Published online May 15, 2019. doi: 10.4239/wjd.v10.i5.291
Peer-review started: April 4 2019
First decision: May 8, 2019
Revised: May 13, 2019
Accepted: May 13, 2019
Article in press: May 14, 2019
Published online: May 15, 2019
Processing time: 44 Days and 8.9 Hours
Cardiovascular diseases (CVDs) remain the leading cause of death in the world and in most developed countries. Patients with type 2 diabetes mellitus (T2DM) suffer from both microvascular and macrovascular diseases and therefore have higher rates of morbidity and mortality compared to those without T2DM. If current trends continue, the Center for Disease Control and Prevention estimates that 1 in 3 Americans will have T2DM by year 2050. As a consequence of the controversy surrounding rosiglitazone and the increasing prevalence of diabetes and CVDs, in 2008 the Food and Drug Administration (FDA) established new expectations for the evaluation of new antidiabetic agents, advising for pre and, in some cases, post-marketing data on major cardiovascular events. As a direct consequence, there has been a paradigm shift in new antidiabetic agents that has given birth to the recently published American Diabetes Association/European Association for the Study of Diabetes consensus statement recommending sodium-glucose cotransporter-2 inhibitors (SGLT2i) and glucagon like peptide-1 receptor agonists (GLP-1RA) in patients with T2DM and established CVD. As a result of over a decade of randomized placebo controlled cardiovascular outcome trials, the aforementioned drugs have received FDA approval for risk reduction of cardiovascular (CV) events in patients with T2DM and established CV disease. SGLT2i have been shown to have a stronger benefit in patients with congestive heart failure and diabetic kidney disease when compared to their GLP-1RA counterparts. These benefits are not withstanding additional considerations such as cost and the multiple FDA Black Box warnings. This topic is currently an emerging research area and this mini-review paper examines the role of these two novel classes of drugs in patients with T2DM with both confirmed, and at risk for, CVD.
Core tip: Cardiovascular diseases are of significant concern in patients with type 2 diabetes mellitus. Novel therapies offer a new opportunity for cardiovascular risk reduction and add complexity in terms of selecting antihyperglycemic treatment. These pharmacological therapies, however, also have additional considerations.
- Citation: Pozo L, Bello F, Suarez A, Ochoa-Martinez FE, Mendez Y, Chang CH, Surani S. Novel pharmacological therapy in type 2 diabetes mellitus with established cardiovascular disease: Current evidence. World J Diabetes 2019; 10(5): 291-303
- URL: https://www.wjgnet.com/1948-9358/full/v10/i5/291.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i5.291
Cardiovascular disease (CVD) is the most important cause of morbidity and mortality in patients with type 2 diabetes mellitus (T2DM), with approximately 20% of the individuals with this condition suffering from established atherosclerotic disease[1-4]. The cardiovascular (CV) risk seems to be driven largely by coexisting conditions in addition to the independent risk related to hyperglycemia[5].
Modern medicine uses a polypharmacy approach due to the nature of the disease. Lipid lowering agents have been studied for years and data supports their cardiovascular benefit in selected patient with and without T2DM[6]. Antithrombotic therapies for primary and secondary prevention are a topic of much debate in recent years, with data both supporting[7], and refuting[8] the idea of one-size-fits-all in patients with T2DM. Blood-pressure goals have also been a point of controversy, as demonstrated by Effects of Intensive Blood-pressure Control in T2DM trial [by the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study group][9] and by the variation of goal blood pressures in major guidelines.
Intensive versus standard glycemic control has been a research question dating back to the 1990s with the United Kingdom Prospective Diabetes Study[10] with 3867 patients with T2DM and the Diabetes Control and Complications Trial[11] with 1441 patients with type 1 diabetes mellitus (T1DM). These trials demonstrated reduced microvascular endpoints but no difference in macrovascular endpoints with intensive glycemic control. Metformin use was associated with a reduction in DM-related complications and all-cause mortality. Fast-forward to 2008-09 and we have the large Action in Diabetes and Vascular Disease ADVANCE[12] trial with 11140 patients and the Veterans Affairs Diabetes Trial[13] with 1,791 patients, both showing intensive glycemic control having no impact on macrovascular outcomes in patients with T2DM.
Given the heterogeneity of diabetes, caution must be had in extrapolating results of one trial to a population with different baseline characteristics, whether that be the type of diabetes or the CVD risk. For example, the Epidemiology of Diabetes Interventions and Complications trial in 2005[14] showed that patients with T1DM had diminished rates of CVD with more stringent HbA1C targets. Then the same intervention of stringent HbA1C target resulted in the opposite outcome in those with T2DM in the large ACCORD trial in 2008[15] with 10251 patients, demonstrating increased mortality and no CV benefit.
With hypoglycemia identified as a driving factor for the increased rate of CV events and related mortality[16], our HbA1c targets became more liberal with many guidelines recommending HbA1c of 7%. As a result of the perceived need to avoid hypo-glycemia, a new drug class, the dipeptidyl peptidase-4 inhibitors, became available in the United States in 2007. They have shown non-inferiority in atherosclerotic CVD, yet, saxagliptin in particular has shown a potential risk in congestive heart failure. For the purposes of this mini-review, this class will not be covered in detail as there are no studies showing superiority in preventing major cardiovascular events (MACE) (Table 1).
| Trial | NumberFollow up | CVD (baseline) | Characteristics (baseline) | Drug vs Placebo (%) PEP | Superiority |
| SAVOR-TIMI53 (Saxagliptin) 2013 | n = 16492, 2.1 yr (median) | Pre-existing CV or high CV risk/multiple CV risk factors | 65 y/o, DM duration: 10 yr; A1c: 8%; BMI: 31 | 7.3 vs 7.2 | No |
| EXAMINE (Alogliptin) 2013 | n = 5380, 1.5 yr (median) | Acute MI or HUA in previous 15 to 90 d | 61 y/o, DM duration: 7 yr; A1c: 8%; BMI: 29 | 11.3 vs 11.8 | No |
| TECOS (Sitagliptin) 2015 | n = 14671, 3.1 yr (median) | Pre-existing CV disease (CAD, ischemic stroke, PAD) | 65.5 y.o, DM duration: 11.6 yr; A1c: 7.2%; BMI: 30.2 | 11.4 vs 11.6 (4-point MACE) | No |
One provocative event was when Rosiglitazone had a post-marketing meta-analysis showing an increased risk of CV events in T2DM patients using this medication. With that debacle and the increasing prevalence of T2DM and CVDs, in 2008 the Food and Drug Administration (FDA) issued new mandates on MACE safety for new antidiabetic drugs. Studies had to be presented prior to approvals and these would be followed by post-marketing cardiovascular outcome trials (CVOTs). This decision has helped bring data that, otherwise, would not have been available.
