Published online Oct 15, 2020. doi: 10.4239/wjd.v11.i10.435
Peer-review started: June 27, 2020
First decision: July 30, 2020
Revised: August 9, 2020
Accepted: September 8, 2020
Article in press: September 8, 2020
Published online: October 15, 2020
Processing time: 109 Days and 1.7 Hours
The number of end-stage renal disease patients with diabetes mellitus (DM) who are undergoing peritoneal dialysis is increasing. Peritoneal dialysis-associated peritonitis (PDAP) is a serious complication of peritoneal dialysis leading to technical failure and increased mortality in patients undergoing peritoneal dialysis. The profile of clinical symptoms, distribution of pathogenic organisms, and response of PDAP to medical management in the subset of end-stage renal disease patients with DM have not been reported previously. Discrepant results have been found in long-term prognostic outcomes of PDAP in patients with DM. We inferred that DM is associated with bad outcomes in PDAP patients.
To compare the clinical features and outcomes of PDAP between patients with DM and those without.
In this multicenter retrospective cohort study, we enrolled patients who had at least one episode of PDAP during the study period. The patients were followed for a median of 31.1 mo. They were divided into a DM group and a non-DM group. Clinical features, therapeutic outcomes, and long-term prognostic outcomes were compared between the two groups. Risk factors associated with therapeutic outcomes of PDAP were analyzed using multivariable logistic regression. A Cox proportional hazards model was constructed to examine the influence of DM on patient survival and incidence of technical failure.
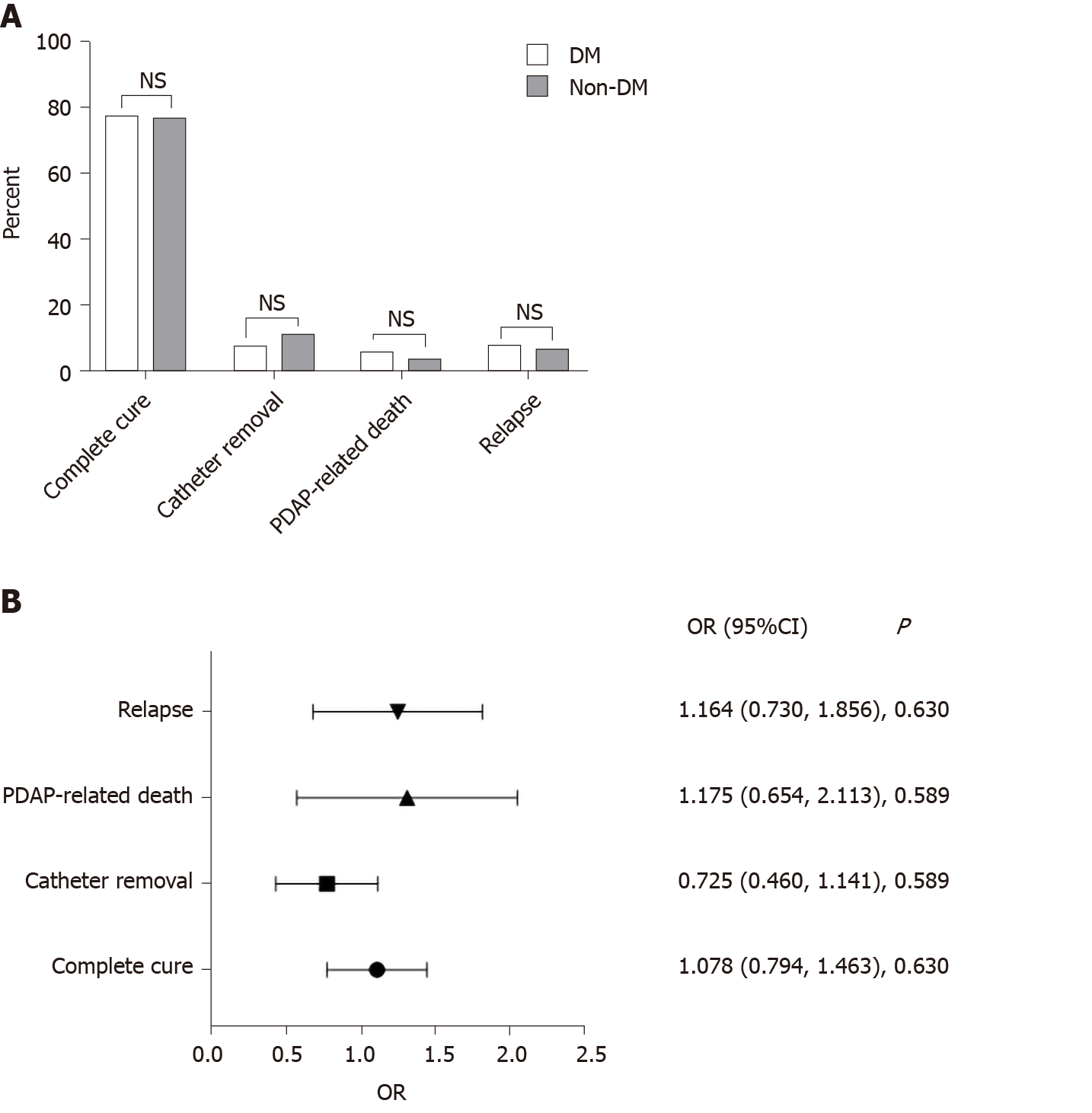
Overall, 373 episodes occurred in the DM group (n = 214) and 692 episodes occurred in the non-DM group (n = 395). The rates of abdominal pain and fever were similar in the two groups (P > 0.05). The DM group had more infections with coagulase-negative Staphylococcus and less infections with Escherichia coli (E. coli) as compared to the non-DM group (P < 0.05). Multivariate logistic regression analysis revealed no association between the presence of diabetes and rates of complete cure, catheter removal, PDAP-related death, or relapse of PDAP (P > 0.05). Patients in the DM group were older and had a higher burden of cardiovascular disease, with lower level of serum albumin, but a higher estimated glomerular filtration rate (P < 0.05). Cox proportional hazards model confirmed that the presence of diabetes was a significant predictor of all-cause mortality (hazard ratio = 1.531, 95% confidence interval: 1.091-2.148, P < 0.05), but did not predict the occurrence of technical failure (P > 0.05).
PDAP patients with diabetes have similar symptomology and are predisposed to coagulase-negative Staphylococcus but not E. coli infection compared those without. Diabetes is associated with higher all-cause mortality but not therapeutic outcomes of PDAP.
