Published online Jul 15, 2019. doi: 10.4239/wjd.v10.i7.396
Peer-review started: March 22, 2019
First decision: May 31, 2019
Revised: June 10, 2019
Accepted: June 21, 2019
Article in press: June 21, 2019
Published online: July 15, 2019
Processing time: 118 Days and 3.9 Hours
Women with gestational diabetes mellitus have an increased risk of developing gestational hypertension, which can increase fetal and neonatal morbidity and mortality. In the past decade, single nucleotide polymorphisms in several genes have been identified as risk factors for development of gestational hypertension. The epidermal growth factor receptor activates tyrosine kinase mediated blood vessels contractility; and inflammatory cascades. Abnormalities in these mechanism are known to contribute towards hypertension. It is thus plausible that polymorphisms in the epidermal growth factor receptor gene would be associated with the development of hypertension in women with gestational diabetes.
To determine whether the epidermal growth factor receptor rs17337023 SNP is associated with the occurrence of hypertension in gestational diabetic women.
This pilot case-control study was conducted at two tertiary care hospitals in Karachi, from January 2017-August 2018. Two hundred and two women at 28 week of gestation with gestational diabetes were recruited and classified into normotensive (n = 80) and hypertensive (n = 122) groups. Their blood samples were genotyped for epidermal growth factor receptor polymorphism rs17337023 using tetra-ARMS polymerase chain reaction. Descriptive analysis was applied on baseline data. Polymorphism data was analyzed for genotype and allele frequency determination using chi-squared statistics. In all cases, a P value of < 0.05 was considered significant.
Subjects were age-matched and thus no difference was observed in relation to age of the study subjects (P >0.05). Body fat percentage was significantly higher in hypertensive females as compared to normotensive subjects (35.138 ± 4.29 Case vs 25.01 ± 8.28 Control; P < 0.05). Similarly, systolic and diastolic blood pressures among groups were significantly higher in hypertensive group than the normotensive group (P < 0.05). Overall epidermal growth factor receptor rs17337023 polymorphism genotype frequency was similar in both groups, with the heterozygous AT genotype (56 in Case vs 48 in Control; P = 0. 079) showing predominance in both groups. Furthermore, the odds ratio for A allele was 1.282 (P = 0.219) and for T allele was 0.780 (P = 0.221) in this study.
This pilot study indicates that polymorphisms in rs17337023 may not be involved in the pathophysiology of gestational hypertension in gestational diabetes via inflammatory cascade mechanism. Further large-scale studies should explore polymorphism in epidermal growth factor receptor and other genes in this regard.
Core tip: Gestational Hypertension (GHTN) can increase risk of fetal and neonatal morbidity and mortality. Many environmental, nutritional and genetic factors are related to the development of GHTN. Among them, Epidermal Growth Factor Receptor (EGFR) has been found to contribute to arterial hypertension. It is thus plausible that Single nucleotide polymorphisms (SNPs) in EGFR gene would be associated with the development of GHTN in women with GDM. This pilot study indicated that EGFR rs17337023 polymorphism may not be involved in the pathophysiology of GHTN in GDM positive females in a local population. Further large-scale studies should explore SNPs in EGFR and other genes in this regard.
- Citation: Martins RS, Ahmed T, Farhat S, Shahid S, Fatima SS. Epidermal growth factor receptor rs17337023 polymorphism in hypertensive gestational diabetic women: A pilot study. World J Diabetes 2019; 10(7): 396-402
- URL: https://www.wjgnet.com/1948-9358/full/v10/i7/396.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i7.396
Gestational diabetes mellitus (GDM), defined as any degree of glucose intolerance with onset or first recognition during pregnancy[1,2], is a significant risk factor for maternal development of a hypertensive pregnancy disorder (HPD)[3-5]. Up to 10% of all pregnancies are complicated by HPDs[6,7], especially in cases with pre-existing GDM[8]. One type of HPD, Gestational Hypertension (GHTN) occurs in 1.8%-4.4% of pregnancies[9]. GHTN is defined as blood pressure (BP) that reaches ≥ 140/90 mmHg for the first-time during pregnancy (after 20 wk gestation), without proteinuria. BP normalizes by 12 week postpartum[10]. Complications of GHTN include increased risk for fetal death and severe neonatal morbidity and mortality[11]. Hypothesized mecha-nisms of HPD development include dysfunction of the placenta, endothelium or lipid metabolism, as well as inflammatory states[12]. However, it is being increasingly established that genetic factors also contribute towards HPDs[13].
Single nucleotide polymorphisms (SNPs) have been a particular focus in genetic mechanisms leading to HPD[14]. SNPs such as NOS SNP rs2070744[15], APM1 SNP rs1501299[16], CYP19A1 SNP rs700158[17], KDR SNP rs2071559[18] and HSD11B1 rs846910[19], have been found to be associated with HPDs. The epidermal growth factor receptor (EGFR) is a single chain transmembrane protein of the ErbB family of receptor tyrosine kinases, which is activated following binding with peptide growth factors of the EGF-family of proteins[20]. The functions of EGFR include inducing cell growth and differentiation[21]. EGFR is abundantly expressed in the vascular wall and myocardium, and is thought to be linked to arterial hypertension, possibly by produc-ing vasoconstriction and renal Na+ retention[22]. Apart from its normal EGF Ligands, EGFR also undergoes transactivation by vasoactive substances such as catecho-lamines[23] and aldosterone[24]. The EGFR SNP rs17337023 (T > A), located on Exon 16 with a global variant allele frequency of 0.456[25], is associated with chronic infla-mmation, which may lead to vascular damage and hypertension[26,27]. Given the mechanistic link of EGFR to BP regulation, we decided to conduct a pilot study to explore any association of SNP rs17337023 with the development of GHTN in pregnant females with GDM.
In a case-control study, n = 202 pregnant women at 28 wk of gestation with GDM were recruited. The study was conducted at Aga Khan University and Jinnah Postgraduate Medical Center during the period of January 2017 till August 2018. The sample size was calculated using the Open-Epi website[28], with a confidence level of 95%, power of 80%, least extreme odds ratio (OR) of 2 and a pregnancy hypertension prevalence of 8% taken according to previously published data sources[29]. The minimum sample size calculated for this research was n = 106. The institutional ethics committee approved the research protocol (Ref # 4523-BBS-ERC-16) (REF: No.F.2-81/GENL-2017-IRB/15107/JPMC). GDM was diagnosed by means of a 75-g 2-h oral glucose tolerance test, as per the criteria set by the IADPSG[30]. All study subjects gave a written informed consent followed by weight and body mass index (BMI) assess-ment based on South Asian criteria for BMI values [normal weight (BMI 18-22.9 kg/m2), and obese (BMI ≥ 26kg/m2)][31]. BP assessment was done following the latest European Society of Cardiology and the European Society of Hypertension task force guidelines[32,33], (Normal BP < 139/85 mmHg and Hypertension > 139/85 mmHg). Subjects diagnosed with GHTN were subsequently being treated by antihypertensive medication. Any individual with a history of pre-existing diabetes, or any inflamm-atory condition, taking oral contraception or hormonal support, was not included in this study. Based on these measurements, grouping of study subjects was done as follow: (A) Normotensive (n = 80); (B) Hypertensive (n = 122) (on diet or medication).
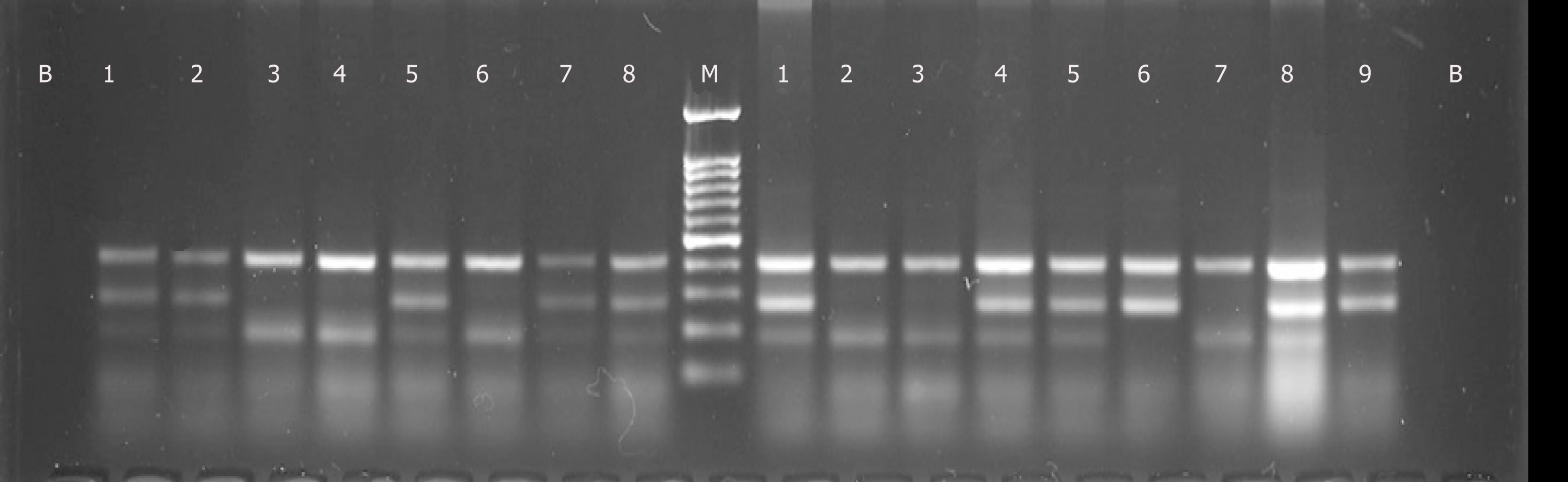
Ten milliliters of venous blood were collected from each subject. DNA was extracted from whole blood by Qiagen DNA extraction kit (Cat. #51185, Valencia, CA, United States). The quantification of extracted DNA was performed by measuring the ultraviolet absorbance of the samples using a Nanodrop-ND1000 (Thermo Fisher Scientific, Waltham, MA). The absorbance ratio (A280/A260) was determined for 2 μL samples using ND-1000 V3.8.1 software (Thermo Fisher Scientific, Waltham, MA). A ratio of approximately 1.8 was considered acceptable for confirming the purity of extracted DNA. Furthermore, around 10% of samples were confirmed on gel electro-phoresis by running 1 μL of sample in a 1% agarose gel against a 1 kb ladder. Tetra arms polymerase chain reaction (PCR) was performed using the Ruby Taq PCR Master mix 2X (Cat. #71191, Affymetrix, United States) as per the manufacturer’s instructions. PCR products were electrophoresed in a 2% agarose gel. Genotyping quality control was performed in 10% of the samples by duplicate checking (rate of concordance in duplicates was > 99 %). The following primer set was used for gene amplification: Statistical analyses were conducted using the IBM Statistical Package for the Social, Sciences (IBM SPSS version 21; IBM Corp Inc, Armonk, NY). Descriptive analysis was applied, and data was expressed either as mean ± standard deviation or absolute number and percentage. SNP data was analysed for genotype and allele frequency determination by applying chi-squared statistics. In all situations a P value of < 0.05 was considered significant. The statistical analyses for this study were performed and reviewed by Syed Adnan Ali (PhD. Statistics) of the University of Karachi.
The detailed results are shown in Table 1, 2 and Figure 1. All study subjects were age-matched and therefore, no difference was observed in relation to age of the study subjects (P > 0.05). Body fat percentage was significantly higher in hypertensive females as compared to normotensive subjects (P < 0.05). Similarly, systolic and diastolic BP among were significantly higher in hypertensive group than the normo-tensive group (P < 0.05). 90% of the hypertensive females practiced sedentary lifestyle versus 17.7% normotensive females (P < 0.05).
| Gene | Primers | Base pairs | PCR cycle | Amplicon size |
| Epidermal growth factor receptor (EGFR) rs17337023 | Forward outer: ATTAACCACCAATCCAACATCCAGAC | 26 | 67 °C; 30 s; 30 cycles | T allele 180; C allele 271; Control 406 |
| Reverse outer: CTTTCCCTCCACTGAGGACAAAGTT | 25 | |||
| Forward inner (A allele): TCTCTTTCACTTCCTACAGATGCTCA | 26 | |||
| Reverse inner (T allele): AGCCTTCAAGACCTGGCGCA | 20 |
| Hypertensive pregnant case (n = 122) | Normotensive pregnant control (n = 80) | P-value | |
| Age (Yr) | 30.55 ± 8.05 | 29.13 ± 10.19 | 0.054 |
| Weight (kg) | 77.56 ± 16.88 | 69.24 ± 11.07 | 0.025 |
| Body Fat % | 35.138 ± 4.29 | 25.01 ± 8.28 | 0.000 |
| Waist circumference (cm) | 104.50 ± 12.09 | 86.92 ± 12.03 | 0.000 |
| Systolic blood pressure (mmHg) | 131.76 ± 13.04 | 122.02 ± 8.27 | 0.000 |
| Diastolic blood pressure (mmHg) | 85.88 ± 8.45 | 73.31 ± 11.27 | 0.000 |
| Walk | |||
| None | 90.1% | 17.7% | 0.000 |
| 30 min/3 days week | 7.9% | 74.2% | |
| 30 min/5 days week | 2.0% | 8.1% | |
| Genotype frequency | |||
| EGFR rs17337023 polymorphism | |||
| AA | 30 | 19 | 0.079 |
| AT | 56 | 48 | |
| TT | 36 | 13 | |
| Allele Odds Ratio | |||
| Allele A | 1.282 [0.860-1.912] | 0.219 | |
| Allele T | 0.780 [0.523-1.163] | 0.221 | |
Overall EGFR rs17337023 polymorphism genotype frequency was similar in both the normotensive and hypertensive groups, with the heterozygous AT genotype showing predominance in both groups. Furthermore, the OR for A allele was 1.282 (P = 0.219) and for T allele was 0.780 (P = 0.221) in this study.
The developmental causes of GHTN in women with pre-existing GDM is poorly understood and it is possible that genetic factors such as SNPs may play a role. Many studies have demonstrated associations of certain SNPs with development of HPDs. Our objective was to investigate whether the EGFR SNP rs17337023 displayed any significant association with the occurrence of GHTN in pregnant women with GDM. However, the findings of our study showed that the frequency of the rs17337023 genotype was not significantly different in the two groups. Furthermore, the OR for the A and T alleles were also non-significant. These results suggest that the SNP rs17337023 does not play any major role in the pathophysiology of GHTN in GDM.
There are possible explanations for the lack of any significant association. The study proposing mechanisms linking EGFR to arterial hypertension does so primarily on the basis of results obtained from experimenting using animal models, and voices uncertainties about its applicability to humans[22]. Moreover, apart from Rheumatoid Arthritis, the SNP rs17337023 has been shown to have no significant association with pathologies such as Gastric Carcinoma[34] and Nasopharyngeal Carcinoma[35]. This suggests that the function of EGFR is not altered significantly enough due to the SNP rs17337023 mutation to cause any major pathological state. It is possible, however, that other EGFR SNPs may indeed be associated with GHTN in women with GDM.
Additionally, since the sample for our pilot study consisted of 202 women from Pakistan, it is possible that the results may show greater significance if the study were replicated in another population with a larger sample size. The lack of association between the EGFR SNP rs17337023 served to suggest that the EGFR gene may not be involved in the pathophysiology of GHTN in the case of pre-existing GDM. Our study was limited by the inability to recruit a larger sample size due to cultural beliefs and barriers towards participation in genetic studies. Moreover, the group of women with GHTN were not managed uniformly in terms of diet and antihypertensive medi-cations. Nevertheless, the rs17337023 polymorphism was in Hardy-Weinberg Equilibrium for cases and controls, suggesting the randomness of the sample as a strength of our study.
This pilot study indicates that polymorphisms in rs17337023 may not be involved in the pathophysiology of gestational hypertension in gestational diabetes via inflammatory cascade mechanism. Further large-scale studies should explore polymorphism in epidermal growth factor receptor and other genes in this regard.
Pregnancy induced hypertension and diabetes are an increasing threat to the wellbeing of both mother and the baby. The basic pathophysiological link to disease predisposition is attributed to the functionality of epidermis and angiogenesis. Several genetic studies have provided evidence that epidermal growth factor dysfunction can lead to hypertension and its complications in preg-nancy.
Materno-fetal mortality is on the rise in lower middle income countries; predominantly due to lack of primary prevention of non-communicable diseases. This led us to investigate one of the route cause i.e. genetic modification as a risk for development of disease.
Explore any association of SNP rs17337023 with the development of gestational hypertension in pregnant females with gestational diabetes.
A case-control study was conducted recruiting 202 pregnant women at 28 wk of gestation. Their blood pressure, blood glucose levels were measures and genotyping of EGFR SNP rs17337023 was performed via tetra arms PCR.
No difference was seen in the EGFR rs17337023 polymorphism genotype frequency among both normotensive and hypertensive groups in this study.
This pilot study indicates that polymorphisms in rs17337023 may not be involved in the pathophysiology of gestational hypertension in gestational diabetes. Further large-scale studies should explore polymorphism in epidermal growth factor receptor and other genes in this regard.
This study has shown some negative results linking a specific area of the gene EGFR; however, it should be noted that other factors may also be in play such as obesity and family history that can be a contributing factor along with genetic predisposition for hypertension. This opens up new avenues for researchers to perform prospective studies to identify the causal link between genetic and environmental factors.
The authors would wish to thank the study participants, and laboratory staff who helped us in this study.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Pakistan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Qu MH, Sanal MG S-Editor: Dou Y L-Editor: A E-Editor: Wang J
| 1. | Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. 1998;21 Suppl 2:B161-B167. [PubMed] |
| 2. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37 Suppl 1:S81-S90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2986] [Cited by in RCA: 3450] [Article Influence: 313.6] [Reference Citation Analysis (16)] |
| 3. | Casey BM, Lucas MJ, Mcintire DD, Leveno KJ. Pregnancy outcomes in women with gestational diabetes compared with the general obstetric population. Obstet Gynecol. 1997;90:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 288] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Joffe GM, Esterlitz JR, Levine RJ, Clemens JD, Ewell MG, Sibai BM, Catalano PM. The relationship between abnormal glucose tolerance and hypertensive disorders of pregnancy in healthy nulliparous women. Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1998;179:1032-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. 2004;191:1655-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 212] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 6. | Magee LA, Helewa M, Rey E; Hypertension Guideline Committee, Strategic Training Initiative in Research in the Reproductive Health Sciences (Stirrhs) Scholars. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30:S1-S2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 7. | Lai C, Coulter SA, Woodruff A. Hypertension and Pregnancy. Tex Heart Inst J. 2017;44:350-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Suhonen L, Teramo K. Hypertension and pre-eclampsia in women with gestational glucose intolerance. Acta Obstet Gynecol Scand. 1993;72:269-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 10. | Watanabe K, Naruse K, Tanaka K, Metoki H, Suzuki Y. Outline of definition and classification of “pregnancy induced hypertension (PIH)”. Hypertension Research in Pregnancy. 2013;3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba'aqeel H, Farnot U, Bergsjø P, Bakketeig L, Lumbiganon P, Campodónico L, Al-Mazrou Y, Lindheimer M, Kramer M; World Health Organization Antenatal Care Trial Research Group. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194:921-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 338] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 12. | Wang Y, Liu RX, Liu H. Association of adiponectin gene polymorphisms with hypertensive disorder complicating pregnancy and disorders of lipid metabolism. Genet Mol Res. 2015;14:15213-15223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kruglyak L. Prospects for whole-genome linkage disequilibrium mapping of common disease genes. Nat Genet. 1999;22:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 862] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 14. | Williams PJ, Broughton Pipkin F. The genetics of pre-eclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:405-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Leonardo DP, Albuquerque DM, Lanaro C, Baptista LC, Cecatti JG, Surita FG, Parpinelli MA, Costa FF, Franco-Penteado CF, Fertrin KY, Costa ML. Association of Nitric Oxide Synthase and Matrix Metalloprotease Single Nucleotide Polymorphisms with Preeclampsia and Its Complications. PLoS One. 2015;10:e0136693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Wang Y, Liu RX, Liu H. Association of adiponectin gene polymorphisms with hypertensive disorder complicating pregnancy and disorders of lipid metabolism. Genet Mol Res. 2015;14:15213-15223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Shimodaira M, Nakayama T, Sato I, Sato N, Izawa N, Mizutani Y, Furuya K, Yamamoto T. Estrogen synthesis genes CYP19A1, HSD3B1, and HSD3B2 in hypertensive disorders of pregnancy. Endocrine. 2012;42:700-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Andraweera PH, Dekker GA, Thompson SD, Roberts CT. Single-nucleotide polymorphisms in the KDR gene in pregnancies complicated by gestational hypertensive disorders and small-for-gestational-age infants. Reprod Sci. 2012;19:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Shimodaira M, Nakayama T, Sato I, Sato N, Izawa N, Mizutani Y, Furuya K, Yamamoto T. Glucocorticoid synthesis-related genes: HSD11B1 and HSD11B2 in hypertensive disorders in pregnancy. Gynecol Endocrinol. 2013;29:657-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1429] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 21. | Voldborg BR, Damstrup L, Spang-Thomsen M, Poulsen HS. Epidermal growth factor receptor (EGFR) and EGFR mutations, function and possible role in clinical trials. Ann Oncol. 1997;8:1197-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 352] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 22. | Bełtowski J, Lowicka E. EGF receptor as a drug target in arterial hypertension. Mini Rev Med Chem. 2009;9:526-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Zhang H, Chalothorn D, Jackson LF, Lee DC, Faber JE. Transactivation of epidermal growth factor receptor mediates catecholamine-induced growth of vascular smooth muscle. Circ Res. 2004;95:989-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Grossmann C, Gekle M. Non-classical actions of the mineralocorticoid receptor: misuse of EGF receptors? Mol Cell Endocrinol. 2007;277:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Choi JE, Park SH, Kim KM, Lee WK, Kam S, Cha SI, Kim CH, Kang YM, Kim YC, Han SB, Jung TH, Park JY. Polymorphisms in the epidermal growth factor receptor gene and the risk of primary lung cancer: a case-control study. BMC Cancer. 2007;7:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Hashemi M, Atabaki M, Daneshvar H, Zakeri Z, Eskandari-Nasab E. Association of PTPN22 rs2476601 and EGFR rs17337023 Gene polymorphisms and rheumatoid arthritis in Zahedan, Southeast Iran. Int J Immunogenet. 2013;40:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Huang CM, Chen HH, Chen DC, Huang YC, Liu SP, Lin YJ, Chang YY, Lin HW, Chen SY, Tsai FJ. Rheumatoid arthritis is associated with rs17337023 polymorphism and increased serum level of the EGFR protein. PLoS One. 2017;12:e0180604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Dean AG SK, Soe MM. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Version. 2013. Available from: http://www.openepi.com/Menu/OE_Menu.htm/. |
| 29. | Perveen S. Frequency and impact of hypertensive disorders of pregnancy. J Ayub Med Coll Abbottabad. 2014;26:518-521. [PubMed] |
| 30. | International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, Dyer AR, Leiva Ad, Hod M, Kitzmiler JL, Lowe LP, McIntyre HD, Oats JJ, Omori Y, Schmidt MI. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2777] [Cited by in RCA: 3176] [Article Influence: 211.7] [Reference Citation Analysis (1)] |
| 31. | Snehalatha C, Viswanathan V, Ramachandran A. Cutoff values for normal anthropometric variables in asian Indian adults. Diabetes Care. 2003;26:1380-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 290] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 32. | Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; SOGC Hypertension Guideline Committee. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can. 2014;36:575-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 367] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 33. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members; Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4368] [Cited by in RCA: 4921] [Article Influence: 546.8] [Reference Citation Analysis (4)] |
| 34. | Zhang J, Zhan Z, Wu J, Zhang C, Yang Y, Tong S, Sun Z, Qin L, Yang X, Dong W. Association among polymorphisms in EGFR gene exons, lifestyle and risk of gastric cancer with gender differences in Chinese Han subjects. PLoS One. 2013;8:e59254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Gao LB, Wei YS, Zhou B, Wang YY, Liang WB, Li C, Li Y, Bai P, Fang WL, Xue H, Zhang L. No association between epidermal growth factor and epidermal growth factor receptor polymorphisms and nasopharyngeal carcinoma. Cancer Genet Cytogenet. 2008;185:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |