (95%CI: 7%-15%) in COVID-19 patients. In the subgroup analyses, the pooled prevalence of diabetes was higher in studies with patients aged > 50 years (13%; 95%CI: 11%-16%) than in studies with patients aged ≤ 50 years (7%; 95%CI: 6%-8%), in severe patients (17%; 95%CI: 14%-20%) than in non-severe patients (6%; 95%CI: 5%-8%), and in dead patients (30%; 95%CI: 13%-46%) than in survivors (8%; 95%CI: 2%-15%) (P < 0.05 for all). Compared with patients without diabetes, the risk of severe cases was higher (RR = 2.13, 95%CI: 1.76-2.56, I2 = 49%) in COVID-19 patients with diabetes. The risk of death was also higher in COVID-19 patients with diabetes (RR = 3.16, 95%CI: 2.64-3.78, I2 = 34%). However, diabetes was not found to be significantly associated with admission to ICU (RR = 1.16, 95%CI: 0.15-9.11).
Nearly one in ten COVID-19 patients have diabetes in China. Diabetes is associated with a higher risk of severe illness and death. The present study suggested that targeted early intervention is needed in COVID-19 patients with diabetes.
Core Tip: At present, the prevalence and impact of diabetes in patients with coronavirus disease 2019 (COVID-19) have not been systematically reviewed in China. This meta-analysis for the first time focused on the pooled prevalence of diabetes among COVID-19 patients and its impact on clinical outcomes (ICU admission, severity, and death) in China. The analysis showed that the pooled prevalence of diabetes was 10% [95% confidence interval (CI): 7%-15%] in COVID-19 patients. Besides, the risks of severe cases (risk ratio = 2.13, 95%CI: 1.76-2.56, I2 = 49%, P = 0.007) and deaths (risk ratio = 3.16, 95%CI: 2.64-3.78, I2 = 34%, P = 0.20) were both higher in COVID-19 patients with diabetes compared with those without. Our findings highlight the need for targeted intervention on diabetes among COVID-19 patients.
- Citation: Du M, Lin YX, Yan WX, Tao LY, Liu M, Liu J. Prevalence and impact of diabetes in patients with COVID-19 in China. World J Diabetes 2020; 11(10): 468-480
- URL: https://www.wjgnet.com/1948-9358/full/v11/i10/468.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i10.468
In December 2019, some pneumonia cases of unknown cause were reported in Wuhan, Hubei Province, China, and deep sequencing analysis from lower respiratory tract samples indicated a novel coronavirus that caused coronavirus disease 2019 (COVID-19)[1]. As of April 12, 2020, there were 1696588 confirmed cases of COVID-19 in the world, with 105952 deaths, according to the World Health Organization. Previous studies have reported that patients with poor immune function, such as the elderly or patients with chronic diseases, may develop severity or even death, such as diabetes and cardiovascular diseases[2,3]. Guo et al[4] reported that diabetes patients with COVID-19 were prone to have severe pneumonia, releasing tissue damage-related enzymes, excessively uncontrolled inflammation, and hypercoagulable states associated with abnormal glucose metabolism[4]. Similarly, COVID-19 is not conducive to blood glucose control in diabetic patients. Zhou and Tan[5] monitored the blood glucose of 26 diabetic COVID-19 patients and found that 56.6% showed abnormal blood glucose levels, 69.0% were considered to have a poor blood glucose level, and 10.3% have experienced hypoglycemia at least once[5].
As a chronic illness, diabetes is characterized by elevated levels of blood glucose, and accompanied by disturbed metabolism of fats and proteins[6]. Several studies reported the prevalence of diabetes among COVID-19 patients. In China, Shi et al[7] discovered that the comorbidity rate of diabetes was 5.95% in Zhengzhou, while it was 7.23% in the study by Lian et al[8]. Huang et al[9] found that the prevalence of diabetes in Wuhan was 11.8%, while Chen et al[10] reported the rate at 14%. Bhatraju et al[11] found that prevalence of diabetes among COVID-19 patients was 58% in the United States. It was reported that the prevalence of diabetes was about three-fold higher in intensive care unit (ICU) cases than in non-ICU cases[12]. The results on the prevalence of diabetes varied across different studies.
Although epidemiological data on COVID-19 infection are growing, the prevalence and impact of diabetes in COVID-19 patients have not been systematically reviewed at the country level. Thus, we conducted a systematic review and meta-analysis to assess the prevalence of diabetes among COVID-19 patients in China and identify its impact on clinical outcomes.
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement, we performed this systematic review and meta-analysis[13].
The literature was searched independently by two investigators (Du M and Yan WX), which was published online from December 1, 2019 to March 31, 2020, from three databases including PubMed, Web of Science, and EMBASE using the following search term: (“novel coronavirus”, “2019-nCoV”, “coronavirus disease 2019”, “COVID-19”, or “SARS-CoV-2”) and “diabetes”. There was no limitation to language or region. Moreover, we also searched highly relevant reference articles by reviewing the list of references. To exclude duplicates, EndNote X 9.0 software was used to manage the records.