Core Tip: We for the first time confirmed that the symptoms of peritoneal dialysis-associated peritonitis in the diabetes mellitus group were the same as those in the non-diabetes mellitus group. This is the first multicenter retrospective cohort study to examine the relationship between diabetes mellitus and long-term outcome in peritoneal dialysis-associated peritonitis patients. It is also the first study to analyze the profile of distribution of pathogenic organisms and response of peritoneal dialysis-associated peritonitis to medical management in the subset of end-stage renal disease patients with diabetes mellitus. We found that diabetes mellitus was inclined to infection with coagulase-negative Staphylococcus but not Escherichia coli. Diabetes mellitus was associated with higher all-cause mortality but not with adverse therapeutic outcome of peritoneal dialysis-associated peritonitis.
- Citation: Meng LF, Yang LM, Zhu XY, Zhang XX, Li XY, Zhao J, Liu SC, Zhuang XH, Luo P, Cui WP. Comparison of clinical features and outcomes in peritoneal dialysis-associated peritonitis patients with and without diabetes: A multicenter retrospective cohort study. World J Diabetes 2020; 11(10): 435-446
- URL: https://www.wjgnet.com/1948-9358/full/v11/i10/435.htm
- DOI: https://dx.doi.org/10.4239/wjd.v11.i10.435
The prevalence of diabetes mellitus (DM) in the general population is increasing so rapidly that diabetic nephropathy is now the leading cause of end-stage renal disease (ESRD) worldwide[1,2]. ESRD patients with DM who are undergoing renal replacement therapy in the form of dialysis pose certain group specific challenges to the overall well-being of these patients[3,4]. Adequate vascular access for hemodialysis is often a concern in ESRD patients with diabetes, consequently many patients may have to opt for peritoneal dialysis.
Peritoneal dialysis-associated peritonitis (PDAP) is a common and serious complication that not only leads to technical failure[5,6], but is also associated with increased mortality in patients undergoing peritoneal dialysis (PD)[7]. Moreover, PDAP-related death constitutes a major chunk of all-cause mortality in patients undergoing PD[8]. Some studies suggest that DM is a risk factor for PDAP[9,10], therefore, PDAP in patients with both ESRD and DM should draw careful attention. We hypothesized that there may be some differences in clinical features (symptoms and pathogens) and prognosis of PDAP between DM and non-DM patients. However, only few studies have analyzed the long-term prognostic outcomes of PDAP in patients with DM, often with discrepant results. Some studies concluded that DM was not a risk factor for death or technical failure[11,12] while others found DM to be a risk factor for all-cause death[13], yet again some researchers like Tsai et al[14] found DM to be a significant risk factor for a combined outcome of death and catheter removal. More importantly, as far as we know, the profile of clinical symptoms, distribution of pathogenic organisms, and response of PDAP to medical management in the subset of ESRD patients with DM has not been reported previously.
To clarify the above issues, a large multicenter retrospective cohort study was performed to compare the clinical features (symptoms and pathogens) and outcomes (therapeutic outcomes and long-term prognostic outcomes) of PDAP in ESRD patients with DM with those without.