The paradigm shift has been to have antihyperglycemic agents show, not only noninferiority, but superiority in reducing MACE. As a result of over a decade of randomized placebo controlled CVOT, drugs in two classes have received FDA approval for risk reduction of CV events in patients with T2DM and established CV disease; glucagon like peptide receptor agonists (GLP1RA) and sodium-glucose cotransporter-2 inhibitors (SGLT2i).
The decision for clinicians in selecting a second antihyperglycemic agent after metformin in T2DM has become significantly more complex with much more data to consider (Tables 2 and 3). We will review the pharmacology followed by the current evidence of cardiovascular, renal, blood pressure, weight and other effects of GLP1RAs and SGLT2is.
| Study | Effects on microvascular complications | Effects on macrovascular complications | Effect on total mortality |
| DCCT[10] (1993), T1DM | Reduced retinopathy, nephropathy, neuropathy | No difference on major cardiovascular and peripheral vascular events | No difference |
| UKPDS[9] (1998) | Reduced microvascular endpoints | No difference on myocardial infarctions | No difference |
| ACCORD[14] (2008) | Reduced retinopathy, nephropathy, neuropathy | No difference on MACE | Increased mortality |
| ADVANCE[11] (2008) | Reduced nephropathy | No effect on MACE | No difference |
| VADT[12] (2009) | Reduced progression of albuminuria | No effects on major cardiovascular events | No difference |
| Trial | Number Follow up | CV disease (baseline) | Characteristics (baseline) | Drug vs Placebo (%) PEP | Superiority |
| ELIXA[22] (Lixisenatide) (2015) | n = 6068, 2.1 yr | Acute Coronary Events (previous 180 d) | Median age: 60; DM duration: 9.3 yr (median); A1c: 7.7%; BMI: 30.1 | 13.4 vs 13.2 (4-point MACE | No |
| LEADER[23] (Liraglutide) (2016) | n = 9340, 3.8 yr (median) | > 50 y/o + > 1 CV condition/CKD or Chronic HF or > 60 y/o > 1 risk factor for CVD | mean age: 64; DM duration: 12.8 yr (median); A1c: 8.7%; BMI: 32.5 | 13.0 vs 14.9 | Yes |
| SUSTAIN-6[24] (Semaglutide) (2016) | n = 3297, 2.1 yr (median) | > 50 y/o + > 1 CV condition/CKD or Chronic HF or > 60 y/o > 1 CV condition | mean age: 65; DM duration: 13.9 yr (median); A1c: 8.7%; BMI: 30.1 | 6.6 vs 8.9 | Yes |
| EXSCEL[26] (Exenatide) (2017) | n = 14752, 3.2 yr (median) | 70% with previous CV events (CAD, ischemic cerebrovascular disease, or PAD) | mean age: 63; DM duration: 12 yr (median); A1c: 8.0%; BMI: 32 | 11.4 vs 12.2 | No |
| REWIND (Dulaglutide) (2019) | ? | ? | ? | ? | ? |
| EMPA-REG[31] (Empagliflozin) (2015) | n = 7020, 3.1 yr (median) | Established CV disease; high CV risk | mean age: 63; DM duration: > 10 yr 57%; 5-10 yr 25%; A1c: 8.07%; BMI: 30.6 | 10.5 vs 12.1 | Yes |
| CANVAS[32] (Canagliflozin); ANVAS – R (Canagliflozin) (2017) | Total = 10142; CANVAS: n = 4330; CANVAS-R n = 5812; 3.6 yr (mean) | > 30 y/o at high CV risk (ASCVD) Or > 50 y/o > 2 CV risk factors | mean age: 63.3; DM duration: 13.5 yr (median); A1c: 8.2; %BMI: 32 | 9.8 vs 10.1 | Yes |
| DECLARE[33] (Dapagliflozin) (2019) | n = 17160; 4.2 yr (median) | > 40 y/o established CVD or multiple risk factors | MEAN age: 64; DM duration: 11 yr (median); A1c: 8.3%; BMI: 32 | 8.8 vs 9.4 | No |
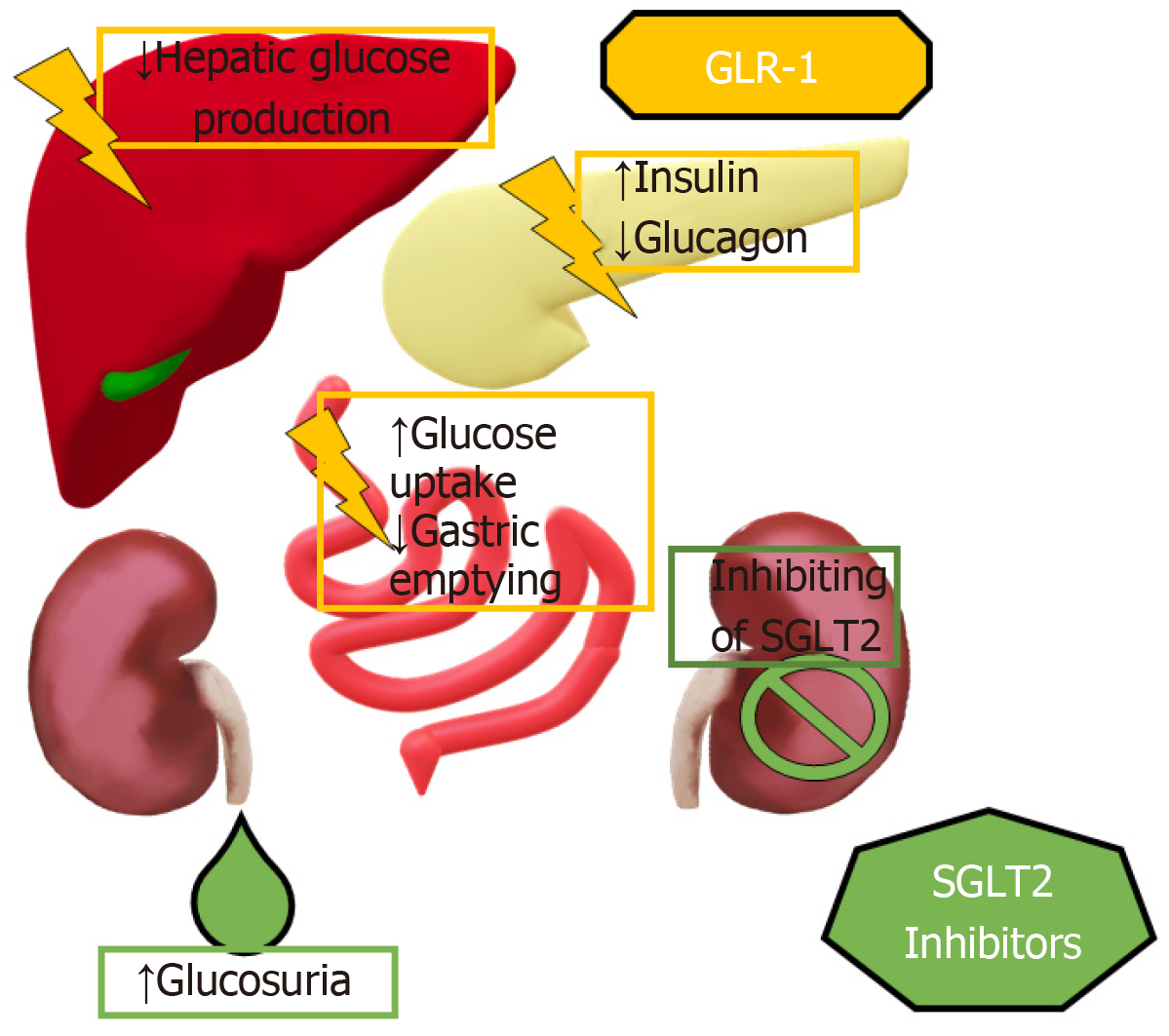
Glucagon like peptide-1 receptor agonists (GLP-1RAs) have been available in the market since 2005, however it has taken over a decade to understand their effects. As an endogenous substance, its insulinotropic effect when associated with glucose-dependent insulinotropic polypeptide is very well established, giving rise to the incretin effect[17] (Figure 1), which is significantly reduced in T2DM. Moreover, the discovery of receptors in the periphery[18] sensitive to GLP1 have raised several questions regarding the reach in which our exogenous, man-made GLP-1RA can have a positive impact in the health of patients with T2DM[19] given their increased potency and half-life compared to endogenous GLP1.