The inclusion criteria for articles in the meta-analysis included: (1) Cross-sectional studies or cohort studies; and (2) Studies that reported the prevalence of diabetes in COVID-19 patients. The exclusion criteria were as follows: (1) The subjects of the study were irrelevant (animal experiments, pathological researches, or molecular researches); (2) Reviews, editorials, or case reports; (3) Overlapped studies. The latest one was selected if studies overlapped; (4) Studies that were not conducted in China; (5) Duplicated studies; and (6) Key information could not be extracted, for example the prevalence of diabetes.
Two investigators (Lin YX and Yan WX) independently identified the studies, based on the inclusion and exclusion criteria. When discrepancies occurred, they were solved by consensus or decided by a third investigators (DM).
The primary outcomes were prevalence of diabetes among COVID-19 patients and its impact on clinical outcomes (admission to ICU, severe cases, and death). In view of the piloted forms, the information which was extracted independently by two investigators (Du M and Yan WX) from the selected studies included three main parts: (1) Basic information of the studies comprising first author, publication year, survey time, study design, and journal; (2) Characteristics of the study population including sample size, location, median/mean age, gender ratio; and (3) Primary outcomes including the numbers of patients with diabetes and non-diabetes in the total COVID-19 patients and in different subgroups (ICU patients vs non-ICU patients, severe vs non-severe patients, and dead patients vs survivors). The methodological quality of the included studies was evaluated by using the tool developed by Hoy and colleagues[14], which had been used in other meta-analyses for pooled prevalence[15]. To generate an overall quality score that ranged from 0 to 10, we assigned each item a score of 1 (yes) or 0 (no), and summed scores across items. Then, according to the overall scores, the methodological quality of studies was classified into three levels: Low (> 8), moderate (6–8), or high (≤ 5) risk of bias[14]. The study quality was independently assessed by two investigators (Du M and Lin YX), and disagreements were resolved by consensus.
In order to get an overall summary estimate of diabetes prevalence across studies, a meta-analysis was used to summarize prevalence data and a random-effects or fixed-effects model was used to pool the study-specific estimates. We used the I2 statistic to assess the magnitude of heterogeneity, with 25%, 50%, and 75% heterogeneity representing low, moderate, and high degrees of heterogeneity, respectively[16]. According to the analysis results, the proper effect model was selected: If I2 ≤ 50%, the fixed-effects model was used, otherwise the random-effects model was used.
If substantial heterogeneity was detected, to investigate the possible sources of heterogeneity, subgroup analysis was performed by using the following grouping variables: Sample size, location, age, admission to ICU or not, severe cases or not, and death or not. Subgroup comparisons were made using the Q test. A P value less than 0.05 was considered to indicate a significant difference between subgroups. We performed sensitivity analysis by deleting the lowest quality score of study and by using a different model (fixed-effect or random-effect model). The risk ratio (RR) and the corresponding 95% confidence interval (CI) were calculated to quantify the impact of diabetes on clinical outcomes (ICU vs non-ICU patients, severe vs non-severe patients, and dead patients vs survivors), and a P value < 0.05 was deemed significant. We used forest plots to describe the pooled rate of diabetes and the risk ratio of diabetes to related outcomes. To assess publication bias, we used funnel plots and Egger’ publication bias test. We analyzed data using R version 3.5.3 and Stata version 16.0.
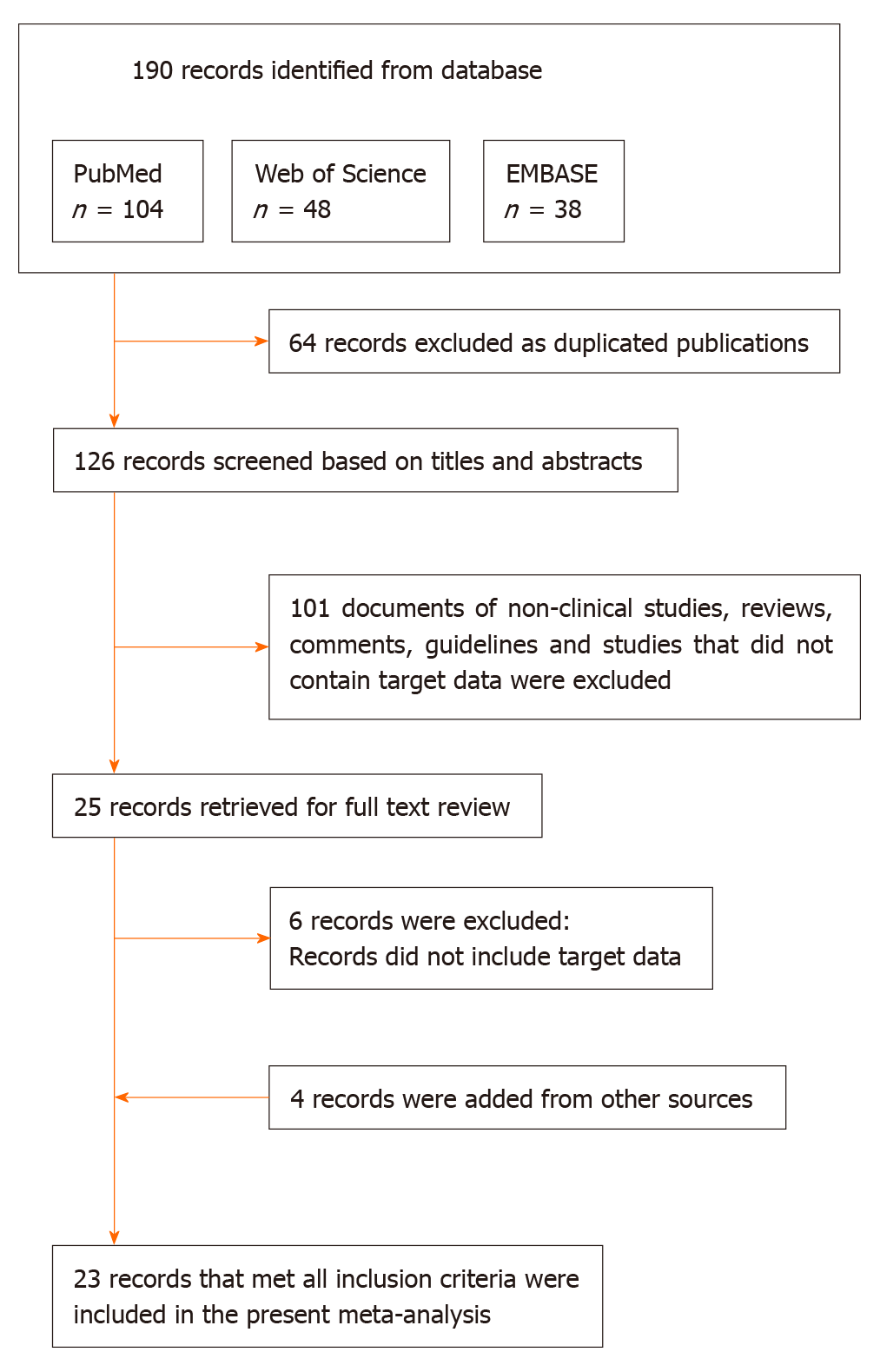
One hundred and ninety articles were searched totally, of which 64 reported duplicate results. After skimming the title and abstracts, we excluded 101 reviews, guidelines, or irrelevant studies. After reading the full articles, six articles that provided insufficient information were excluded. Four articles from other sources were included. As a result, 23 eligible articles were included in the meta-analysis[1,3,4,7-9,17-34]. Figure 1 shows the flow chart of study selection.
The total sample size of the included studies was 49564, with the number of subjects in each study varying from 18 to 44672. Baseline characteristics are shown in Table 1.
| Ref. | Survey period | Sample size | Location | Age (mean or median) | Sex (male, %) | Prevalence of diabetes (%) | Quality score |
| Chen et al[17] | 2020.1.1-2020.1.20 | 99 | Wuhan China | 55.5 | 67.68 | 12.12 | 7 |
| Huang et al[1] | 2019.12.16-2010.1.2 | 41 | Wuhan China | 49.0 | 73.17 | 19.51 | 7 |
| Liu et al[18] | 2019.12.30-2020.1.24 | 137 | Wuhan China | 57 | 44.53 | 10.22 | 7 |
| Wang et al[19] | 2020.1-2020.1.31 | 138 | Wuhan China | 56 | 54.35 | 10.14 | 7 |
| Zhang et al[20] | 2020.1.06-2020.2.3 | 140 | Wuhan China | 57 | 50.71 | 12.14 | 7 |
| Chen et al[21] | 2019.1 -2020 .2 | 150 | Wuhan China | 59 | 56.00 | 13.33 | 5 |
| Guan et al[22] | 2020.12.11-1.31 | 1590 | China | 48.9 | 56.86 | 8.18 | 9 |
| Guo et al[23] | 2020.1.23-2020.2.23 | 187 | Wuhan China | 58.5 | 48.66 | 14.97 | 7 |
| Guo et al[4] | 2020.2.10-2020.2.29 | 174 | Wuhan China | 59 | 43.68 | 21.26 | 6 |
| Huang et al[9] | 2019.12.21-2020.1.28 | 34 | Wuhan China | 56.24 | 41.18 | 11.76 | 4 |
| China CDC[24] | As of 2020.2.11 | 44672 | China | - | 51.44 | 2.47 | 6 |
| Lian et al[8] | 2020.1.17-2020.2.12 | 788 | Zhejiang China | 45.8 | 51.65 | 7.23 | 7 |
| Shi et al[7] | As of 2020.2.17 | 487 | Zhejiang China | 46 | 53.18 | 5.95 | 3 |
| Li et al[25] | 2020.1.18-2020.2.7 | 78 | Zhuhai China | 44.6 | 48.72 | 5.13 | 5 |
| Liu et al[3] | 2020.1.15-2020.2.18 | 56 | Hainan China | 53.75 | 55.36 | 7.14 | 6 |
| Wan et al[26] | 2020.1.23-2020.2.28 | 135 | Chongqing China | 47 | 53.33 | 8.89 | 5 |
| Wang et al[27] | 2020.1.21-2020.2.5 | 18 | Zhengzhou China | 39 | 55.56 | 16.67 | 5 |
| Wu et al[28] | 2019.12.25-2020.1.26 | 201 | Wuhan China | 51 | 63.68 | 10.95 | 7 |
| Xu et al[29] | 2020.1.23-2020.2.4 | 90 | Guangzhou China | 50 | 43.33 | 5.56 | 6 |
| Yuan et al[30] | 2020.1.11-2020.2.4 | 94 | Shenzhen China | 40 | 44.68 | 5.32 | 6 |
| Yuan et al[31] | 2020.1.1-2020.1.25 | 27 | Wuhan China | 60 | 44.44 | 22.22 | 5 |
| Zhao et al[32] | 2020.1.23-2020.1.31 | 37 | Wuhan China | 41 | 37.84 | 10.81 | 6 |
| Zhou et al[33] | 2019.12.29-2020.1.31 | 191 | Wuhan China | 56 | 62.30 | 18.85 | 7 |
Among the 23 studies included, the rate of diabetes varied widely from 2.47% to 22.22%. The pooled prevalence of diabetes was 10% (7%-15%) among 49564 COVID-19 patients from 23 studies (Figure 2). For the subgroup analyses, the pooled prevalence of diabetes was higher in studies on patients with a median age > 50 years (13%; 95%CI: 11%-16%) than in those on patients with a median age ≤ 50 years (7%; 95%CI: 6%-8%), in severe patients (17%; 95%CI: 14%-20%) than in non-severe patients (6%; 95%CI: 5%-8%), and in dead patients (30%; 95%CI: 13%-46%) than in survivors (8%; 95%CI: 2%-15%, Table 2).
