INTRODUCTION
Gestational diabetes mellitus (GDM) is defined as glucose intolerance that develops after the first trimester of pregnancy and resolves post-delivery. Its prevalence has increased over recent years due to a variety of factors including a change in diagnostic criteria, increasing maternal obesity, and increasing maternal age. Although estimates vary according to the population and diagnostic tests used, in Australia the current prevalence is around 13% (reported in 2016)[1] compared to 3.6% 20 years earlier[2].
GDM increases the risk of a number of adverse outcomes for both mother and baby. A common concern is the increased risk of a large baby, referred to as “large for gestational age” or “LGA” - usually defined as birthweight above the 90th percentile for gestational age[3]. A related term is macrosomia - usually defined as absolute birthweight greater than 4000 g or 4500 g[4]. LGA and macrosomia in turn pose risks for delivery complications as well as potential longer-term metabolic complications.
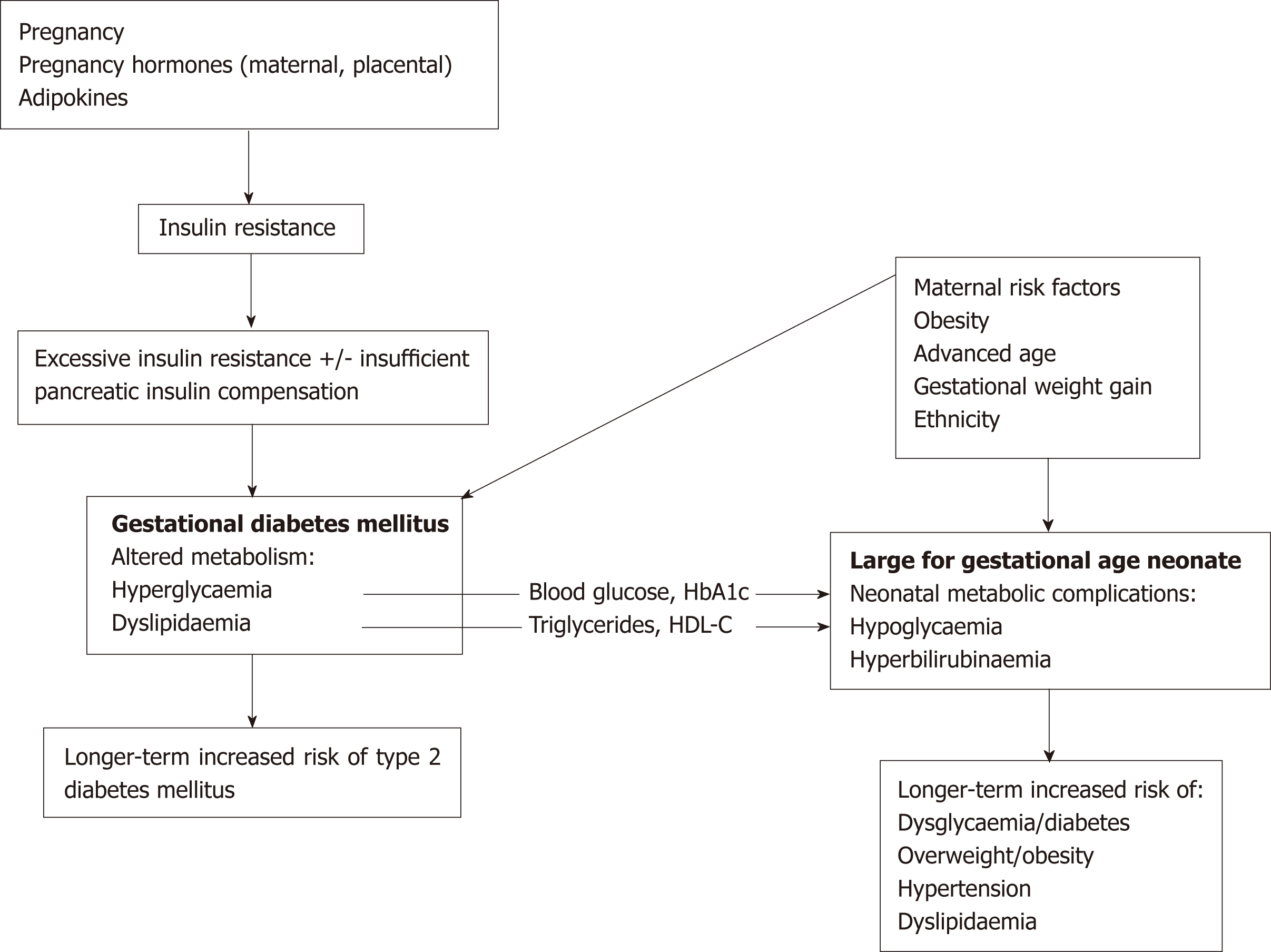
While fetal growth is determined by the interaction of a number of factors, both genetic and environmental, the link between GDM and LGA suggests there may be shared pathogenic elements (Figure 1). Indeed, risk factors in common for both conditions include older maternal age, maternal obesity, gestational weight gain, and ethnicity[4]. The aim of this paper is to review the key elements of maternal meta-bolism that are both altered in the GDM state and linked to higher birthweight in such pregnancies, and to discuss how they relate to nutritional guidelines for women with GDM.
Figure 1 Gestational diabetes mellitus increases the risk of large for gestational age offspring, and both have potential long-term metabolic consequences.
The two conditions also have shared maternal risk factors. Altered carbohydrate and lipid metabolism is present in pregnancies affected by gestational diabetes mellitus (GDM) and markers of this metabolism e.g., maternal blood glucose, triglycerides and high-density lipoprotein cholesterol (HDL-C), have been associated with large for gestational age offspring in GDM pregnancies. Therefore, these biomarkers may provide a link between the two conditions. HbA1c: Glycosylated haemoglobin; HDL-C: High-density lipoprotein cholesterol; GDM: Gestational diabetes mellitus.
GLYCAEMIA
Normal pregnancy involves a progressive increase in maternal insulin resistance from mid-pregnancy, promoting diversion of glucose to the fetus[4,5]. Maternal and placental hormones such as progesterone, oestrogen, prolactin, human placental lactogen, human placental growth hormone, and cortisol, are the main drivers for this insulin resistance[6,7]. Alterations in adipokines (proteins secreted by adipose tissue) such as tumour necrosis factor alpha, adiponectin, and leptin may also contribute[7].
In GDM, the insulin resistance of pregnancy is exaggerated, and/or maternal pancreatic beta cells can not sufficiently compensate[8]. GDM may therefore be the unmasking of underlying beta cell dysfunction. Other key elements of carbohydrate metabolism are also altered in GDM. For instance, compared to matched controls, women with GDM exhibit smaller increases in first-phase insulin response (the initial rapid insulin secretion in response to glucose ingestion that lasts only minutes) as pregnancy progresses[8]. Insulin suppression of gluconeogenesis is also impaired in women with GDM[9]. Overall, this leads to hyperglycaemia.
Maternal hyperglycaemia is believed to play a significant role in the development of LGA[9]. According to the hyperglycaemia-hyperinsulinaemia hypothesis (also called the Pedersen hypothesis), maternal hyperglycaemia leads to fetal hyperglycaemia as glucose is transferred down the placental concentration gradient[10]. This consequently stimulates the fetal pancreas to increase insulin production; the resulting hyperinsu-linaemia leads to fetal overgrowth as insulin is a prominent growth hormone for the fetus[11].
There is much data from human and animal studies indicating the importance of glycaemia management during pregnancy[10]. The Hyperglycemia and Adverse Pregnancy Outcomes study was the seminal paper demonstrating the continuous relationship between glucose levels at 24-28 wk’ gestation and pregnancy outcomes, including LGA offspring[12]. A similar linear relationship between maternal glucose and C-peptide levels in infant cord blood was also evident in this population, corro-borating the role of fetal hyperinsulinaemia. This study subsequently informed the development of GDM diagnostic criteria by demonstrating that hyperglycaemia below that of overt diabetes is associated with greater risk of adverse outcomes.
Moreover, other glycaemic markers have also been assessed for an association with LGA in GDM pregnancies. This includes glycosylated haemoglobin, 1,5-anhydrog-lucitol and fructosamine[13]; however, there is limited support for these markers. Overall, the association between glucose control and LGA offspring is incomplete, suggesting that other factors may be involved.
LIPIDS
Adaptive changes occur in lipid metabolism during normal pregnancy. The first two trimesters of gestation comprise an “anabolic phase”, involving enhanced maternal fat storage[14]. This occurs due to a combination of hormonal changes (e.g., progesterone, prolactin, cortisol) promoting lipid synthesis and storage, inhibition of lipid breakdown, as well as hyperphagia[15]. The final trimester is a “catabolic phase” where there is a net breakdown of fat stores. Insulin resistance plays an important role in this shift, by leading to enhanced lipolysis of adipose tissue and reduced lipoprotein lipase activity, thereby reducing overall lipid uptake[15]. These changes are associated with an initial decrease in maternal circulating lipid levels in the first trimester, with subsequent gradual increase across gestation, reaching peak levels prior to delivery[15]. The greatest increase is seen in triglyceride levels, which reach up to triple prepreg-nancy values[16].
Lipids are vitally important in fetal development, as they are involved in key processes such as synthesis of cell membranes and steroid hormones[17]. Fetal lipids are sourced through a combination of placental transfer and de novo synthesis. While maternal cholesterol is transferred across the placenta, particularly early on in pregnancy, endogenous fetal cholesterol synthesis becomes more prominent in late gestation[9,15]. On the other hand, triglycerides are the major lipid storage form in fetal adipocytes but maternal triglycerides do not cross the placenta intact. However, free fatty acids (FFAs) may be transferred across the placenta, with specific enzymes, receptors and binding proteins within the placenta thought to enable this process[15]. Fetal triglycerides are synthesised using FFAs[12,15].
In women with GDM, significantly higher triglyceride levels are present throu-ghout pregnancy, and lower high-density lipoprotein cholesterol (HDL-C) levels are present across the final two trimesters, compared to those without GDM[12]. The exaggerated insulin resistance in GDM again plays an important role, with one effect being reduced suppression of lipolysis[18].
The effect of these alterations in lipid metabolism on fetal adiposity in pregnancies with GDM is increasingly being recognised[12]. A meta-analysis involving GDM and non-GDM pregnancies indicated high maternal triglycerides and low HDL-C during pregnancy were associated with increased birthweight[19,20]. Furthermore, alterations in placental structure and function which are seen in GDM pregnancies, such as the expression of lipid-related genes and enzymes, as well as differences in placental phospholipid composition, raise the possibility of altered transplacental lipid pathways which may promote increased lipid transfer in GDM[21]. Indeed, the metabolic environment of GDM, involving hyperinsulinaemia, hyperlipidaemia, and hyperglycaemia, is proposed to contribute to enhanced transfer, synthesis and storage of lipids in fetal adipose tissue[15]. This has even led to revision of the Pedersen hypothesis, with lipids being proposed as a driver of LGA in pregnancies with dia-betes despite good glycaemic control[15,20].
Several studies have investigated associations between maternal lipids and birthweight in pregnancies affected by GDM. A study by Schaefer-Graf et al[22] showed third trimester maternal FFAs and triglycerides were associated with LGA newborns in GDM pregnancies, which remained significant after adjusting for confounding variables including glucose levels[23]. Of note, cord blood lipids were also sampled, with positive correlations evident between maternal and cord blood levels of triglycerides, FFAs, and glycerol. There was also indication of greater insulin resistance in LGA newborns, as cord blood insulin-to-glucose ratio was significantly positively correlated to birthweight.
Greater insight has come from a subsequent study from Schaefer-Graf et al[22], which found cord blood FFA levels were significantly lower in pregnancies without diabetes compared with those with GDM. This may be indicative of increased FFA trans-placental transport in GDM pregnancies. In addition, in a study of 104 Korean women with GDM, maternal hypertriglyceridaemia (defined as triglycerides > 75th percentile value, 3.33 mmol/L) at 24-32 wk’ gestation was significantly associated with LGA offspring, independent of maternal pre-pregnancy body mass index (BMI), gestational weight gain, maternal age or parity; although the model showed only a sensitivity of 48% and specificity of 83.5% for LGA prediction. This study found no significant association between total cholesterol or HDL-C levels and LGA newborns. In contrast, a European research group did find HDL-C was negatively associated with LGA newborns in GDM pregnancies[24]. Triglycerides, however, were not significantly associated in this study, which may be related to the earlier timing of the sample collection (booking visit compared to 24-32 wk’ gestation for the former study). Finally, a pilot study examining home monitoring of fasting and post-prandial triglyceride levels during late pregnancy has also been presented[25]. While the triglyceride levels were not correlated with birthweight, the study was limited by small sample size (twelve participants) and heterogeneous population (eight participants had GDM). It does however provide a new avenue to gather more comprehensive data on triglyceride profiles during pregnancy. In sum, there is increasing support for a role of lipids contributing to fetal overgrowth in GDM pregnancies.
Lipids are also being investigated as predictive biomarkers for GDM. Lipidomics, using techniques such as liquid-chromatography-mass spectrometry and nuclear magnetic resonance imaging, have been employed to identify potential lipid biomarkers of GDM in maternal blood[26]. However, limited data is available regarding optimal cut-off values[27,28]. One study of low risk pregnancies found triglycerides and LDL-C were independent predictors of GDM, although the sensitivity was low[5]. The ratio of triglycerides to HDL-C, proposed to be a clinical indicator of insulin resistance, may be superior than a single lipid biomarker[29], and has been shown to have a high negative predictive value, identifying women with low risk of GDM development in early gestation[30]. Ultimately, lipids may be most useful in a combined prediction model, including other biomarkers factors and clinical risk factors[31].
GDM NUTRITIONAL GUIDELINES
Given the aforementioned links between maternal glycaemia and lipids with GDM, it is unsurprising that dietary modification is central to GDM management. Indeed, medical nutrition therapy is considered first-line treatment, along with physical activity[32], aiming to achieve an appropriate balance between promoting normogly-caemia and adequate gestational weight gain, while allowing for optimal nutrition and fetal growth[33].
Despite being a foundation of management, a prominent issue is the paucity of specific dietary recommendations, reflecting the state of evidence. Indeed, there is substantial heterogeneity across studies including differences in experimental designs, macronutrient compositions, outcome measures, prescribed versus self-reported intakes (and lack of adherence monitoring), and lack of control conditions. Other issues include the small sample sizes, late timing of initiation of the dietary intervention and the short duration. Consequently, there is a lack of consensus, with differing guidelines available[34].
Carbohydrate intake has been the main focus of guidelines and research. Fasting and/or 2-h post-prandial glucose titres are key targets in the management of GDM[35,36]. The recommended dietary reference intake of carbohydrate during pregnancy is a minimum of 175 g per day[34], but the available evidence does not indicate the ideal carbohydrate intake for women with GDM. In part, this may be because the effect of carbohydrate intake may not only relate to the amount consumed (grams or percentage), but also to the type of carbohydrate [e.g., fibre, low/high glycaemic index (GI)], timing of consumption and the protein/fats consumed at the same time, thus elucidating the impact across studies can be difficult[35]. Indeed, recommendations on calorie intake vary, with studies examining a broad range from 1500 to 2800 kcal/d showing positive pregnancy outcomes[36]. Some guidelines do recommend moderate calorie restriction (1600-1800 kcal/d) for overweight or obese women with GDM to improve glycaemic control and limit gestational weight gain[29]. Currently, it is generally recommended that carbohydrate intake should be indivi-dualised with respect to amount and type of carbohydrate, according to ongoing assessment of nutrition and glycaemic management[37].
Different dietary interventions with varying carbohydrate compositions have been proposed for GDM management. GI is a relative ranking of food items (on a scale of 0-100), based on how quickly blood glucose levels increase after ingestion of a standard quantity of the particular food[38]. The GI is unchanged by pregnancy[39]. Low GI diets for women with GDM have been associated with reductions in the proportion of women requiring insulin and in neonatal birthweight in randomised control trials (RCTs)[37]. Meanwhile, a systematic review and meta-analysis of RCTs assessing different types of dietary interventions on maternal glycaemic control and birthweight in pregnancies with GDM concluded that on pooled analysis, dietary interventions demonstrated favourable effects compared with control diets[40]. This included improved maternal glycaemic control from baseline (fasting and post-prandial glucose levels), lower birthweight and less macrosomia (although similar LGA risk). While the quality of the evidence was graded as low, it does indicate nutritional guidance can play an important role in GDM management and there is potential for improvement in outcomes with firmer evidence to guide recommen-dations.
A concern about the attention on reducing carbohydrate intake is the possibility that it may lead to a proportional increase in dietary fat intake. The impact of this on pregnancy outcomes is unclear, although it may be associated with worsening of insulin resistance[34]. There has been limited specific investigation of maternal dietary lipid management during pregnancy[35]. A study involving women without GDM found a high fat maternal diet was associated with increased neonatal adiposity[41]. Meanwhile, a pilot study (n = 12) of women with diet-controlled GDM found a low fat/higher-complex carbohydrate diet was associated with lower insulin resistance of adipose tissue, compared with a higher fat/low-carbohydrate diet, although the birthweights of the two groups were not significantly different[42]. Furthermore, a high monounsaturated fatty acid diet compared with a high carbohydrate diet in GDM pregnancies showed there was better insulin sensitivity in the latter group, although the overall glucose control was similar between the groups[43]. There was no difference in birthweights, but the high monounsaturated fatty acid diet was associated with lower diastolic blood pressure. Moreover, a small study (n = 10) assessing the effect of the fat composition in a test meal on glycaemic parameters in women with GDM found that saturated fats were associated with significantly lower post-prandial glucose and insulin levels compared to monounsaturated fatty acids[44]. Hence, while there is a suggestion that dietary fats may influence metabolism in GDM, much more research is needed to properly assess this. Further evaluation of potential treatment options targeting maternal dyslipidaemia is also needed[45].
Furthermore, gestational weight gain is a related important consideration, with higher gestational weight gain associated with both GDM and LGA[41]. There are no specific guidelines for gestational weight gain in women with GDM, but since maternal overweight and obesity are also risk factors for adverse outcomes including GDM and LGA, the Institute of Medicine recommendations for appropriate gesta-tional weight gain are different according to pre-pregnancy BMI[46,47]. For women with obesity (BMI ≥ 30 kg/m2), 5-9 kg weight gain is recommended, compared to 11.5-16 kg for normal weight women (BMI 18.5-24.9 kg/m2). The importance of obesity in pregnancy is also highlighted by research demonstrating a synergistic relationship between obesity and GDM in increasing the risk of adverse pregnancy outcomes[48]. Meanwhile, although numerous diet and/or lifestyle interventions aiming to promote appropriate gestational weight gain have been reported, the results have overall been mixed, with only modest effectiveness demonstrated[49].
Finally, while many gaps remain in the current state of knowledge, techno-logical advancements are likely to be a key driver of developments in this space. Indeed, contributions from fields such as metabolomics are already shedding light on the mechanisms underlying GDM[50]. Analytical techniques such as mass spectrometry and nuclear magnetic resonance spectroscopy have been employed to investigate metabolic profiles associated with GDM and hence determine the pathways leading to insulin resistance[50]. Lipid and amino acid molecules have been most consistently identified by these processes thus far[51]. Importantly, such research is enabling identification of potential therapeutic targets, which in turn may involve dietary intervention. However, given the metabolic heterogeneity within pregnancy, personalisation of interventions will be important[52].
CONCLUSION
The relationships between GDM and LGA raise the possibility of shared mechanisms. Central components include abnormal carbohydrate and lipid metabolism, with insulin resistance recognised as a key instigating factor. While both glycaemic and lipid biomarkers have been associated with LGA, their utility for prediction in clinical practice is yet to be determined. Dietary modification is the cornerstone of GDM treatment, but there is insufficient data, particularly in the area of dietary lipids, to form definitive evidenced-based dietary recommendations. Hence there remains great need for rigorous studies investigating the impact of specific dietary interventions on pregnancy outcomes in women with GDM.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nobile S S-Editor: Dou Y L-Editor: A E-Editor: Wang J