This multicenter study was performed in Northeast China; the participating centers were The Second Hospital of Jilin University, The First Hospital of Jilin University-Eastern Division, Jilin FAW General Hospital, and Jilin Central Hospital. All PD patients who developed PDAP during the study period from January 1, 2013 to June 30, 2019 were recruited and followed until December 31, 2019. Patients with incomplete records, patients younger than 18 years, and those with chronic liver disease at initiation of PD were excluded from the study. Patients on immu-nosuppressant medications or steroids or with a history of intake of the same within the last 3 mo were also excluded from the study. We adhered to all the ethical requirements for retrospective observational studies at our center. Individual informed consent was waived given that the study was retrospective and non-interventional by design. We used a de-identified dataset. Diabetes was diagnosed according to American Diabetes Association criteria 2014[15]. According to the status of diagnosis of DM at initiation of PD, the patients were divided into a DM group and a non-DM group. They were followed until any of the following events: Death, a change to HD, renal transplantation, dropout, transfer to other centers, diagnosis with DM after initiation of PD, or until 31 December 2019.
Double-cuff Tenckhoff straight catheters and integrated Y-sets were used for PD treatment. PD trainings were conducted by experienced and educated physicians and nurses. The patients were asked to report back if they experienced cloudy effluent or abdominal pain. PD effluent was sent for bacterial and fungal culture by inoculation into blood culture media, and observed for at least 72 h to document pathogens. The diagnosis of PDAP required any two of the following features: (1) Clinical features consistent with peritonitis, i.e., abdominal pain and/or cloudy dialysis effluent; (2) dialysis effluent white cell count > 100/μL or > 0.1 × 109/L (after a dwell time of at least 2 h), with > 50% polymorphonuclear cells; and (3) positive dialysis effluent culture[16]. All patients suspected of having PDAP were managed as per the International Society for Peritoneal Dialysis recommendation, which includes treatment with empiric intra-peritoneal antibiotics at presentation covering both Gram-positive and Gram-negative organisms, after taking culture samples[16]. Subsequent choice of antibiotics was directed by the effluent culture and sensitivity results.
Baseline data collected at the time of first episode of PDAP encompassed demographic data [age, gender, presence of DM, and history of cardiovascular disease (CVD)], timing of PDAP episodes, clinical and biochemical data, fever, abdominal pain, PD cell count on admission, 24 h urine output, serum white cell count, hemoglobin, serum albumin, blood urea nitrogen, serum creatinine, and estimated glomerular filtration rate (eGFR). Biochemical measurements were performed by standard laboratory techniques. The culture results of effluent samples were subcategorized into mono-microbial (Gram-positive, Gram-negative, fungal, and mycobacterial organisms), polymicrobial, culture-negative, and no culture. Patients with ≥ 2 cultured pathogens were considered to have polymicrobial peritonitis.
Therapeutic outcomes of medical management of PDAP included complete cure, catheter removal, PDAP-related death, and relapse. Complete cure was defined as complete resolution of PDAP by antibiotics alone without relapse or recurrence within 4 wk of completion of therapy[17]. PDAP-related death was defined as patient’s death with peritonitis occurring within 30 d[8]. Relapse was defined as an episode occurring within 4 wk of completion of therapy with the same organism being isolated in effluent culture as in the previous episode[16].
Long-term prognostic outcomes of PDAP included continued PD, technical failure, and all-cause mortality. All-cause mortality was the primary endpoint in the patient survival analysis. If a patient died within 4 wk after switching over to hemodialysis, the death was attributed to PD because these early deaths are considered to reflect the health status of the patient during PD therapy[18]. Technical failure was defined as a switch to HD for at least 3 mo due to any reason[19].
Normally distributed parametric continuous variables are represented as the mean ± standard deviation, and were compared by Student’s t-test. Continuous variables with a non-normal distribution are represented as medians (Q1-Q3), and were compared using Wilcoxon’s rank-sum test. Categorical variables are represented as frequencies (percentages) and were compared using the chi-square (χ2) test. The risk factors associated with therapeutic outcomes of PDAP were analyzed using multivariable logistic regression. Kaplan-Meier survival curves were constructed to evaluate cumulative hazard of all-cause mortality and technical failure between the two groups, and differences in the survival distribution was assessed by log rank test. Cox proportional hazard analysis was used to analyze the relationship between DM and all-cause mortality. Data were analyzed using SPSS (version 22.0, IBM, New York, United States). A P value < 0.05 was considered statistically significant. All the artworks were created using GraphPad Prism (version 8.0).
The statistical methods of this study were reviewed by Su-Yan Tian from the First Hospital of Jilin University.