| Item | Number of studies | Sample size | Diabetes | Prevalence(95%CI, %) | I2 | P values | |
| Heterogeneity | Subgroup difference | ||||||
| Overall | 23 | 49564 | 1573 | 10 (7-15) | 97% | < 0.01 | - |
| Subgroup analysis | |||||||
| Sample size | 0.002 | ||||||
| ≤ 100 | 10 | 574 | 55 | 9 (6-11) | 28% | 0.14 | |
| 100-200 | 8 | 1252 | 178 | 14 (11-16) | 60% | 0.02 | |
| > 200 | 5 | 49588 | 1340 | 7 (4-9) | 94% | < 0.0001 | |
| Location | < 0.0001 | ||||||
| Wuhan, China | 13 | 1556 | 222 | 14 (12-16) | 35% | 0.12 | |
| Outside of Wuhan, China | 8 | 1746 | 119 | 7 (6-8) | 0% | 0.79 | |
| China (all cities) | 2 | 46262 | 1432 | 5 (0-11) | 99% | < 0.0001 | |
| Age group | < 0.0001 | ||||||
| ≤ 50 yr | 10 | 3358 | 257 | 7 (6-8) | 10% | 0.27 | |
| > 50 yr | 12 | 1534 | 214 | 13 (11-16) | 45% | 0.05 | |
| ICU admission | 0.94 | ||||||
| ICU | 2 | 112 | 20 | 15 (4-26) | 47% | 0.17 | |
| Non-ICU | 2 | 1519 | 118 | 14 (3-35) | 78% | 0.03 | |
| Severity | < 0.0001 | ||||||
| Severe | 8 | 553 | 99 | 17 (14-20) | 0% | 0.92 | |
| Non-severe | 7 | 2190 | 143 | 6 (5-8) | 41% | 0.05 | |
| Death | 0.02 | ||||||
| Death | 5 | 1146 | 120 | 30 (13-46) | 89% | < 0.0001 | |
| Survival | 5 | 45508 | 1191 | 8 (2-15) | 99% | < 0.0001 | |
Compared with patients without diabetes, the risks of severe cases (RR = 2.13, 95%CI: 1.76-2.56; I2 = 49%) and deaths (RR = 3.16, 95%CI: 2.64-3.78, I2 = 34%) were both higher in COVID-19 patients with diabetes (Figure 3B and C). However, there was no relationship between diabetes and admission to ICU (RR = 1.16, 95%CI: 0.15-9.11; Figure 3A).
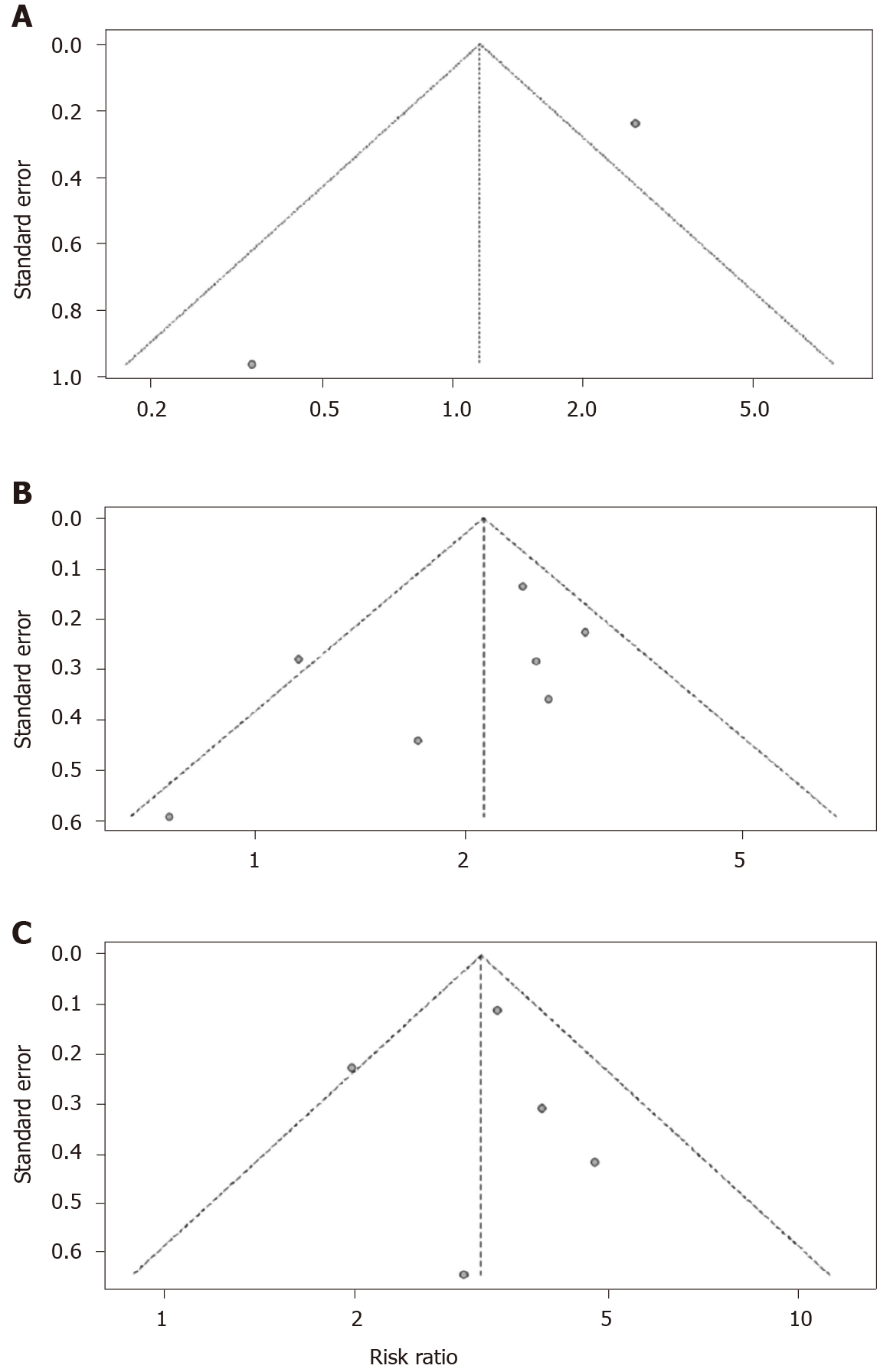
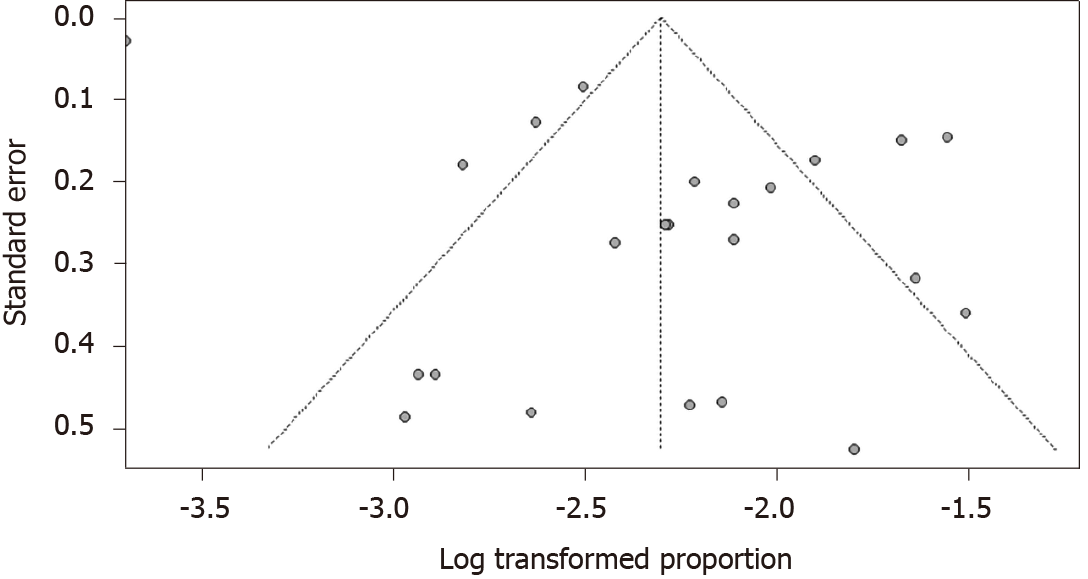
In the sensitivity analysis, we found the pooled results of meta-analysis had no difference between the fixed-effects model and random-effects model or when deleting the study with the lowest quality score, which showed that the results of pooled diabetes prevalence and RRs were stable. Both funnel plots (Figure 4B and C) and Egger’s tests showed publication bias on the pooled prevalence of diabetes (P < 0.001; Figure 5) and the association of diabetes and ICU admission (P < 0.05; Figure 4A), while showed no evidence of publication bias on the association of diabetes and severe cases (t = -1.36, P = 0.23) or death (t = 0.03, P = 0.98).
Diabetes is an important public health problem. Uncontrolled diabetes could lead to complications in many organs, resulting in loss of vision and kidney function, heart attacks, strokes, and lower limb amputations which cause disability and death[6]. Previous studies reported that the presence of comorbidities in severe acute respiratory syndrome patients increased the risk of death by nearly two-fold[35]. Yuan et al[31] found that the incidence of comorbidities in the death group was significantly higher than that of the survival group (80% vs 29%, P = 0.018), especially for comorbid diabetes, hypertension, and heart disease[31]. Some studies suggested that chronic diseases such as hypertension and diabetes might be important risk factors for poor prognosis in patients with COVID-19[36]. In this systematic review and meta-analysis, we included 23 studies related to COVID-19 cases and diabetes. Our results showed that the prevalence of diabetes in COVID-19 cases was 10%. The pooled prevalence of diabetes in COVID-19 cases in our study (10%) was slightly higher than the estimation in the general population (8.5%)[6]. Li et al[12] found that the prevalence of diabetes is about 9.7% in COVID-19 cases by meta-analysis including five studies[12], which was similar to our findings. Yang et al[37] found that the prevalence of diabetes is about 9% by a meta-analysis that include eight studies. The differences between these studies might be related to the number of studies included, sample size, and characteristics of the included patients.
We found that diabetes was associated with severe illness (RR = 2.13, 95%CI: 1.76, 2.56) and death (RR = 3.16, 95%CI: 2.64-3.78). A meta-analysis by Li et al[12] reported that the prevalence of diabetes in ICU patients was not significantly higher than that in non-ICU patients (RR = 2.21, 95%CI: 0.88, 5.57)[12]. Yang et al[37] found that the proportion of diabetic patients between severe and non-severe patients had no significant difference by meta-analysis[37]. These two meta-analyses described the prevalence of diabetes among COVID-19 patients with limited studies (less than 10 studies) included in the analysis. Huang et al[38] reported that diabetes was associated with severe illness (RR = 2.45, 95%CI: 1.79-3.35) in a meta-analysis of 30 studies, which was similar to our findings[38]. It should be noted that, the pooled prevalence of diabetes was 30% (95%CI: 12%-51%) in the dead patients, which was much higher than 8% (95%CI: 3%-14%) in the survivors in our study. Pooled RR for the association between diabetes and death was 3.16 (95%CI: 2.64-3.78), which was similar to the study of Kumar et al[39], who did a meta-analysis of 33 case-control studies to examine the association between diabetes and mortality in COVID-19 patients. They found that diabetes was significantly associated with mortality of COVID-19 (RR = 1.90, 95%CI: 1.37-2.64)[39]. The findings indicated that the risk of death among COVID-19 patients with diabetes should be paid more attention.
Diabetes and susceptibility of the body have certain physiological mechanisms. Because of the accumulation of activated innate immune cells in metabolic tissues, the inflammatory mediators such as IL-1β and TNF-α are released, which accelerates β-cell damage and systemic insulin resistance. Conversely, metabolic problems may further impair the immunologic function of macrophages and lymphocytes, which makes the individual easy to develop other complications[40]. Recently, it was found that the proportion of CD3+ or CD4+ T cells and the absolute count of CD3+, CD4+, or CD8+ T cells in the death group were significantly higher than those in the survival group by analyzing the clinical data of viral pneumonia patients retrospectively, and the death group had higher levels of different inflammatory factors compared to the survival group[17]. Huang et al[1] also found that the plasma concentrations of inflammatory factors (such as IL-2, IL-7, and IL-10) in ICU patients with COID-19 were higher than those of the non-ICU COVID-19 patients[1]. Studies have found that coronaviruses (including SARS-CoV and SARS-CoV-2) can bind to target cells because of angiotensin-converting enzyme 2 (ACE2), which may promote the proliferation of SARS-CoV-2and enhance its ability to infect[41]. ACE inhibitors and type I angiotensin II receptor blockers (ARBs) that are used in patients with type 1 or type 2 diabetes can result in a significant increase in ACE2[41,42]. Besides ACE2, dipeptidyl peptidase-4 (DPP4) was also found to be a coronavirus receptor protein[43]. DPP4 inhibitors, one of the glucose-lowering agents, are widely used in treatment of diabetes and known to modify the biological activities of multiple immunomodulatory substrates[44]. The potential role of DPP4 inhibition in preventing SARS-CoV-2 infection and progression needs to be clarified in the future[44]. Previous studies found that ACE2 and DPP4 were established transducers of metabolic signals and pathways regulating inflammation and glucose homeostasis[43]. Guo et al[45] analyzed the biochemical indicators of diabetic and non-diabetic patients in new coronavirus cases and found that compared with non-diabetic patients, the levels of serum inflammation-related biomarkers, including IL-6, C-reactive protein, serum ferritin, and D-dimer increased significantly in diabetic patients (P < 0.01), suggesting that patients with diabetes might have a worse prognosis[45]. Diabetes may not be related to the risk of COVID-19 infection, but it could deteriorate clinical outcome of COVID-19 patients[46].
This study has some limitations. First, publication bias might exit in this meta-analysis. Second, due to the limitation of sample size and short study periods, limited studies had completed follow-up of the COVID-19 patients and reported the results of its impact on clinical outcomes.
This study indicated that nearly one in ten COVID-19 patients have diabetes and diabetes increases the risk of severe illness and death. Targeted public health measures should be carried out timely to make the protection of patients with diabetes from COVID-19 and other respiratory infections even better. More adequate and vigorous research should be conducted to prove the associations found in this study.
Coronavirus disease 2019 (COVID-19) has spread around the world rapidly. The prevalence of diabetes varies across different studies. Previous studies showed that COVID-19 patients with diabetes were prone to having poor clinical outcomes. However, a systematical review of the prevalence of diabetes in COVID-19 patients and the impact of diabetes on clinical outcomes has not been done in China.
We hypothesized that the presence of diabetes is associated with a poor prognosis. To our knowledge, this is the first meta-analysis which focused on evaluating the prevalence of diabetes among patients with COVID-19 infection in China and its impact on clinical outcomes.
The aim of this study was to systematically evaluate the prevalence of diabetes among COVID-19 patients in China and its impact on clinical outcomes, including ICU admission, progression to severe cases, or death.
A systematic review and meta-analysis of observational studies were performed.
Twenty-three eligible articles including 49564 COVID-19 patients (1573 with and 47991 without diabetes) were included. The pooled prevalence of diabetes was 10% [95% confidence interval (CI): 7%-15%] in COVID-19 patients. In the subgroup analyses, the pooled prevalence of diabetes was higher in studies on patients with a median age > 50 years (13%; 95%CI: 11%-16%) than studies on patients with a median age ≤ 50 years (7%; 95%CI: 6%-8%), in severe patients (17%; 95%CI: 14%-20%) than in non-severe patients (6%; 95%CI: 5%-8%), and in dead patients (30%; 95%CI: 13%-46%) than in survivors (8%; 95%CI: 2%-15%, all P < 0.05). Compared with patients without diabetes, the risks of severe cases [risk ratio (RR) = 2.13, 95%CI: 1.76-2.56, I2 = 49%] and death (RR = 3.16, 95%CI: 2.64-3.78, I2 = 34%) were both higher in COVID-19 patients with diabetes. However, diabetes was not found to be significantly associated with admission to ICU (RR = 1.16, 95%CI: 0.15-9.11).
Nearly one in ten COVID-19 patients have diabetes in China. Diabetes is associated with a higher risk of severe illness and death. The present study suggested that targeted early intervention is needed in COVID-19 patients with diabetes.
A systematic review and meta-analysis of observational studies which summarizes the evidence on this topic is meaningful. More adequate and vigorous research should be conducted to prove the associations found in this study.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Avtanski D, Popovic DS S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30123] [Article Influence: 6024.6] [Reference Citation Analysis (3)] |
| 2. | Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1341] [Reference Citation Analysis (0)] |
| 3. | Liu K, Chen Y, Lin R, Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Infect. 2020;80:e14-e18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 753] [Cited by in RCA: 908] [Article Influence: 181.6] [Reference Citation Analysis (0)] |
| 4. | Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;e3319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 884] [Cited by in RCA: 899] [Article Influence: 179.8] [Reference Citation Analysis (1)] |
| 5. | Zhou J, Tan J. Diabetes patients with COVID-19 need better blood glucose management in Wuhan, China. Metabolism. 2020;107:154216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 6. | World Health Organization. Global report on diabetes 2016. Available from: https://www.who.int/diabetes/global-report/en/. |
| 7. | Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit Care. 2020;24:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 379] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 8. | Lian J, Jin X, Hao S, Cai H, Zhang S, Zheng L, Jia H, Hu J, Gao J, Zhang Y, Zhang X, Yu G, Wang X, Gu J, Ye C, Jin C, Lu Y, Yu X, Yu X, Ren Y, Qiu Y, Li L, Sheng J, Yang Y. Analysis of Epidemiological and Clinical Features in Older Patients With Coronavirus Disease 2019 (COVID-19) Outside Wuhan. Clin Infect Dis. 2020;71:740-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 9. | Huang Y, Tu M, Wang S, Chen S, Zhou W, Chen D, Zhou L, Wang M, Zhao Y, Zeng W, Huang Q, Xu H, Liu Z, Guo L. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: A retrospective single center analysis. Travel Med Infect Dis. 2020;101606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 10. | Chen R, Zhang Y, Huang L, Cheng BH, Xia ZY, Meng QT. Safety and efficacy of different anesthetic regimens for parturients with COVID-19 undergoing Cesarean delivery: a case series of 17 patients. Can J Anaesth. 2020;67:655-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 11. | Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, Kritek PA, West TE, Luks A, Gerbino A, Dale CR, Goldman JD, O'Mahony S, Mikacenic C. Covid-19 in Critically Ill Patients in the Seattle Region - Case Series. N Engl J Med. 2020;382:2012-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1819] [Cited by in RCA: 1862] [Article Influence: 372.4] [Reference Citation Analysis (0)] |
| 12. | Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531-538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1374] [Cited by in RCA: 1237] [Article Influence: 247.4] [Reference Citation Analysis (0)] |
| 13. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47198] [Article Influence: 2949.9] [Reference Citation Analysis (0)] |
| 14. | Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, Baker P, Smith E, Buchbinder R. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1168] [Cited by in RCA: 1834] [Article Influence: 141.1] [Reference Citation Analysis (0)] |
| 15. | Noubiap JJ, Essouma M, Bigna JJ, Jingi AM, Aminde LN, Nansseu JR. Prevalence of elevated blood pressure in children and adolescents in Africa: a systematic review and meta-analysis. Lancet Public Health. 2017;2:e375-e386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 16. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25813] [Article Influence: 1122.3] [Reference Citation Analysis (0)] |
| 17. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12977] [Article Influence: 2595.4] [Reference Citation Analysis (1)] |
| 18. | Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, Wang YN, Zhong MH, Li CH, Li GC, Liu HG. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133:1025-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 728] [Cited by in RCA: 856] [Article Influence: 171.2] [Reference Citation Analysis (0)] |
| 19. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14767] [Article Influence: 2953.4] [Reference Citation Analysis (0)] |
| 20. | Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2342] [Article Influence: 468.4] [Reference Citation Analysis (0)] |
| 21. | Chen C, Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. [Analysis of myocardial injury in patients with COVID-19 and association between concomitant cardiovascular diseases and severity of COVID-19]. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 121] [Reference Citation Analysis (0)] |
| 22. | Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX, China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1711] [Cited by in RCA: 2189] [Article Influence: 437.8] [Reference Citation Analysis (0)] |
| 23. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2516] [Cited by in RCA: 2842] [Article Influence: 568.4] [Reference Citation Analysis (0)] |
| 24. | Meo SA, Alhowikan AM, Al-Khlaiwi T, Meo IM, Halepoto DM, Iqbal M, Usmani AM, Hajjar W, Ahmed N. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020;24:2012-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 160] [Reference Citation Analysis (0)] |
| 25. | Li K, Fang Y, Li W, Pan C, Qin P, Zhong Y, Liu X, Huang M, Liao Y, Li S. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19). Eur Radiol. 2020;30:4407-4416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 459] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 26. | Wan S, Xiang Y, Fang W, Zheng Y, Li B, Hu Y, Lang C, Huang D, Sun Q, Xiong Y, Huang X, Lv J, Luo Y, Shen L, Yang H, Huang G, Yang R. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92:797-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 574] [Cited by in RCA: 643] [Article Influence: 128.6] [Reference Citation Analysis (0)] |
| 27. | Wang L, Gao YH, Lou LL, Zhang GJ. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur Respir J. 2020;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 28. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5518] [Article Influence: 1103.6] [Reference Citation Analysis (1)] |
| 29. | Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, Chen B, Zhang Z, Guan W, Ling Z, Jiang R, Hu T, Ding Y, Lin L, Gan Q, Luo L, Tang X, Liu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 457] [Cited by in RCA: 491] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 30. | Yuan J, Zou R, Zeng L, Kou S, Lan J, Li X, Liang Y, Ding X, Tan G, Tang S, Liu L, Liu Y, Pan Y, Wang Z. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res. 2020;69:599-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 31. | Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One. 2020;15:e0230548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 319] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 32. | Zhao S, Ling K, Yan H, Zhong L, Peng X, Yao S, Huang J, Chen X. Anesthetic Management of Patients with COVID 19 Infections during Emergency Procedures. J Cardiothorac Vasc Anesth. 2020;34:1125-1131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 33. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18201] [Article Influence: 3640.2] [Reference Citation Analysis (0)] |
| 34. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6231] [Cited by in RCA: 6660] [Article Influence: 1332.0] [Reference Citation Analysis (0)] |
| 35. | Hu X, Deng Y, Wang J, Li H, Li M, Lu Z. Short term outcome and risk factors for mortality in adults with critical severe acute respiratory syndrome (SARS). J Huazhong Univ Sci Technolog Med Sci. 2004;24:514-517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92:726-730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 37. | Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2672] [Cited by in RCA: 2510] [Article Influence: 502.0] [Reference Citation Analysis (2)] |
| 38. | Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia - A systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 685] [Cited by in RCA: 604] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 39. | Kumar A, Arora A, Sharma P, Anikhindi SA, Bansal N, Singla V, Khare S, Srivastava A. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14:535-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 401] [Cited by in RCA: 460] [Article Influence: 92.0] [Reference Citation Analysis (0)] |
| 40. | Odegaard JI, Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harb Perspect Med. 2012;2:a007724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 41. | Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3085] [Cited by in RCA: 2915] [Article Influence: 583.0] [Reference Citation Analysis (0)] |
| 42. | Li G, Hu R, Zhang X. Antihypertensive treatment with ACEI/ARB of patients with COVID-19 complicated by hypertension. Hypertens Res. 2020;43:588-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 43. | Drucker DJ. Coronavirus Infections and Type 2 Diabetes-Shared Pathways with Therapeutic Implications. Endocr Rev. 2020;41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 44. | Strollo R, Pozzilli P. DPP4 inhibition: Preventing SARS-CoV-2 infection and/or progression of COVID-19? Diabetes Metab Res Rev. 2020;e3330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 45. | Guo L, Wei D, Zhang X, Wu Y, Li Q, Zhou M, Qu J. Clinical Features Predicting Mortality Risk in Patients With Viral Pneumonia: The MuLBSTA Score. Front Microbiol. 2019;10:2752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 284] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 46. | Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43:867-869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 334] [Article Influence: 66.8] [Reference Citation Analysis (0)] |