SGLT2 inhibitors have been available in our armamentarium since 2012 and were first used unrelated to β-cell function and insulin sensitivity[20]. Originating from observations and studies made on patients with Familial Renal Glucosuria[21], the effects of inhibiting SGLT2 are still under thorough investigation given the presence of such molecules not only in the proximal tubule of the nephron, but also on the glomerular basement membrane and in the heart[22].
Agents in both GLP-1RA and SGLT2i classes have obtained approval by the FDA for the indication of CV risk reduction in patients with T2DM and established CVD. Current data has proven that these agents can reduce the risk of MACE (CV death, nonfatal myocardial infarction and nonfatal stroke) with questions remaining on the ideal level of cardiovascular risk to benefit from GLP1RA. The CVOT design was intended to have both treatment groups maintain similar glycemic control, to minimize this confounder. In addition to this, SGLT2i have shown evidence of reduced hospitalization due to heart failure. New submissions to the FDA for both drug classes are in process.
The first GLP-1RA CVOT was the Evaluation of Lixisenatide in acute coronary syndrome trial[23] in 2015, studying the effects of lixisenatide in a high risk population with subjects that had an acute coronary syndrome in the 6 mo prior to the study with an average starting HbA1c of 7.7%, demonstrating noninferiority when compared to placebo but no superiority. One of the limitations of the trial was its short duration and the severity of the illness in this very high-risk population.
In 2016, GLP-1RA gained much more attention after the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER)[24] trial demonstrated the superiority of liraglutide in the primary end point (PEP) when compared against placebo in subjects with T2DM and high risk for CV events. These patients had a lower rate of CV death, nonfatal myocardial infarctions (MI) and nonfatal strokes (but no statistical difference with all strokes). Starting average HbA1c was 8.7%. The rate of hospitalization due to heart failure remained statistically nonsignificant.
Also, in 2016, the Trial to Evaluate Cardiovascular and Other Long-term Outcomes With Semaglutide in Subjects With Type 2 Diabetes (SUSTAIN-6)[25] compared the once-weekly injection of semaglutide to placebo and had similar outcomes to liraglutide on a patient with a sizable prevalence of ischemic heart disease and hypertension (60% and 93% respectively). This was achieved with fewer patients and less years of follow-up (2 compared to 4 in the LEADER trial). The once-weekly injection of semaglutide was FDA approved in 2017 and in 2019 Novo Nordisk filed for FDA approval for a new CV indication based on the SUSTAIN-6 trial. Simultaneously they filed for FDA approval for oral semaglutide[26] which would be the first GLP1RA in a pill form and the pertaining CVOT PIONEER6 is discussed below.
Subsequently in 2016-2017, two CVOTs were published on another GLP1RA exenatide. The Exenatide Study of Cardiovascular Event Lowering Trial[27] confirmed noninferiority with once weekly subcutaneous injection of exenatide but lacking superiority when comparing to placebo. The study had the largest population in CVOT at the time with 14752 patients, from which 70% had a previous CV event, including coronary artery disease, ischemic cerebrovascular disease or peripheral artery disease. On average, the starting HbA1c was 8%. The main pitfall of the study was the inclusion of a sizable number of patients using SGLT2i in the placebo group. A phase 3 safety trial, FREEDOM-CVO[28], had more than 4,000 patients supplied with exenatide through a continuous implanted pump and announced non-inferiority in CV safety. The subcutaneous pump would potentially address the high rate of discontinuation with weekly exenatide, which was 43%.
Finally, in 2018, three more CVOTs with GLP-1RAs were announced and full results are yet to be reported. REWIND[29], investigating a weekly dulaglutide with an international scope, 46% women, and including T2DM with coexisting CVD or 2 or more CV risk factors. Only 36% of the 9901 patients had established CVD, yet at a median follow-up of 5 years, dulaglutide was still showing significantly reduced MACE. Next, Albiglutide was studied in the HARMONY[30] trial which was also international across 28 countries and enrolling 9463 participants but all had established CVD and it was superior to placebo in reducing MACE. Lastly, the PIONEER6[31] examined oral semaglutide in patients with T2DM with high risk of CV events and showed non-inferiority but not superiority in MACE. Secondary outcomes though showed statistically significant reduction in CV death and all-cause mortality in those 3183 patients.
SGLT2i also had its first CVOT published in 2015, Empagliflozin, Cardiovascular Outcomes and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME)[32]. They enrolled 7028 patients with recognized CVD or elevated CV risk with an average starting HbA1c of 8%, demonstrating superiority over placebo, similar to the PEP of the LEADER trial with an additional benefit for hospitalization for heart failure and diabetic nephropathy. Later, the Canagliflozin Cardiovascular Assessment Study (CANVAS)[33] and the Study of the Effects of Canagliflozin on Renal Endpoints in Adult Participants with T2DM (CANVAS-R) trials had similar results by examining approximately 10000 patients with established CVD in a younger population. However, the magnitude of the benefit with canagliflozin was smaller compared to other trials (only a third of 1%, meaning we would have to treat several hundred more patients to prevent a MACE). It also raised safety concerns by showing increased risk for lower limb amputations and fractures while also being consistent with previous CVOTs in regards of the increased risk for mycotic infections but no change in rates of Diabetic Ketoacidosis.
In 2019, the Dapagliflozin Effect on Cardiovascular Events trial (DECLARE – TIMI 58)[34] studied dapagliflozin for primary and secondary prevention in patients with T2DM and CVD or at high-risk for CVD and was the largest CVOT to date with 17160 patients. It showed noninferiority in MACE without superiority. A reduction in hospitalization for heart failure and all-cause mortality was established with robust reductions in the renal composite endpoints, suggesting a delay in the development and progression of renal disease.
Results have for the most part been consistent, as was demonstrated by Cheng et al[35], who analyzed a total of 12 double-blind randomized controlled trials, concluding that liraglutide, empagliflozin and canagliflozin to be superior in CV outcome in comparison to placebo in patients with T2DM and established or high-risk for CVD.
From the abundance of evidence, clinicians have already established that the intensification of glycemic control is the best approach to reduce microvascular complications. But when microvascular disease has already taken place, our options have remained limited, with our first line of defense consisting of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers (ARB) in the case of nephropathy and blood pressure control, and symptomatic treatment for the case of neuropathy and retinopathy.
Most of the large RCTs involving either SGLT2i or GLP-1RA have demonstrated, to varying degrees, a reduction in microvascular endpoints and associated morbidity. This is especially relevant for patients with chronic kidney disease and albuminuria, who represent a vulnerable subset of patients who, until recently, lacked treatment options for both preventing the development of the disease and delayed the its progression when these two factors are already present.
SGLT2i have demonstrated effects in hyperglycemic states by enhancement of glycosuria and natriuresis[36]. These effects may have a renal protective role by indirectly lowering blood pressure by competitively blockading the SGLT2 receptors in the proximal convoluted tubules in the kidneys, thus preventing reabsorption of the filtered glucose and sodium, decreasing the overall effective intravascular volume in addition to the intended antihyperglycemic effect.
This is further exemplified by a new prospective analysis by Sugiyama et al[37]. In this study, dapagliflozin was used in patients with T2DM with ineffective glycemic control. Those patients who were treated with dapagliflozin had a significant decrease in urine albumin-to-creatinine ratio (UACR) and urine N-acetyl- β-glycosaminidase, a marker of kidney injury. We can speculate based on these findings that dapagliflozin might prevent the renal tubulointerstitial atrophy that is correlated with the development of chronic kidney disease (CKD) in patients with T2DM.
Patients with early and uncontrolled T2DM have an increased glomerular filtration rate (GFR). This exposes the proximal tubule to insulin and other growth factors, leading to hyperplasia and hypertrophy in the tubular cells with significant hyperfiltration[38]. In turn, hyperfiltration could be the leading cause of renal damage in people with T2DM. SGLT2i can reverse this hyperfiltration in certain patients by blocking the glucose reabsorption in the proximal tubule. A model of renal hyperfiltration developed with pharmacokinetics (PBPK) and pharmacodynamics (PD) by the Quantitative Systems Pharmacology Diabetes Platform have confirmed this hypothesis[39].
The evidence evaluating renal benefits of SGLT2i until recently, was limited by the fact that there has been no RCT trial where the primary outcome is renal with SGLT2i (recently, this has changed, see below). A meta-analysis of the CVOTs of SGLT2i in patients with T2DM including 34322 patients performed by Zelniker et al[40] in Lancet 2019 concluded that SGLT2i decreased the risk of progression of renal failure by 45% with lesser reductions in progression of renal disease in patients with more severe kidney disease at baseline.
Another 2019 systematic review of 27 studies totaling 7363 participants with T2DM and CKD[41] found SGLT2is demonstrated a nonsignificant decline in estimated glomerular filtration rate (eGFR) slope, though a significantly reduced risk of the composite renal outcome. A retrospective analysis made by Kobayashi et al[42], defined the renal effects of SGLT2i in Japanese patients with T2DM with CKD. Results were statistically significant for reduction in the UACR. GLP-1 RA have also demonstrated a certain degree of renal protection. Liraglutide and semaglutide have shown to decrease albuminuria while also halting the worsening of the eGFR[43].
GLP-1 acts directly in the kidney by inhibiting the NH3-dependent sodium reabsorption in the proximal tubule. The renal outcomes were a secondary outcome assessed in the LEADER trial[23], showing a delay in new onset macroalbuminuria with a reduction of 26% in patients with liraglutide and a notable decrease in UACR[44].
Due to lacking studies with primary renal outcomes, no GLP1RAs or SGLT2s have FDA approval for indication of renal benefits with T2DM. The recently published Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial[45], whose data was published recently, might be a game changer. It is been almost 18 years since the advent of renin-angiotensin-aldosterone system blockers, the last advancement in the area. The study randomly assigned patients to receive canagliflozin or placebo on top of renin-angiotensin-aldosterone-system (RAAS) blocker therapy, observing an impressive 30% relative risk reduction in the primary endpoint consisting of end-stage kidney disease, doubling of serum creatinine, or renal or cardiovascular death that seems to be independent of the glucose lowering properties due to the minimal A1c difference at the end of the study (0.1%). This concept will be tested in the ongoing trials for dapagliflozin and empagliflozin (Dapa-CKD and EMPA-KIDNEY trials respectively) which have a sizable portion of participants without diabetes.
Additionally, it is also important to remember renal dosing requirements. SGLT2i require, in general, an eGFR greater than 45. For now, dulaglutide and liraglutide remain as the only novel medications that can be used in moderate to severe CKD given the evidence provided by the Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7) trial[46] and the LEADER trial.
Both SGLT2is and GLP1RA have shown reduction in blood pressure, independent from their hypoglycemic mechanisms[47]. In Tikkanen’s[48] study of patients with T2DM and hypertension, at week 12 the mean difference versus placebo in mean 24-h systolic blood pressure was -3.44 mmHg and -4.16 mmHg with 10 mg and 25 mg of empagliflozin, respectively. Blood pressure can be reduced also in patients with nocturnal hypertension, as demonstrated in the SGLT-2i and ARB Combination Therapy in Patients with T2DM and Nocturnal Hypertension (SACRA) study, conducted in Japan. The reduction in nighttime systolic blood pressure (SBP) with the use of empagliflozin was associated with daytime reductions in SBP and 24-h SBP[49].
The activation of the RAAS increases the SGLT2 mRNA expression in the proximal renal tubular epithelial cells with subsequent sodium intake. This causes an expansion in the intravascular volume that leads to hypertension[50]. Although the inhibition of SGLT2 will activate the RAAS, it is suggested to combine the SGLT2i with any RAAS blockers to suppress RAAS and thus prevent hypertension[51].
Within GLP1RAs, exenatide and liraglutide have displayed a reduction in the systolic and diastolic blood pressure from 1 to 5 mmHg in comparison with other antidiabetic medications, like insulin, glimepiride, metformin, or placebo[52]. Co-initiating the GLP1RA exenatide and the SGLT2i dapagliflozin compared to either agent alone, the DURATION-8[53] trial showed the combination lowered the systolic blood pressure 4.1 mmHg, which was greater than either agent alone. Similar to renal outcomes, blood pressure has been a secondary outcome yet a beneficial one in alleviating some burden of hypertension with an antidiabetic agent[54].
Increasing BMI can lead to the development of T2DM and poses a greater risk of CVD and all-cause mortality. Weight loss in patients with T2DM is critical in the improvement of hyperglycemia and cardiovascular comorbidities like hypertension and hyperlipidemia[55].
Currently, the American Diabetes Association and the European Association for the Study of Diabetes have made an emphasis in the importance of lifestyle modifications, diet and exercise in patients with T2DM. Unfortunately, many of our antihy-perglycemic agents are associated with weight gain including thiazolidinediones, sulfonylureas and insulin. Though modest at 1-3kg weight loss[56], this has made the SGLT2i and the GLP-1RAs benefits in weight loss even more exciting.
In the DURATION-6[57] trial, extended-release exenatide demonstrated an average weight loss of about 2.87 kg, and liraglutide[58] has shown to reduce 4 to 6 kg of weight loss. The SCALE[59] trial evidenced an 8.4 kg weight loss compared to a placebo group of 2.8 kg when treating patients without T2DM with a high-dose of 3.0 mg injected liraglutide as an adjunct to diet and exercise. One must keep in mind the CVOT trials were mostly the 1.8 mg dosing and the applicability of these results to the 3.0 mg dose is unknown.
Moreover, in 2017, semaglutide[23] was associated with significant weight loss, which has shown to be superior to liraglutide in its dose for treatment for T2DM. Even though semaglutide not been approved for pharmacological weight loss therapy, it opens the possibility for one more GLP-1 RA being used as an anti-obesity drug that would prevent cardiovascular events in patients with T2DM. SGLT2i are associated with a more modest reduction of body weight, with dapagliflozin showing a mean 1.63 kg reduction compared with placebo. The DURATION-8[52] clinical trial confirmed that a combination of dapagliflozin and exenatide, plus metformin as a background therapy, resulted in a secondary outcome of weight loss of 3.4 kg, which was greater than either drug alone. Nevertheless, the trend is that GLP1RAs offer more weight loss compared to SGLT2i, which is a relief compared to the classes that are associated with weight gain such as insulin and sulfonylureas.
While these medications are helpful in weight management, it is important to keep in mind this does not triumph over comprehensive lifestyles changes with aerobic exercise and dietary changes. Also, equally important, weight loss using GLP-1 RA should be monitored at least every 3 mo from the starting of the treatment due to side effects[60].
Several concerns exist with both GLP-1RAs and SGLT2is which must be weighed against the benefits detailed above. Like with any agent, discussion of risks and benefits when starting treatment is recommended. GLP-1RAs most common adverse effect is gastrointestinal with nausea, vomiting and diarrhea[61]. Commonly, nausea tends to wane over time. Patients should be informed about this as well as the possibility of injection site reactions when beginning therapy. An increased rate of acute and/or chronic pancreatitis has been established with the available RCTs, but there is no firm evidence pointing towards causality[62]. Preclinical data from studies done in rodents[63] raised the possibility of a medication induced carcinogenesis, specifically medullary thyroid cancer (MTC), however, these effects may be irrelevant in humans[64]. Nevertheless, a Black Box warning remains with GLP1RAs and risk of thyroid c-cell tumors, including a contraindication in patients with personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2.
SGLT2is most common adverse effect is genitourinary. There is a fivefold increased risk of genital fungal infections with SGLT2i, including an FDA warning about rare occurrences of Fournier’s gangrene[65]. Multiple RCTs show increased risk of bacterial urinary tract infection versus placebo, which can prove to be a challenge when considering treatment[66]. No clinical trial has examined special circumstances (indwelling bladder catheterization, benign prostatic hypertrophy, chronic obstruction or ureteral reflux) but caution under these circumstances is advised. Given the inherent diuretic effect, patients who are prone to volume depletion (use of loop diuretics, the elderly) are at increased risk of complications, including hypotension[67].
The FDA[68] has issued a warning regarding SGLT2i users being more prone to DKA, secondary to the intrinsic shift in the metabolism of glucose to fat oxidation with the promotion of hyperglucagonemia and ketosis[69]. Canagliflozin has been associated with an increased risk for fractures[70] and lower-limb amputations[32], including a Black Box warning for the amputation risk. There is need for further research on whether these side effects are a class effect or unique to canagliflozin.
One of the most controversial topics is the cost-effectiveness and the prohibitive out-of-pocket costs of both drug classes. Studies with reliable results on the long-term economic burden are scarce. The number needed to treat on both classes is in the hundreds based on the CVOTs from which the indication for cardiovascular prevention was approved by the FDA. GLP-1RAs demonstrated CV benefit after several years of median follow up, compared to SGLT2is, specifically empagliflozin, which demonstrated a divergence in survival curve for MACE at 3 mo in the EMPA-REG study. Some of the drugs that had a statistically significant benefit in the PEP for cardiovascular outcome had a very small percentage of benefit over placebo (i.e., canagliflozin with 0.3% benefit over placebo). Side effects like lower-limb amputations, DKA and pancreatitis can be economically damaging and add several thousand dollars to the already high economic burden.
In summary, given the current data, both GLP-1 RA and SGLT2i have, to varying degrees, a benefit in renal and cardiovascular protection independent of their glucose-lowering potential in patients with T2DM and high risk of CVD. Additionally, they have more modest benefits in blood pressure and weight control. The low risk for hypoglycemia is appealing.
When starting therapy, the cost-effectiveness is a concern shared by clinicians and patients. The number needed to treat to prevent MACE in both drug classes are in the hundreds and the economic burden is in the thousands to millions per patient per year. Considering the benefits in each study were observed after several years of follow up, the out-of-pocket expense could be prohibitively high.
Both common and rare adverse effects are also a consideration. SGLT2i carry an increased risk for mycotic urinary tract infections, dehydration and DKA. Canagliflozin additionally has the concerns of bone fractures and lower-limb amputations. GLP-1RAs have been associated with both acute and chronic pancreatitis, as well as a common side effect of nausea. The evidence for pancreatitis is debatable and weak since it is not supported by trials or a meta-analysis. Reports of increased MTC risk in rodents has the resultant black box warning of thyroid c-cell tumors. Specific trials designed to take a closer look at these effects will be necessary in the future to prepare a better risk-benefit assessment. The economic burden needs to be added to the equation.
As more studies concerning different agents on the same class of drugs are added to the already existing data, the question on whether each new finding is a class affect or a molecule-based outcome will be determined. With the current evidence at our disposal, we cannot guarantee that GLP-1 RAs all have the same benefits and what the ideal patient population is to maximize those benefits. SGLT2is, on the other hand, seem to offer a more homogenous effect with certain differences that can be attributed to each individual study to a certain extent, but more research is necessary.
As clinicians, we are moving a step forward in T2DM management. Now, patients can be offered antihyperglycemic agents that will treat micro and macrovascular complications while also treating independent risk factors, maintaining an acceptable level of antihyperglycemic effect with a low risk for hypoglycemic events.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: García-Mayor RV, Karras SN S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
| 1. | World Health Organization. Cardiovascular Diseases (CVDs). 2017; Available from: http://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). |
| 2. | Center for Disease Control and Prevention. Number of Americans with Diabetes Projected to Double or Triple by 2050. 2010; Available from: http://www.cdc.gov/media/pressrel/2010/r101022.html. |
| 3. | Davies MJ, D'Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, Rossing P, Tsapas A, Wexler DJ, Buse JB. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41:2669-2701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1622] [Cited by in RCA: 1821] [Article Influence: 260.1] [Reference Citation Analysis (0)] |
| 4. | Taylor KS, Heneghan CJ, Farmer AJ, Fuller AM, Adler AI, Aronson JK, Stevens RJ. All-cause and cardiovascular mortality in middle-aged people with type 2 diabetes compared with people without diabetes in a large U.K. primary care database. Diabetes Care. 2013;36:2366-2371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Saito I, Folsom AR, Brancati FL, Duncan BB, Chambless LE, McGovern PG. Nontraditional risk factors for coronary heart disease incidence among persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Ann Intern Med. 2000;133:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 141] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Cholesterol Treatment Trialists' (CTT) Collaboration. Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1073] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 7. | Antithrombotic Trialists' (ATT) Collaboration. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849-1860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2851] [Cited by in RCA: 2618] [Article Influence: 163.6] [Reference Citation Analysis (0)] |
| 8. | ASCEND Study Collaborative Group. Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J. Effects of Aspirin for Primary Prevention in Persons with Diabetes Mellitus. N Engl J Med. 2018;379:1529-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 773] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 9. | ACCORD Study Group. Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, Cutler JA, Simons-Morton DG, Basile JN, Corson MA, Probstfield JL, Katz L, Peterson KA, Friedewald WT, Buse JB, Bigger JT, Gerstein HC, Ismail-Beigi F. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575-1585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2787] [Cited by in RCA: 2456] [Article Influence: 163.7] [Reference Citation Analysis (0)] |
| 10. | Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 12672] [Article Influence: 469.3] [Reference Citation Analysis (0)] |
| 11. | Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17510] [Cited by in RCA: 16280] [Article Influence: 508.8] [Reference Citation Analysis (3)] |
| 12. | ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC Jr, Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2195] [Cited by in RCA: 1939] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 13. | Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD; VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3327] [Article Influence: 207.9] [Reference Citation Analysis (0)] |
| 14. | Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3867] [Cited by in RCA: 3436] [Article Influence: 171.8] [Reference Citation Analysis (0)] |
| 15. | Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6292] [Cited by in RCA: 5614] [Article Influence: 330.2] [Reference Citation Analysis (0)] |
| 16. | Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 363] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 17. | Sandoval DA, D'Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev. 2015;95:513-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 355] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 18. | Nauck MA, Homberger E, Siegel EG, Allen RC, Eaton RP, Ebert R, Creutzfeldt W. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 596] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Wei Y, Mojsov S. Tissue-specific expression of the human receptor for glucagon-like peptide-I: brain, heart and pancreatic forms have the same deduced amino acid sequences. FEBS Lett. 1995;358:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 316] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 20. | Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 1026] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 21. | Santer R, Calado J. Familial renal glucosuria and SGLT2: from a mendelian trait to a therapeutic target. Clin J Am Soc Nephrol. 2010;5:133-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 22. | Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014;8:1335-1380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 23. | Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Køber LV, Lawson FC, Ping L, Wei X, Lewis EF, Maggioni AP, McMurray JJ, Probstfield JL, Riddle MC, Solomon SD, Tardif JC; ELIXA Investigators. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N Engl J Med. 2015;373:2247-2257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1573] [Cited by in RCA: 1733] [Article Influence: 173.3] [Reference Citation Analysis (0)] |
| 24. | Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4164] [Cited by in RCA: 4918] [Article Influence: 546.4] [Reference Citation Analysis (0)] |
| 25. | Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, Lingvay I, Rosenstock J, Seufert J, Warren ML, Woo V, Hansen O, Holst AG, Pettersson J, Vilsbøll T; SUSTAIN-6 Investigators. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3025] [Cited by in RCA: 4087] [Article Influence: 454.1] [Reference Citation Analysis (1)] |
| 26. | Andrew CA, Saunders KH, Shukla AP, Aronne LJ. Treating obesity in patients with cardiovascular disease: the pharmacotherapeutic options. Expert Opin Pharmacother. 2019;20:585-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, Chan JC, Choi J, Gustavson SM, Iqbal N, Maggioni AP, Marso SP, Öhman P, Pagidipati NJ, Poulter N, Ramachandran A, Zinman B, Hernandez AF; EXSCEL Study Group. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:1228-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1599] [Cited by in RCA: 1495] [Article Influence: 186.9] [Reference Citation Analysis (0)] |
| 28. | Intarcia Therapeutics. A study to evaluate cardiovascular outcomes in patients with type 2 diabetes treated with ITCA 650. [Accessed 19 October 2016]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT01455896 ClinicalTrials.gov Identifier: NCT01455896. |
| 29. | Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, Probstfield J, Riddle MC, Rydén L, Xavier D, Atisso CM, Avezum A, Basile J, Chung N, Conget I, Cushman WC, Franek E, Hancu N, Hanefeld M, Holt S, Jansky P, Keltai M, Lanas F, Leiter LA, Lopez-Jaramillo P, Cardona-Munoz EG, Pirags V, Pogosova N, Raubenheimer PJ, Shaw J, Sheu WH, Temelkova-Kurktschiev T; REWIND Trial Investigators. Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab. 2018;20:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 30. | Hernandez AF, Green JB, Janmohamed S, D'Agostino RB Sr, Granger CB, Jones NP, Leiter LA, Rosenberg AE, Sigmon KN, Somerville MC, Thorpe KM, McMurray JJV, Del Prato S; Harmony Outcomes committees and investigators. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 1218] [Article Influence: 174.0] [Reference Citation Analysis (0)] |
| 31. | Bain SC, Mosenzon O, Arechavaleta R, Bogdański P, Comlekci A, Consoli A, Deerochanawong C, Dungan K, Faingold MC, Farkouh ME, Franco DR, Gram J, Guja C, Joshi P, Malek R, Merino-Torres JF, Nauck MA, Pedersen SD, Sheu WH, Silver RJ, Tack CJ, Tandon N, Jeppesen OK, Strange M, Thomsen M, Husain M. Cardiovascular safety of oral semaglutide in patients with type 2 diabetes: Rationale, design and patient baseline characteristics for the PIONEER 6 trial. Diabetes Obes Metab. 2019;21:499-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 32. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8302] [Article Influence: 830.2] [Reference Citation Analysis (1)] |
| 33. | Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4498] [Cited by in RCA: 5405] [Article Influence: 675.6] [Reference Citation Analysis (0)] |
| 34. | Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Ruff CT, Gause-Nilsson IAM, Fredriksson M, Johansson PA, Langkilde AM, Sabatine MS; DECLARE–TIMI 58 Investigators. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4652] [Cited by in RCA: 4259] [Article Influence: 709.8] [Reference Citation Analysis (0)] |
| 35. | Cheng JWM, Colucci VJ, Kalus JS, Spinler SA. Managing Diabetes and Preventing Heart Disease: Have We Found a Safe and Effective Agent? Ann Pharmacother. 2019;53:510-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Saleem F. Dapagliflozin: Cardiovascular Safety and Benefits in Type 2 Diabetes Mellitus. Cureus. 2017;9:e1751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Sugiyama S, Jinnouchi H, Kurinami N, Hieshima K, Yoshida A, Jinnouchi K, Tanaka M, Nishimura H, Suzuki T, Miyamoto F, Kajiwara K, Jinnouchi T. Impact of Dapagliflozin Therapy on Renal Protection and Kidney Morphology in Patients With Uncontrolled Type 2 Diabetes Mellitus. J Clin Med Res. 2018;10:466-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Jaikumkao K, Pongchaidecha A, Chatsudthipong V, Chattipakorn SC, Chattipakorn N, Lungkaphin A. The roles of sodium-glucose cotransporter 2 inhibitors in preventing kidney injury in diabetes. Biomed Pharmacother. 2017;94:176-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Balazki P, Schaller S, Eissing T, Lehr T. A Quantitative Systems Pharmacology Kidney Model of Diabetes Associated Renal Hyperfiltration and the Effects of SGLT Inhibitors. CPT Pharmacometrics Syst Pharmacol. 2018;7:788-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Sabatine MS. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1923] [Article Influence: 320.5] [Reference Citation Analysis (0)] |
| 41. | Toyama T, Neuen BL, Jun M, Ohkuma T, Neal B, Jardine MJ, Heerspink HL, Wong MG, Ninomiya T, Wada T, Perkovic V. Effect of SGLT2 inhibitors on cardiovascular, renal and safety outcomes in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and meta-analysis. Diabetes Obes Metab. 2019;21:1237-1250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 42. | Kobayashi K, Toyoda M, Kimura M, Hatori N, Furuki T, Sakai H, Takihata M, Umezono T, Ito S, Suzuki D, Takeda H, Kanamori A, Degawa H, Yamamoto H, Machimura H, Mokubo A, Chin K, Obana M, Hishiki T, Aoyama K, Nakajima S, Umezawa S, Shimura H, Aoyama T, Sato K, Miyakawa M. Retrospective analysis of effects of sodium-glucose co-transporter 2 inhibitor in Japanese type 2 diabetes mellitus patients with chronic kidney disease. Diab Vasc Dis Res. 2019;16:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Scheen AJ. Effects of glucose-lowering agents on surrogate endpoints and hard clinical renal outcomes in patients with type 2 diabetes. Diabetes Metab. 2019;45:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Thomas MC. The potential and pitfalls of GLP-1 receptor agonists for renal protection in type 2 diabetes. Diabetes Metab. 2017;43 Suppl 1:2S20-2S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, Cannon CP, Capuano G, Chu PL, de Zeeuw D, Greene T, Levin A, Pollock C, Wheeler DC, Yavin Y, Zhang H, Zinman B, Meininger G, Brenner BM, Mahaffey KW; CREDENCE Trial Investigators. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2826] [Cited by in RCA: 3989] [Article Influence: 664.8] [Reference Citation Analysis (0)] |
| 46. | Tuttle KR, Lakshmanan MC, Rayner B, Busch RS, Zimmermann AG, Woodward DB, Botros FT. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018;6:605-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 432] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 47. | Katout M, Zhu H, Rutsky J, Shah P, Brook RD, Zhong J, Rajagopalan S. Effect of GLP-1 mimetics on blood pressure and relationship to weight loss and glycemia lowering: results of a systematic meta-analysis and meta-regression. Am J Hypertens. 2014;27:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 48. | Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC, Woerle HJ; EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 369] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 49. | Kario K, Okada K, Kato M, Nishizawa M, Yoshida T, Asano T, Uchiyama K, Niijima Y, Katsuya T, Urata H, Osuga JI, Fujiwara T, Yamazaki S, Tomitani N, Kanegae H. 24-Hour Blood Pressure-Lowering Effect of an SGLT-2 Inhibitor in Patients with Diabetes and Uncontrolled Nocturnal Hypertension: Results from the Randomized, Placebo-Controlled SACRA Study. Circulation. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 50. | Bautista R, Manning R, Martinez F, Avila-Casado Mdel C, Soto V, Medina A, Escalante B. Angiotensin II-dependent increased expression of Na+-glucose cotransporter in hypertension. Am J Physiol Renal Physiol. 2004;286:F127-F133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Lim S, Eckel RH, Koh KK. Clinical implications of current cardiovascular outcome trials with sodium glucose cotransporter-2 (SGLT2) inhibitors. Atherosclerosis. 2018;272:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, Ni Y, Liu D, Zhu Z. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab. 2013;15:737-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 53. | Frías JP, Guja C, Hardy E, Ahmed A, Dong F, Öhman P, Jabbour SA. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 300] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 54. | Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, Golden SH, Liu K, Mukamal KJ, Campbell-Jenkins B, Dyer AR. Association of weight status with mortality in adults with incident diabetes. JAMA. 2012;308:581-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 300] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 55. | Riaz H, Khan MS, Siddiqi TJ, Usman MS, Shah N, Goyal A, Khan SS, Mookadam F, Krasuski RA, Ahmed H. Association Between Obesity and Cardiovascular Outcomes: A Systematic Review and Meta-analysis of Mendelian Randomization Studies. JAMA Netw Open. 2018;1:e183788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 56. | Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 511] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 57. | Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, Hoogwerf BJ, Gao A, Boardman MK, Fineman M, Porter L, Schernthaner G. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 399] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 58. | Mehta A, Marso SP, Neeland IJ. Liraglutide for weight management: a critical review of the evidence. Obes Sci Pract. 2017;3:3-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 189] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 59. | Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015;373:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1579] [Article Influence: 157.9] [Reference Citation Analysis (0)] |
| 60. | Wilding JPH. Medication use for the treatment of diabetes in obese individuals. Diabetologia. 2018;61:265-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L; LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32:1224-1230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 629] [Cited by in RCA: 651] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 62. | Egan AG, Blind E, Dunder K, de Graeff PA, Hummer BT, Bourcier T, Rosebraugh C. Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N Engl J Med. 2014;370:794-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 369] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 63. | Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Mølck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M, Moerch U. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 451] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 64. | Hegedüs L, Moses AC, Zdravkovic M, Le Thi T, Daniels GH. GLP-1 and calcitonin concentration in humans: lack of evidence of calcitonin release from sequential screening in over 5000 subjects with type 2 diabetes or nondiabetic obese subjects treated with the human GLP-1 analog, liraglutide. J Clin Endocrinol Metab. 2011;96:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 65. | Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, Sarigianni M, Matthews DR, Tsapas A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 663] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 66. | Boyle LD, Wilding JP. A safety evaluation of canagliflozin: a first-in-class treatment for type 2 diabetes. Expert Opin Drug Saf. 2014;13:1535-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Zhang M, Zhang L, Wu B, Song H, An Z, Li S. Dapagliflozin treatment for type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2014;30:204-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 68. | Available from: http://www.fda.gov/Drugs/DrugSafety/ucm475463.htm. |
| 69. | Keller U, Schnell H, Sonnenberg GE, Gerber PP, Stauffacher W. Role of glucagon in enhancing ketone body production in ketotic diabetic man. Diabetes. 1983;32:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 70. | Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, Meininger G. Effects of Canagliflozin on Fracture Risk in Patients With Type 2 Diabetes Mellitus. J Clin Endocrinol Metab. 2016;101:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 323] [Article Influence: 35.9] [Reference Citation Analysis (0)] |