A total of 1145 episodes of PDAP occurred in 660 patients from four PD centers in Northeast China during the study period. Finally, 1065 episodes of peritonitis in 609 patients were included in this study (Figure 1). Patients in the DM group had significantly lower levels of serum albumin and serum phosphorus, but a higher level of eGFR (P < 0.05). There was no difference in the frequency or distribution of symptoms such as fever and abdominal pain between DM group and non-DM group (P > 0.05) (Table 1).
| Index | DM (n = 373) | Non-DM (n = 692) | P |
| Clinical manifestation | |||
| Fever | 115 (30.8) | 253 (36.6) | 0.061 |
| Abdominal pain | 300 (80.4) | 552 (79.8) | 0.797 |
| PD cell count on admission(/μL) | 1920 (620, 5350) | 1847 (613, 4974) | 0.791 |
| Laboratory test | |||
| WBC (1012/L) | 8.27 (6.57, 10.98) | 8.29 (6.09, 11.43) | 0.903 |
| Hb (g/L) | 97 (84, 109) | 99 (85, 113) | 0.128 |
| Alb (g/dL) | 28.24 ± 6.24 | 29.42 ± 6.29 | 0.003 |
| BUN (mmol/L) | 14.85 (10.79, 20.16) | 15.68 (12.01, 19.92) | 0.090 |
| Scr (μmol/L) | 672.66 (511.00, 854.50) | 735.60 (511.00, 954.35) | 0 |
| eGFR | 6.29 (4.68, 8.38) | 5.70 (4.35, 7.28) | 0 |
Among the 1065 PDAP episodes, 373 (35%) episodes occurred in the DM group. The differential distribution of causative organisms of PDAP between the two groups is shown in Table 2. The incidence of infection by Gram-positive bacteria, especially coagulase-negative Staphylococcus (CNS), was significantly higher in patients in the DM group (P < 0.05) while the incidence of E. coli and Pseudomonas aeruginosa infection was significantly higher in the non-DM group (P < 0.05). No significant difference was found in other organisms between the two groups (P > 0.05).
| Organism (n, %) | DM (n = 373) | Non-DM (n = 692) | P |
| Gram-positive | 172 (46.1) | 259 (37.4) | 0.006 |
| Coagulase-negative staphylococcus | 103 (27.6) | 130 (18.8) | 0.001 |
| Staphylococcus aureus | 22 (5.9) | 26 (3.8) | 0.108 |
| Streptococcus species | 30 (8.0) | 59 (8.5) | 0.786 |
| Enterococcus species | 8 (2.1) | 11 (1.6) | 0.514 |
| Other gram-positive | 9 (2.4) | 33 (4.8) | 0.060 |
| Gram-negative | 74 (19.8) | 170 (24.6) | 0.080 |
| Escherichia coli | 26 (6.7) | 73 (10.5) | 0.038 |
| Klebsiella species | 6 (1.6) | 20 (2.9) | 0.196 |
| Acinetobacter baumannii | 7 (1.9) | 17 (2.5) | 0.543 |
| Pseudomonas aeruginosa | 5 (1.3) | 27 (3.9) | 0.020 |
| Other gram-negative | 31 (8.3) | 32 (4.6) | 0.015 |
| Fungi | 13 (3.5) | 30 (4.3) | 0.501 |
| Mycobacterium tuberculosis | 2 (0.5) | 8 (1.2) | 0.508 |
| Polymicrobial | 24 (6.4) | 55 (7.9) | 0.369 |
| Culture-negative | 84 (22.5) | 163 (23.6) | 0.703 |
| No culture | 4 (1.1) | 7 (1.0) | 1.000 |
There was also no significant difference in the outcomes including rates of complete cure, catheter removal, PDAP-related death, and relapse of PDAP between the two groups (P > 0.05) (Figure 2A). By using a multivariable logistic regression model, we did not find diabetes to be a significant risk factor for adverse therapeutic outcomes of PDAP (P > 0.05) (Figure 2B).
The patients were followed for a median of 31.1 mo (interquartile range, 16.5-49.6 mo). Baseline demographic characteristics and laboratory test parameters of patients with and without DM were compared and are presented in Table 3. Compared with patients without DM, the patients with DM were older and had a higher burden of CVD (P < 0.05). Patients in the DM groups had a lower level of serum albumin, but a higher level of eGFR (P < 0.05).
| Variable | DM (n = 214) | Non-DM (n = 395) | P |
| Demographic characteristics | |||
| Age (yr) | 61 (54, 68) | 55 (42, 68) | 0 |
| Gender (male, n, %) | 117 (54.7) | 185 (48.6) | 0.065 |
| No. of PDAP episodes, n (%) | 0.596 | ||
| 1 | 127 (59.3) | 230 (58.2) | |
| 2 | 51 (23.8) | 86 (21.8) | |
| ≥ 3 | 36 (16.8) | 79 (20.0) | |
| CVD | 77 (36.0) | 104 (26.3) | 0.013 |
| Laboratory test | |||
| WBC (1012/L) | 8.41 (6.90,10.62) | 8.26 (5.99,11.29) | 0.411 |
| Hb (g/L) | 96 (80, 108) | 98 (83, 111) | 0.089 |
| Alb (g/dL) | 28.34 ± 6.74 | 29.60 ± 5.88 | 0.017 |
| BUN (mmol/L) | 14.9 (10.2, 20.5) | 16.0 (12.5, 20.2) | 0.062 |
| Scr (μmol/L) | 654.7 (480.0, 858.0) | 745.3 (575.2, 972.2) | 0.001 |
| eGFR | 6.46 (4.61, 8.55) | 5.51 (4.23, 7.17) | 0 |
| PD cell count on admission(/μL) | 1799.5 (571.0, 5040.0) | 2040 (739.0, 5219.5) | 0.330 |
One hundred and fifty (24.6%) patients died during the study period, of whom 71 belonged to the DM group and 79 to the non-DM group. The reasons for death included PDAP, cardiovascular death, cerebrovascular death, and others. Compared to the non-DM group, the all-cause mortality rate in the DM group was significantly higher, and correspondingly the rate of continuing on dialysis was significantly lower (P < 0.05). One hundred and thirty-four (22.0%) patients experienced technical failure, of whom 42 were in the DM group and 92 in the non-DM group. There was no significant difference in the rates of technical failure between the two groups (P > 0.05) (Figure 3A).
Kaplan-Meier survival curves showed that the DM group had a higher all-cause mortality rate compared to the non-DM group (P < 0.05) (Figure 3B). There was no significant association between the occurrence of diabetes and incident rates of technical failure (P > 0.05) (Figure 3C).
Cox regression analysis was used to analyze the relationship between DM and all-cause mortality. It was found that DM was a significant independent risk factor for all-cause mortality (hazard ratio = 1.531, 95% confidence interval: 1.091-2.148, P < 0.05) (Figure 3D).
The present study aimed to explore differences in the clinical features and outcomes in PDAP patients with and without diabetes as we hypothesized. We found that the symptoms of PDAP between the DM group and non-DM group were similar; the DM group had more infections with CNS and less infections with E. coli as compared to the non-DM group; the therapeutic outcomes of PDAP including complete cure, catheter removal, PDAP-related death, and relapse were comparable between the two groups; DM was an independent risk factor of all-cause mortality but not technique failure in PDAP patients.
We found no difference in the symptomatology of PDAP between the two groups. Theoretically, the symptoms of PDAP in diabetic patients may be atypical due to the presence of concomitant peripheral and autonomic neuropathy[14]. However, we confirmed that the symptoms of PDAP in the DM group were the same as those in the non-DM group for the first time.
To the best of our knowledge, no prior study has compared differences in microbial isolates on culture of effluent fluid between PDAP patients with and without diabetes. Our study characterized the microbiological etiology of PDAP in patients with DM over a period of 7 years. We found important differences in the distribution of organisms responsible for PDAP between the two groups, with a higher propensity for infection with Gram-positive bacteria, especially CNS in the DM group. One plausible reason for greater number of peritonitis episodes caused by CNS in diabetic population is due to higher risk of touch contamination and incorrect operation of peritoneal fluid exchange consequent to impaired vision due to diabetic retinopathy. The identification of an increased CNS peritonitis rate in this population suggests that more extensive training and more frequent review of operations might be beneficial. In addition, Staphylococcus epidermidis is the most common CNS, which can cause disease under certain circumstances. A study showed that the Staphylococcus epidermidis causing PDAP had low immunogenicity, which makes it more easily establish an infection since it cannot be immediately recognized by the immune system[20]. Meanwhile, DM is related to impaired immunity[21]. We consequently infer that CNS is inclined to colonize in PD patients with DM. Moreover, DM patients are more susceptible to infection especially in poorly controlled diabetics[22]. The impairment of neutrophil oxidative burst in individuals with poorly controlled diabetics may explain this phenomenon. A negative correlation was observed between neutrophil oxidative burst and hemoglobin A1c levels in the study by Osar et al[23]. And reduced neutrophil respiratory burst activity in diabetic patients could be restored to almost normal by blood glucose control[24]. However, our findings contrast with a previously published study, which showed that the development of CNS PDAP was not associated with DM[25]. A possible explanation for this discrepancy may be varying microbiological flora over geographical area and time.
E. coli is one of the most frequent causes of PDAP caused by Gram-negative bacteria. In our study, E. coli peritonitis was less common in patients with diabetes with no apparent explanation. Both E. coli virulence characteristics and host factors contribute to the development of PDAP. Previous studies showed that the PDAP E. coli isolates exhibited a superior virulence capability[26,27]. However, E. coli obtained from patients with PDAP did not show a common virulence profile and exhibited diverse serotypes[28]. Difference in virulence patterns of E. coli may explain a differential distribution frequency of infection between the DM group and non-DM group.
A noteworthy finding of our study is that the therapeutic outcomes of peritonitis between the DM group and non-DM group are comparable. More importantly, our results were further confirmed by using a multivariable logistic regression model. Our observation had both similarities and differences with previous data from ANZDATA registry study, which included 11122 episodes of peritonitis in 5367 patients in Australia during the period of 2004-2014[29]. Our study demonstrated that the complete cure rates were comparable between DM group and non-DM group, which was similar to the study by Htay et al[29]. DM was not associated with PDAP-related death in our study. In contrast, Htay et al[29] showed that DM correlated with PDAP-related death. Regional differences may account for this discrepancy.
The effect of DM on long-term prognostic outcomes of PDAP is controversial. A previous study found that DM is a risk factor for all-cause death[13]. Additionally, the study by Tsai et al[14] in a single Taiwan center also found a positive relation between DM and PDAP treatment failure, which was defined as death or catheter removal. However, in another study involving 483 patients (69 DM patients) diagnosed with PDAP, patients with DM had similar patient survival with those without DM[11]. In our study, we found a significantly higher all-cause mortality rate in the DM group than in the non-DM group. Higher burden of CVD could explain greater all-cause mortality in patients with diabetes. Patients with diabetes also had a lower level of serum albumin, which again has been associated with higher mortality in some studies[30,31]. Blood albumin is a marker of both ongoing inflammatory response and malnutrition, which is contained in the malnutrition inflammation score. High malnutrition inflammation score indicates malnourished status in patients undergoing PD[32], which further leads to bad clinical outcomes[33]. Clinicians need to pay more attention to the serum albumin status of patients with diabetes to improve the prognosis of PDAP. The discrepancy between our study and previous studies may be explained by distinct patient characteristics and different covariates included in the Cox proportional hazards model. Similar to the previous study, we also did not find diabetes to be a risk factor for technical failure, further affirming that diabetes should not be considered a hurdle for instituting peritoneal dialysis[11-13].
However, the present study has some limitations. Since it is a retrospective cohort study, potential bias and other confounding factors cannot be entirely excluded. Moreover, we did not consider the effect of indicators such as glycosylated hemoglobin and fasting blood-glucose on the outcomes of the study.
In conclusion, PDAP patients with DM have similar symptomology and are predisposed to CNS but not E. coli infection compared those without. DM is not associated with adverse therapeutic outcomes of PDAP. DM is associated with higher all-cause mortality but not technical failure in patients with PDAP.
The number of end-stage renal disease patients with diabetes mellitus (DM) who are undergoing peritoneal dialysis is increasing. Peritoneal dialysis-associated peritonitis (PDAP) is a serious complication of peritoneal dialysis leading to technical failure and increasing mortality in patients undergoing peritoneal dialysis. The profile of clinical symptoms, distribution of pathogenic organisms, and response of PDAP to medical management in the subset of end-stage renal disease patients with DM has not been reported previously. Discrepant results have been found in long-term prognostic outcomes of PDAP in patients with DM. It is important to clarify the clinical features and outcomes of PDAP patients with DM.
PDAP in DM patients is very common in the clinical practice, and treatment of PDAP in DM population is difficult and often with poor prognosis. Our research aimed to study the clinical manifestations, distribution of pathogenic organisms, and outcomes of PDAP in DM patients to provide a basis for future research of reasonable treatment and improvement of prognosis in this population.
This study aimed to compare the clinical features and outcomes of PDAP between patients with DM and those without. We found that the distribution of pathogenic organisms of PDAP was different between the DM group and non-DM group, and DM was a significant predictor of all-cause mortality but not technical failure.
This is a multicenter retrospective cohort study. We enrolled patients who had at least one episode of PDAP during the study period. The patients were divided into a DM group and a non-DM group. Clinical features, therapeutic outcomes, and long-term prognostic outcomes were compared between the two groups. Risk factors associated with therapeutic outcomes of PDAP were analyzed using multivariable logistic regression. A Cox proportional hazards model was constructed to examine the influence of DM on patient survival.
We confirmed that the symptoms of PDAP in the DM group were the same as those of the non-DM group (P > 0.05). The DM group had more infections with coagulase-negative Staphylococcus and less infections with Escherichia coli (E. coli) as compared to the non-DM group. DM was not associated with therapeutic outcomes (complete cure, catheter removal, PDAP-related death, or relapse) of PDAP (P > 0.05). The presence of DM was a significant predictor of all-cause mortality (hazard ratio = 1.531, 95% confidence interval: 1.091-2.148, P < 0.05), but did not predict occurrence of technical failure (P > 0.05). However, we did not consider the effect of indicators such as glycosylated hemoglobin and fasting blood-glucose on the outcomes of the study.
The symptoms of PDAP are similar in the DM group and non-DM group. Patients with diabetes are predisposed to coagulase-negative Staphylococcus but not E. coli infection. DM is associated with higher all-cause mortality but not therapeutic outcomes of PDAP.
Future research should focus on the effects of blood glucose control on PDAP outcomes, the mechanism of bacterial colonization, and ways to improve prognosis of PDAP in DM patients.
We are grateful to all staff of the four peritoneal dialysis centers, whose assistance in data collection and follow-up made this paper possible.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koch T S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Alicic RZ, Rooney MT, Tuttle KR. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol. 2017;12:2032-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1257] [Cited by in RCA: 1796] [Article Influence: 224.5] [Reference Citation Analysis (0)] |
| 2. | Tang SCW, Yu X, Chen HC, Kashihara N, Park HC, Liew A, Goh BL, Nazareth MGC, Bunnag S, Tan J, Lun V, Lydia A, Sharma SK, Hoque E, Togtokh A, Ghnaimet M, Jha V. Dialysis Care and Dialysis Funding in Asia. Am J Kidney Dis. 2020;75:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Coimbra S, Reis F, Nunes S, Viana S, Valente MJ, Rocha S, Catarino C, Rocha-Pereira P, Bronze-da-Rocha E, Sameiro-Faria M, Oliveira JG, Madureira J, Fernandes JC, Miranda V, Belo L, Santos-Silva A. The Protective Role of Adiponectin for Lipoproteins in End-Stage Renal Disease Patients: Relationship with Diabetes and Body Mass Index. Oxid Med Cell Longev. 2019;2019:3021785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | de Moraes TP, Figueiredo AE, de Campos LG, Olandoski M, Barretti P, Pecoits-Filho R; BRAZPD Investigators. Characterization of the BRAZPD II cohort and description of trends in peritoneal dialysis outcome across time periods. Perit Dial Int. 2014;34:714-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Li PK, Chow KM, Van de Luijtgaarden MW, Johnson DW, Jager KJ, Mehrotra R, Naicker S, Pecoits-Filho R, Yu XQ, Lameire N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13:90-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 384] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 6. | Ghali JR, Bannister KM, Brown FG, Rosman JB, Wiggins KJ, Johnson DW, McDonald SP. Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit Dial Int. 2011;31:651-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 151] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Ye H, Zhou Q, Fan L, Guo Q, Mao H, Huang F, Yu X, Yang X. The impact of peritoneal dialysis-related peritonitis on mortality in peritoneal dialysis patients. BMC Nephrol. 2017;18:186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Boudville N, Kemp A, Clayton P, Lim W, Badve SV, Hawley CM, McDonald SP, Wiggins KJ, Bannister KM, Brown FG, Johnson DW. Recent peritonitis associates with mortality among patients treated with peritoneal dialysis. J Am Soc Nephrol. 2012;23:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Ozener C, Arikan H, Karayaylali I, Utas C, Bozfakioglu S, Akpolat T, Ataman R, Ersoy F, Camsari T, Yavuz M, Akcicek F, Yilmaz ME. The impact of diabetes mellitus on peritoneal dialysis: the Turkey Multicenter Clinic Study. Ren Fail. 2014;36:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Chen HL, Tarng DC, Huang LH. Risk factors associated with outcomes of peritoneal dialysis in Taiwan: An analysis using a competing risk model. Medicine (Baltimore). 2019;98:e14385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Tian Y, Xie X, Xiang S, Yang X, Lin J, Zhang X, Shou Z, Chen J. Risk Factors and Outcomes of Early-Onset Peritonitis in Chinese Peritoneal Dialysis Patients. Kidney Blood Press Res. 2017;42:1266-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Ma X, Shi Y, Tao M, Jiang X, Wang Y, Zang X, Fang L, Jiang W, Du L, Jin D, Zhuang S, Liu N. Analysis of risk factors and outcome in peritoneal dialysis patients with early-onset peritonitis: a multicentre, retrospective cohort study. BMJ Open. 2020;10:e029949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | TekkariÅŸmaz N, Torun D. Long-term clinical outcomes of peritoneal dialysis patients: 9-year experience of a single centre in Turkey. Turk J Med Sci. 2020;50:386-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Tsai CC, Lee JJ, Liu TP, Ko WC, Wu CJ, Pan CF, Cheng SP. Effects of age and diabetes mellitus on clinical outcomes in patients with peritoneal dialysis-related peritonitis. Surg Infect (Larchmt). 2013;14:540-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2986] [Cited by in RCA: 3450] [Article Influence: 313.6] [Reference Citation Analysis (16)] |
| 16. | Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, Fish DN, Goffin E, Kim YL, Salzer W, Struijk DG, Teitelbaum I, Johnson DW. ISPD Peritonitis Recommendations: 2016 Update on Prevention and Treatment. Perit Dial Int. 2016;36:481-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 589] [Cited by in RCA: 635] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 17. | Szeto CC, Kwan BC, Chow KM, Lau MF, Law MC, Chung KY, Leung CB, Li PK. Coagulase negative staphylococcal peritonitis in peritoneal dialysis patients: review of 232 consecutive cases. Clin J Am Soc Nephrol. 2008;3:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Prasad N, Sinha A, Gupta A, Sharma RK, Bhadauria D, Chandra A, Prasad KN, Kaul A. Effect of body mass index on outcomes of peritoneal dialysis patients in India. Perit Dial Int. 2014;34:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Wu H, Ye H, Huang R, Yi C, Wu J, Yu X, Yang X. Incidence and risk factors of peritoneal dialysis-related peritonitis in elderly patients: A retrospective clinical study. Perit Dial Int. 2020;40:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Jung K, Lüthje P, Lundahl J, Brauner A. Low immunogenicity allows Staphylococcus epidermidis to cause PD peritonitis. Perit Dial Int. 2011;31:672-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, Rutten GE. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 687] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 22. | Rayfield EJ, Ault MJ, Keusch GT, Brothers MJ, Nechemias C, Smith H. Infection and diabetes: the case for glucose control. Am J Med. 1982;72:439-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 410] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Osar Z, Samanci T, Demirel GY, Damci T, Ilkova H. Nicotinamide effects oxidative burst activity of neutrophils in patients with poorly controlled type 2 diabetes mellitus. Exp Diabesity Res. 2004;5:155-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Ihm SH, Yoo HJ, Park SW, Park CJ. Effect of tolrestat, an aldose reductase inhibitor, on neutrophil respiratory burst activity in diabetic patients. Metabolism. 1997;46:634-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Fahim M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW. Coagulase-negative staphylococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 936 cases. Nephrol Dial Transplant. 2010;25:3386-3392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Li YF, Su N, Chen SY, Hu WX, Li FF, Jiang ZP, Yu XQ. Genetic background of Escherichia coli isolates from peritoneal dialysis patients with peritonitis and uninfected control subjects. Genet Mol Res. 2016;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Lin WH, Tseng CC, Wu AB, Chang YT, Kuo TH, Chao JY, Wang MC, Wu JJ. Clinical and microbiological characteristics of peritoneal dialysis-related peritonitis caused by Escherichia coli in southern Taiwan. Eur J Clin Microbiol Infect Dis. 2018;37:1699-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Dias RCB, Vieira MA, Moro AC, Ribolli DFM, Monteiro ACM, Camargo CH, Tiba-Casas MR, Soares FB, Dos Santos LF, Montelli AC, da Cunha MLRS, Barretti P, Hernandes RT. Characterization of Escherichia coli obtained from patients undergoing peritoneal dialysis and diagnosed with peritonitis in a Brazilian centre. J Med Microbiol. 2019;68:1330-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Htay H, Cho Y, Pascoe EM, Hawley C, Clayton PA, Borlace M, Badve SV, Sud K, Boudville N, Chen JH, Sypek M, Johnson DW. Multicentre registry data analysis comparing outcomes of culture-negative peritonitis and different subtypes of culture-positive peritonitis in peritoneal dialysis patients. Perit Dial Int. 2020;40:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Jiang J, Wang LH, Fei YY, Zhou XW, Peng L, Lan L, Ren W. Serum Albumin at Start of Peritoneal Dialysis Predicts Long-Term Outcomes in Anhui Han Patients on Continuous Ambulatory Peritoneal Dialysis: A Retrospective Cohort Study. Kidney Dis (Basel). 2018;4:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Matsui M, Akai Y, Samejima KI, Tsushima H, Tanabe K, Morimoto K, Tagawa M, Saito Y. Prognostic Value of Predialysis Indices for Technique Failure and Mortality in Peritoneal Dialysis Patients. Ther Apher Dial. 2017;21:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Harvinder GS, Swee WC, Karupaiah T, Sahathevan S, Chinna K, Ahmad G, Bavanandan S, Goh BL. Dialysis Malnutrition and Malnutrition Inflammation Scores: screening tools for prediction of dialysis-related protein-energy wasting in Malaysia. Asia Pac J Clin Nutr. 2016;25:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 33. | Choi HY, Lee JE, Han SH, Yoo TH, Kim BS, Park HC, Kang SW, Choi KH, Ha SK, Lee HY, Han DS. Association of inflammation and protein-energy wasting with endothelial dysfunction in peritoneal dialysis patients. Nephrol Dial Transplant. 2010;25:1266-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |