Published online Mar 15, 2019. doi: 10.4239/wjd.v10.i3.140
Peer-review started: February 20, 2019
First decision: February 26, 2019
Revised: March 6, 2019
Accepted: March 8, 2019
Article in press: March 8, 2019
Published online: March 15, 2019
Processing time: 24 Days and 12 Hours
Diabetes mellitus (DM) is a chronic systemic disease that has increases in prevalence over time. DM can affect all ocular structures, with cataract being the most common ocular complication. Cataract is the leading cause of blindness worldwide. Due to several mechanisms, there is an increased incidence of cataract formation in the diabetic population. Advancements in technology have now made cataract surgery a common and safe procedure. However, the diabetic population is still at risk of vision-threatening complications, such as diabetic macular edema (ME), postoperative ME, diabetic retinopathy progression, and posterior capsular opacification.
Core tip: Because the number of people with diabetes mellitus is predicted to increase in the future, cataract surgery will remain an important procedure for diabetic patients. Patients with diabetes have multiple issues which should be evaluated preoperatively, perioperatively, and in the postoperative period. The preoperative, intraoperative, and postoperative factors are of paramount importance in the management of such complications and in improving visual outcomes. This article aims to review diabetic cataracts and related complications, and to outline important management strategies.
- Citation: Kiziltoprak H, Tekin K, Inanc M, Goker YS. Cataract in diabetes mellitus. World J Diabetes 2019; 10(3): 140-153
- URL: https://www.wjgnet.com/1948-9358/full/v10/i3/140.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i3.140
The prevalence of diabetes mellitus (DM) is increasing on a daily basis, with the International Diabetes Federation estimating that there will be 439 million DM patients by 2030[1]. An aging population and longer patient life expectancy also means that the prevalence of DM will exceed 33% by 2050[2]. DM can lead to pathologies in many tissues in the eye structure, with both a systemic chronic metabolic disease and a microangiopathic character[3]. Cataract is one of the major causes of visual impairment in diabetic patients[4]. Patients with DM are reported to be up to five times more likely to develop cataract, in particular at an early age[5-8]. Due to the increasing prevalence of DM, the incidence of diabetic cataracts has also risen. Cataract extraction is one of the most common surgical procedures among the general population, and the number of cataract surgeries each year also continues to increase. Recent technological advancements in cataract surgery have improved surgical outcomes. However, in diabetic individuals, the scale of improvement is still a matter of debate, and many studies have revealed both the results and complications of cataract surgery in diabetic patients. In the light of these findings, this study will review related articles in order to highlight current developments and controversies regarding cataract surgery management in patients with DM.
Different types of mechanisms have been proposed for the pathogenesis of cataract in cases of DM.
It has been suggested that the polyol pathway-via which the enzyme aldose reductase (AR) catalyzes the reduction of glucose into sorbitol-is a central part of the mechanism of cataract development[9-11]. Multiple studies have been conducted to explain the AR pathway’s role in this process. The increased intracellular accumulation of sorbitol leads to a hyperosmotic effect, resulting in hydropic lens fibers that degenerate and form cataract[9,12]. The production of sorbitol in diabetic patients (as compared to nondiabetic patients) takes place more quickly than it can be converted into fructose by the enzyme sorbitol dehydrogenase. Intracellular removal of sorbitol through diffusion is also prevented because of its polar character. A hyperosmotic effect is created when an accumulation of sorbitol results in an infusion of fluid. Finally, animal studies have shown that the intracellular accumulation of polyols causes liquefaction of lens fibers resulting in the formation of lens opacities[9,10,12,13]. In the study of Oishi et al[13], it was found that AR levels in red blood cells of patients under the age of 60 and with short duration of DM had a positive correlation with the prevalence of posterior subcapsular cataract. Moreover, a negative correlation was reported between the level of AR in erythrocytes and the density of lens epithelial cells, which is known to be lower in diabetics than in nondiabetics. These findings suggest that AR may play a role in this pathomechanism.
Osmotic stress as a result of extensive swelling of the cortical lens fibers is another compounding mechanism in the rapid development of cataracts, especially in young patients with type 1 DM[14-16]. Osmotic stress resulting from the accumulation of sorbitol induces stress in the endoplasmic reticulum (ER), the main site of protein synthesis, resulting in the formation of free radicals[17]. Stress in the ER can also be caused by fluctuation of glucose levels that initiate an unfolded protein response producing reactive oxygen species and cause oxidative stress damage to lens fibers. Moreover, increased glucose levels in the aqueous humor may lead to glycation of lens proteins, a process that results in the formation of advanced glycation end products[18]. Fenton reactions resulting from elevated levels of hydrogen peroxide (H2O2) in the aqueous humor of diabetics also induces the generation of hydroxyl radicals (OH–) after entering the lens[19]. Another factor that is elevated in the lens and aqueous humor of diabetic patients is free radical nitric oxide (NO•), which may cause an increase in peroxynitrite formation, which contributes to cell damage due to oxidizing properties[20,21]. However, diabetic lenses have increased susceptibility to oxidative stress due to their impaired antioxidant capacity. Superoxide dismutase (SOD) is the most predominant antioxidant enzyme in the lens that degrades superoxide radicals (O2-) into H2O2 and oxygen. Several in vitro and in vivo animal studies have shown that SOD has protective properties against cataract development in the presence of DM[22-24].
Some studies have shown that osmotic stress in the lens resulting from sorbitol accumulation causes apoptosis in lens epithelial cells and leads to cataract formation[25]. Rapid glycemic control can also increase these effects in the lens by creating a hypoxic environment that reduces protective enzymes and increases oxidative radicals. High AR expression could constitute a risk factor that predisposes the lens to distortions in signaling through the extracellular signal-regulated kinase and c-Jun N-terminal kinase pathways-involved in cell growth and apoptosis, respectively-thereby altering the balance required for lens homoeostasis[11,26]. These findings show that impairments in osmoregulation may render the lens susceptible to even the smallest increase in AR-mediated osmotic stress, potentially leading to progressive cataract formation.
Another recently proposed mechanism is autoimmune hypothesis in acute bilateral type 1 diabetic cataracts[26]. The authors reported that insulin autoantibodies became positive within three months of beginning insulin treatment, and that this period coincided with cataract formation. Their suggestion that there could be an autoimmune process behind acute bilateral cataract in DM warrants further investigation[26].
The type of cataract seen in diabetic patients has also been investigated. The most common is the senile type[10]. However, snowflake cataracts, which are characteristic for DM, are very common in type 1 diabetics. Posterior subcapsular cataracts have also been shown to be significantly associated with diabetes. Increased levels of glycated hemoglobin were demonstrably associated with an increased risk of nuclear and cortical cataracts[6]. Further analysis revealed that diabetic patients were prone to developing cortical cataracts and that this process was associated with the duration of diabetes[5,7].
Finally, the initiating mechanism in diabetic cataract formation is the generation of polyols from glucose by AR. However, osmotic stress, apoptosis of the lens epithelial cells, and the autoimmune theories may be confounding mechanisms in the development of the cataract formation in DM.
Several clinical studies have reported that cataract formation occurs more frequently and at an earlier age in diabetic patients than in nondiabetic patients[7,27-29]. Some studies indicate that cataracts are three to four times more prevalent in patients with diabetes under the age of 65. In patients over 65, cataracts are twice as prevalent[27,30]. The main risk factors are longer duration of diabetes and poor metabolic control. Although older patients suffer from irreversible cataract formation, good metabolic control may reverse cataract in young diabetics.
Several important study groups have investigated cataract incidence in diabetic patients. The Wisconsin Epidemiologic Study of Diabetic Retinopathy investigated the incidence of cataract and factors associated with a higher risk of cataract surgery[7]. They found 8.3% of patients suffering from type 1 diabetes and 24.9% of those with type 2 diabetes had a 10-year cumulative incidence of cataract surgery. For type 1 diabetics, they found some risk factors, including age, severity of diabetic retinopathy (DR), and proteinuria; for Type 2 diabetics, risk factors included age and use of insulin[7].
The Beaver Dam Eye Study also reported an association between DM and cataract formation[5]. The study took place over five years and consisted of 3684 participants aged 43 and older. It showed an increased incidence and progression of cortical and posterior subcapsular cataracts for DM patients. It also found an increased risk of nuclear and cortical cataracts with increased levels of glycated hemoglobin. Further analysis of the study showed that diabetics had a higher rate of cortical lens opacities and previous cataract surgery than nondiabetics[6]. A longer duration of diabetes was also associated with increased frequency of both cortical cataracts and cataract surgery.
The Blue Mountains Eye Study aimed to examine the relationship between nuclear, cortical, and posterior subcapsular cataracts[31]. The study supported the findings of previous research, but also found an association between posterior subcapsular cataracts and DM. In contrast to the Beaver Dam Eye Study, nuclear cataracts showed a weak association with DM.
The Barbados Eye Study evaluated the relationship between diabetes and lens opacities among 4314 black participants[32]. The authors found that a history of DM (18% prevalence) was related to all lens changes, especially at younger ages. Another study by Srinivasan et al[33] found, for diabetics, the cumulative incidence of cataracts is much higher than that of progression. Moreover, they indicated that the main risk factor for cumulative incidence and progression of most types of cataract is age, with higher rates of both in older patients.
Approaches to the timing of cataract surgery in diabetic patients seem to be changing worldwide. Where once a more conservative approach was applied, now there is a growing tendency toward early surgery. Pollack et al[34] reported that the main cause of poor visual outcomes is macular edema (ME). For this reason, they do not recommend cataract extraction for eyes with DR until visual acuity has deteriorated to 20/100–20/200. Similarly, Schatz et al[35] stated that diabetic patients with cataracts might wish to postpone surgery, especially if there is any retinopathy present preoperatively.
The growing tendency toward earlier cataract surgery in patients with diabetes has contributed to improved visual outcomes[36]. This approach facilitates panretinal photocoagulation (PRP) and also allows for the identification and adequate treatment of diabetic macular edema (DME) before cataract surgery. In addition, if surgery is undertaken before lens opacities make it more difficult to detect retinal thickening using macular assessment, then risk of ME decreases and visual outcomes may be considerably improved[37].
Preoperative counseling is crucial for diabetic patients. Before surgery, patients should have good glycemic control and no evidence of ocular or periocular infection. Transient refractive changes related to morphologic and functional changes in the crystalline lens should be observed during periods of unstable blood sugar[38]. Hyperglycemia induces myopia and, when intensive medical therapy is applied, patients tend to become more hyperopic as opposed to hyperglycemia. Changes in corneal topographic parameters during periods of glycemic changes can be a potential source of error in keratorefractive and biometric calculations[39].
A thorough and comprehensive ophthalmologic examination-including an assessment of bestcorrected visual acuity (BCVA) and relative afferent pupillary defect; using slitlamp biomicroscopy to assess the corneal health and neovascularization of the iris (NVI); and using tonometry, dilated fundoscopy, and gonioscopy for the evaluation of neovascularization at the angle-is mandatory. In select cases, advanced diagnostic evaluations such as fluorescein angiography, optical coherence tomography (OCT), and Bscan ultrasonography may be helpful. Due to the range of diabetic anterior segment changes, an experienced surgeon will perform better[40].
Consultation with vitreoretinal subspecialists is recommended by some authors, especially in complicated cases[41]. PRP is recommended preoperatively in patients with pre-existing proliferative diabetic retinopathy (PDR), because of its possible rapid progression after cataract surgery. In situations where lens opacity precludes PRP, it can be performed after surgery. Another approach is preoperative panretinal cryopexy or combined cataract surgery with vitrectomy and endolaser photocoagulation, particularly in cases with posterior pole tractional retinal detachment (TRD). ME should be efficiently treated preoperatively, since pre-existing maculopathy may worsen postoperatively and is strongly associated with a poor visual outcome[42].
Treatment options for ME are laser photocoagulation, pharmacotherapy with intravitreal injections of antivascular endothelial growth factor (anti-VEGF) agents, or steroids[43,44]. Because preexisting DME can increase the risk of ME progression by 20%–50%, intravitreal antiVEGF agents are recommended perioperatively[45,46]. Steroids, on the other hand, have been shown to be effective for persistent or refractory DME[47]. Dexamethasone implants and fluocinolone implants resulted in significant improvement in clinically significant ME and visual outcomes[48,49]. It has also been shown that dexametason has a potentially lower risk of intraocular pressure elevation and cataract formation compared to fluocinolone acetonide and triamcinolone acetate[50]. Recently, preoperative use of nonsteroidal antiinflammatory drugs, such as diclofenac and nepafenac, has been examined. Most studies suggested that they did not reduce the chances of postoperative ME in patients with DR[51-54].
Patients with NVI also need prompt treatment, including PRP. In patients who develop neovascular glaucoma (NVG), medical therapy is the first line of defense, however, it is usually ineffective. Eyes with active NVI are at greater risk for intraoperative and postoperative complications. Anti-VEGF agents such as bevacizumab showed dramatic short-term responses in terms of intraocular pressure reduction and regression of neovascularization in the treatment of NVG[55,56]. Cataract surgery after administering anti-VEGF agents should be done with or without vitrectomy as early as possible to enable treatment of the posterior segment. When NVG is a problem, a combination of trabeculectomy with phacoemulsification may also be considered after regression of NVI. Despite all these options, the visual outcomes following phacoemulsification in eyes with NVG are generally poor.
Cataract surgery in diabetic patients yields better results since the introduction of phacoemulsification, when compared to extracapsular or intracapsular cataract surgery[57,58]. Different options are available during surgery that can lead to better surgical results and improved postoperative retinopathy evaluation. As anterior capsular phimosis is more common in diabetic eyes, capsulorhexis size should be larger than normal but smaller than the intraocular lense (IOL) optic diameter, in order to prevent anterior IOL displacement and posterior capsular opacification (PCO)[59-61]. However, a large diameter optic is also important for the postoperative diagnosis and treatment of peripheral retinal pathology[8].
Progression of retinopathy after cataract surgery is another problem in diabetic patients[62]. The duration and complexity of cataract surgery are the main risk factors for progression of retinopathy[63]; it is therefore important to reduce the time and complexity of the surgery. Poor pupillary dilatation can be seen in diabetic patients as the result of damage to pupillary parasympathetic supply and elevated prostoglandin levels[64,65]. This means that pupil dilation is also a problem for these patients. As such, iris hooks, malyugin rings, or other iris expanders should be considered for intraoperative use. In cases with NVI, bleeding in the anterior chamber during or after surgery should also be kept in mind. Photic retinopathy during cataract surgery, especially surgeries of a longer duration, was also more prevalent in diabetic patients than nondiabetics[66].
While the presence of DM does not increase complications such as posterior capsular rupture, zonular dehiscence, or vitreous loss, the effect of DM on the entire eye can result in other problems. The effects of DM on the ocular surface include neurogenic effects (subbasal nerve abnormalities) and impaired corneal stem cell and epithelial cell division, which can result in keratoepitelyopathy and lead to corneal epithelial defects/abrasions, which may heal slowly[40,67]. It has also been shown that corneal endothelial cell loss is higher in people with diabetes than in nondiabetics[68-70]; this means that routine evaluation of diabetic patients using specular microscopy is recommended. Moreover, surgeons should take greater care in order to reduce endothelial stress during surgery.
The most common problem for diabetic patients is DR. For this reason, optimal visualization and treatment of the retina should be kept in mind during cataract surgery. As the diameter of the lens increases, it will provide a larger optical area-a difference that may be crucial for optimal management of DR.
PCO is another concern following cataract extraction. It has been reported that the development and severity of PCO is increased in DM patients as compared to non-diabetic patients[60,71]. Several studies have shown a relationship between the development of PCO and lens material type, and that the shape of the lens[72]. A square edge design seems to inhibit lens epithelial cell proliferation and may therefore prevent PCO formation[72].
Several studies have evaluated the biocompatibility of three common materials used to manufacture foldable IOLs with diabetic patients. One performed a comparison between hydrophobic acrylic and plate-haptic silicone IOLs in diabetic patients; although PCO developed less frequently with hydrophobic acrylic IOLs, it was demonstrated that this material was associated with a higher risk of anterior chamber flare in the early postoperative period[72]. In addition, hydrophobic acrylic lenses have the lowest propensity for silicone oil adhesion, meaning that they may be the IOL of choice for diabetic patients. Because diabetic patients may need vitreoretinal surgery during the course of managing their disease, silicone IOLs that develop condensation during pars plana vitrectomy may be relatively contraindicated in such individuals[73]. Hydrophilic acrylic IOLs are prone to opacification, particularly in patients with PDR, since elevated levels of phosphorus in the serum combined with the aqueous humor of diabetic patients may lead to opacification. Several reports have proved progressive calcific opacification of hydrophilic acrylic IOLs in diabetic patients[74-77]. Rodríguez-Galietero et al[78] evaluated contrast sensitivity and color discrimination in diabetic patients and suggested that blue-light filtering IOLs do not cause chromatic discrimination defects, but that they may even improve color vision in the blue-yellow chromatic axis. Multifocal and accommodative IOLs in people with diabetes are controversial. Postoperative laser treatment and fundus visualization during vitrectomy are difficult because of the optics of these types of lenses[79]. Additionally, the design of multifocal IOLs reduces contrast sensitivity and could be a cause of visual dissatisfaction for patients with preexisting maculopathy[79].
The implantation site in diabetic patients is also important. For DM patients, the ideal site is the capsular bag, as usual. The use of anterior chamber angle-fixated lenses and sulcus fixated posterior chamber IOLs in diabetic patients is controversial. It is recommended that iris claw lenses be avoided in patients with DM, due to the increased risk of iris neovascularization. The theoretical risk of cystoid ME, ovalization of the pupil, and poor mydriasis are other risk factors for diabetic patients after iris claw IOL implantation.
Carefully performed cataract surgery in diabetic patients should yield optimal postoperative results. Patient follow-up should also be done carefully. Preoperatively, patients diagnosed with NPDR who have adequate retinal view should undergo detailed retinal examination within three months of cataract extraction. Patients with PDR or those with inadequate retinal view prior to cataract extraction should be examined closely after surgery in order to evaluate their DR status[80].
Endophthalmitis is the most serious complication of cataract surgery. The risk of postoperative endophthalmitis in diabetic patients has increased and is associated with a poor visual prognosis.
As previously mentioned, as a patient’s age and duration of diabetes increases, there is greater prevalence of corneal epithelial defects and persistent erosions due to impaired corneal innervation[40,68]. Corneal endothelial cell damage and persistent corneal edema in diabetic patients following cataract surgery have also increased[81,82]. Specular microscopy should therefore be used to evaluate DM patients and all the necessary precautions should be taken intraoperatively. Also more frequently observed in diabetic patients are severe iritis, posterior synechiae, pupillary block, and pigmented precipitates on the IOL[83].
The Early Treatment Diabetic Retinopathy Study (ETDRS) outlines the prognostic factors after cataract surgery. The presence of clinically significant macular edema (CSME) at the time of surgery was found to be a predictor of poor final BCVA in cases of uncomplicated phacoemulsification[84]. Another determinant of poor postoperative BCVA was the severity of DR at the time of surgery. As the severity of retinopathy increased, the risk of macular ischemia or edema also increased[36,58,85]. More severe retinopathy also correlated with a reduced tendency for spontaneous resolution of postoperative ME, which is itself associated with poor postoperative BCVA. PDR without any treatment prior to cataract surgery is another factor-one which comes with an increased risk of vitreous hemorrhage and TRD following surgery[86].
Despite the advancement in phacoemulsification technology, poor visual acuity following cataract extraction is still common in patients with DM. PCO, postoperative cystoid macular edema (CME), DME, and worsening of the DR are the main complications seen in diabetic patients[87].
PCO is one of the most common causes of decreased vision after cataract extraction. Although modifications in surgical technique and improvements in IOL technology have reduced the incidence of PCO, it is still a problem for these patients. Proliferation of lens epithelial cells and the degree of postoperative inflammation are associated with development of PCO. Proliferation of lens epithelial cells is affected by several factors, including optic edge design, optic-haptic junction, and IOL material. However, surgical trauma and contact with the IOL can induce inflammation and cause epithelial cells to produce cytokines, which induce collagen production and fibrous metaplasia[88].
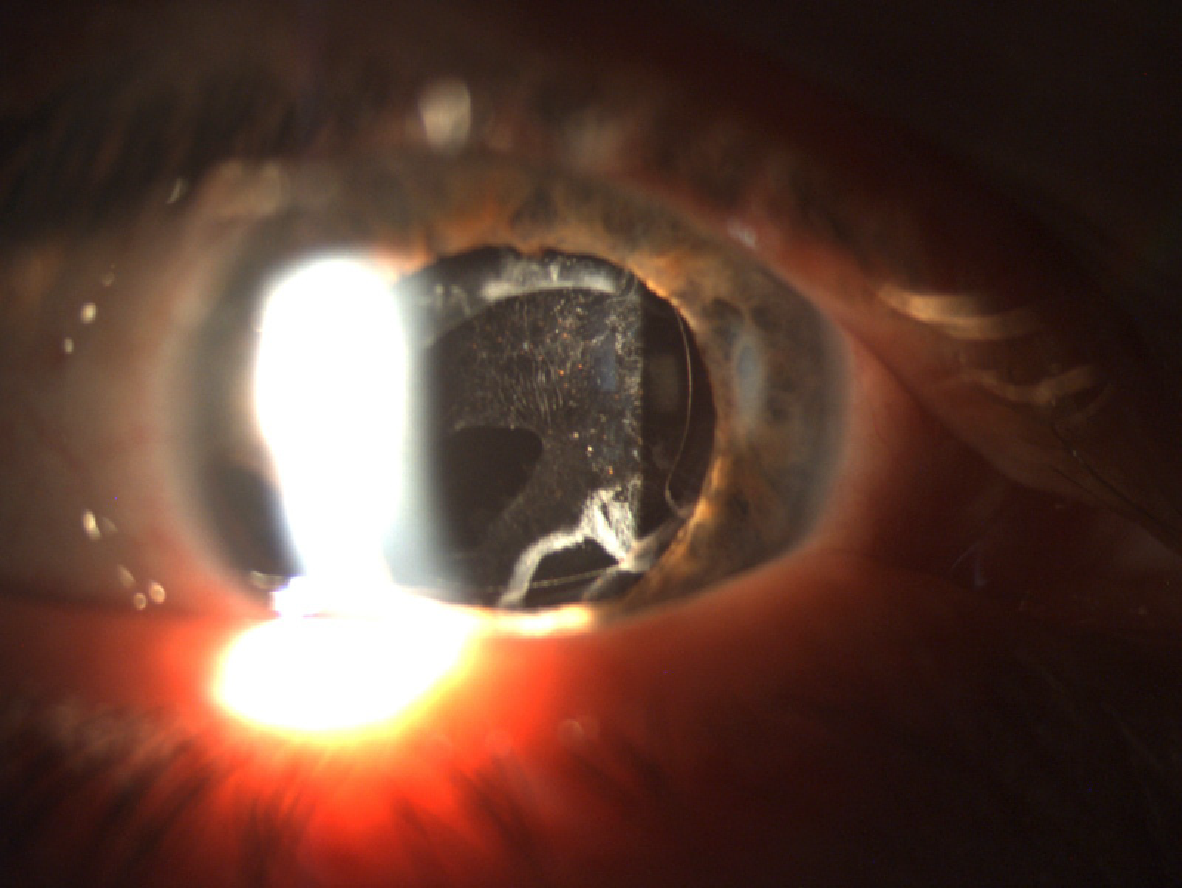
While some studies revealed a higher incidence of PCO in diabetic patients[60,89], others showed fewer cases of PCO in diabetic eyes, regardless of the retinopathy stage, over the course of two years[90]. In a study by Hyashi et al[91], the development of PCO was significantly higher in diabetic patients 18 mo after surgery, even though it was similar to the control group for the first 12 mo. Severity of retinopathy did not have an impact on the development of PCO, according to some studies[92]. Figure 1 demonstrates PCO development in a diabetic patient six months after cataract surgery.
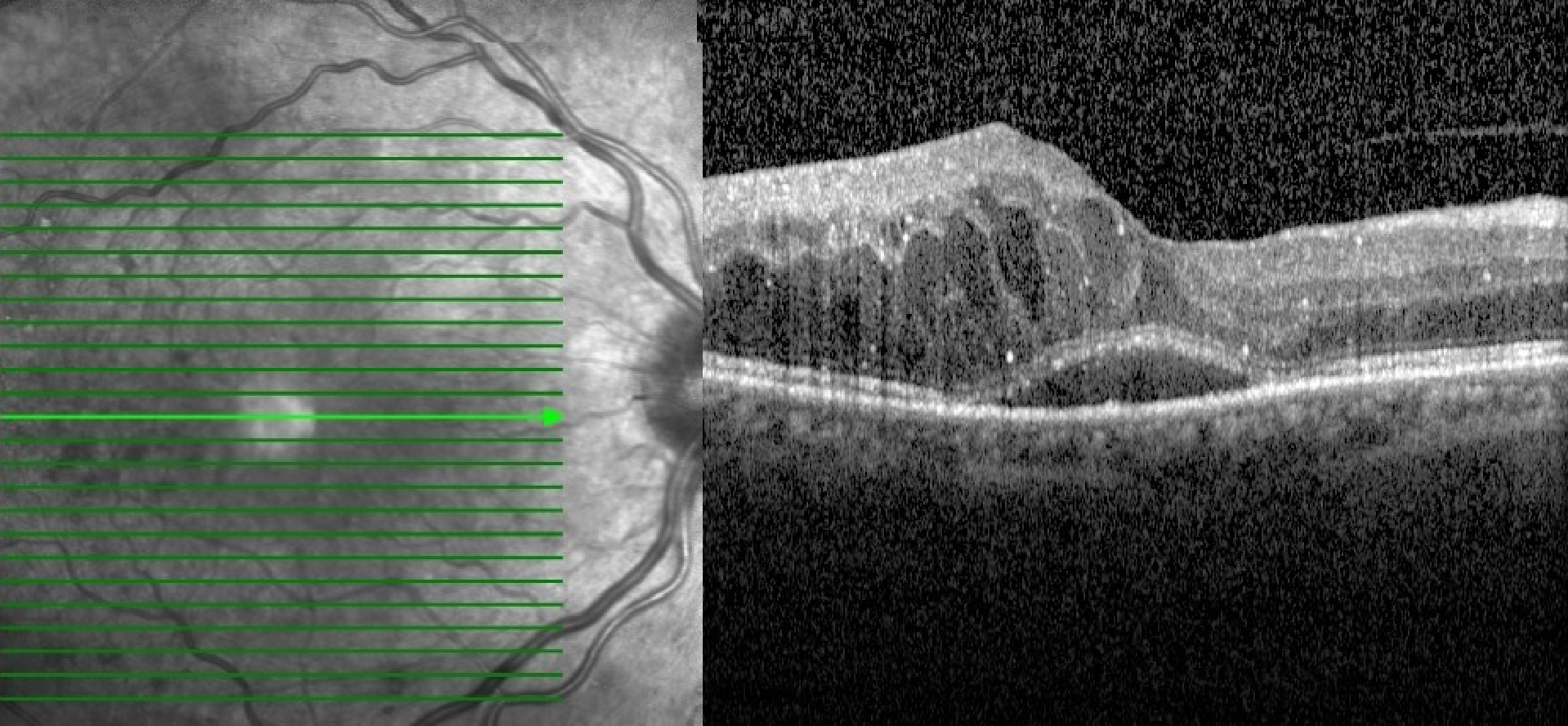
The development of DME, pseudophakic macular edema (PCME), CME, or Irvine-Gass syndrome are other frequent causes of postoperative vision deterioration among the general population[93,94]. Altered concentrations of angiogenic factors after cataract surgery may aggravate maculopathy[95]. OCT imaging has also revealed increased retinal thickness following an uneventful cataract surgery in diabetic eyes without retinopathy as compared to non-diabetic eyes[96]. Chu et al[93] reviewed 81,984 eyes and reported that, even in the absence of retinopathy, diabetic patients’ eyes had an increased relative risk of ME after surgery. In addition, patients with preexisting DR had a higher relative risk of ME, with this risk being proportional to the increasing severity of retinopathy[93]. Figure 2 shows the development of CME in a diabetic patient after cataract surgery.
The incidence of CME varied between 0.2% and 20% in older studies. However, recent studies report lower rates of CME, ranging from less than 1% to 2%-3%[97]. The methods of detection used in these studies have a significant effect on the rate of CME detection. Fluorescein angiography and OCT were more sensitive, for example, reporting higher rates of CME than clinical detection[97]. It is also important to differentiate DME from PCME (Irvine-Gass syndrome), since the pathogenesis, treatment, natural course, and outcomes for both are very different. While the underlying presence of DR, exudates, and ME point toward DME, if there is minimal or no DR and there are no exudates in the posterior pole, this suggests PCME. When in doubt, fluorescein angiography can help to distinguish; if the angiography shows a petaloid pattern associated with hyperfluorescence of optic disc and there is no retinopathy or microaneurysms, edema may be considered as a result of Irvine-Gass syndrome[36].
According to Medicare data, the cost of cataract surgery and related patient care in the United States can be doubled due to ME[98,99]. Therefore, the prevention of CME in diabetic patients is very important. Recently, both prophylactic and therapeutic usage of both topical steroidal and non-steroidal anti-inflammatory eye drops (NSAIDs) has become central to perioperative management of CME in diabetic patients. Especially NSAIDs have been shown to decrease the incidence of CME in the general population. In a systematic review of 15 randomized trials, Kessel et al[100] showed that topical NSAIDs are more efficient in preventing CME than topical steroids. However, the use of NSAIDs did not change the incidence of CME in patients with DR[101].
In addition to facing a higher risk of CME, diabetic patients with preexisting DME are at an increased risk of worsening edema following cataract surgery[29,36]. In ETDRS Report 25, the presence of preexisting, CSME-though it showed no statistically significant difference in the prevalence of ME before and one year after surgery-was associated with worse visual outcomes[36]. Although ME is commonly seen after cataract surgery, it can follow a benign course. The development of postoperative CSME may be the result of the natural progression of the disease rather than a direct effect of surgery on many patients. On the other hand, the clinical course was quite different in eyes with CSME at the time of surgery. None of them resolved spontaneously within a year and the majority showed clinical and angiographic signs of deterioration. Dowler et al[37] have shown that CSME at the time of cataract surgery is associated with worse visual acuity outcomes at one year post-surgery. It seems possible that severe ME after cataract surgery represents a postoperative deterioration of pre-existing ME that was previously untreated because of lens opacity[36].
Attempts to stabilize and resolve DME will help improve outcomes, if DME is present prior to cataract surgery. Many strategies for the preoperative medical management of DME are available. Postoperative laser photocoagulation for diabetic ME is controversial. The ETDRS established the utility of focal/grid laser photocoagulation for the treatment of ME[102]. Focal/grid laser treatment (as described in the ETDRS) was considered as first line treatment for CSME, prior to the use of anti-VEGF agents for central involved DME. It remains an alternative treatment in cases in which anti-VEGFs are not applicable or the center of the macula is not involved[103]. On the other hand, Pollack et al[34] and Dowler et al[37] showed that ME resolves spontaneously if it arises postoperatively but not when it is present preoperatively. They suggested that early laser treatment is unnecessary for all cases of postoperative DME. Generally, experts do not perform argon laser treatment until six months after cataract surgery.
The advent of anti-VEGF injections has shifted the paradigm in the treatment of DME. Many studies performed on anti-VEGF agents in diabetic patients have shown their effectiveness at preventing and treating CSME[104-111]. Current opinion supports that anti-VEGF agents are first-line therapy in preoperative treatments, perioperative stabilization of DME, and postoperative management and that they show great success in anatomic recovery and visual function. Focal laser treatment and steroid injections still provide significant additional support.
Numerous studies have evaluated the effect of cataract surgery on the progression of DR. The progression of DR after intracapsular (ICCE) and extracapsular (ECCE) cataract extraction has been extensively studied[80,112,113]. Sebestyen et al[112] and Alpar et al[113] demonstrated the progression of retinopathy after ICCE and ECCE, with ICCE showing worse results than ECCE. However, the effect of phacoemulsification is controversial. Modern phacoemulsification procedures are considered faster, safer, and more cost-effective than ICCE and ECCE[114]. Even with the advances in modern phacoemulsification techniques, some studies have demonstrated a similar trend of DR progression after phacoemulsification surgery; others have reported no significant change[37,42,115]. Figure 3 shows the progression of DR in a diabetic patient’s right eye after phacoemulsification surgery.
Prospective studies by Dowler et al[37] and Squirrell et al[42] have reported that uncomplicated cataract extraction using phacoemulsification have no effect on the progression of DR. However, Squirrell et al[42] have shown an increased risk of DR progression following cataract surgery in patients with elevated hemoglobin A1c. These studies included one eye that underwent phacoemulsification surgery and one eye as a control. Conversely, some studies that included diabetic patients undergoing phacoemulsification cataract surgery showed a retinopathy progression rate that had nearly doubled at the 12-mo period as compared to unoperated eyes[84]. Similarly, ETDRS Report 25, which enrolled 3711 patients with a nine-year follow-up period, also showed increased rates of retinopathy progression in cases of phacoemulsification than in unoperated eyes[36]. A recent study by Denniston et al[116] reported significant postoperative progression of center involving DME, which was associated with the preoperative grade of DR. Shah et al[41] found that recent studies do not support the generalized conclusion that phacoemulsification causes progression of retinopathy and ME in all diabetic patients.
The risk factors for DR progression have also been investigated. In a retrospective study by Krepler et al[115], these included being male, the disease duration, and poor glycemic control. Dowler et al[37] reported that a smaller incision size and shorter surgical duration for phacoemulsification decreased inflammation and may induce less breakdown of the blood–ocular barrier, meaning that uncomplicated phacoemulsification cataract surgery does not accelerate DR progression. Additionally, recent studies suggest that anti-VEGF injections may also affect the incidence of DR progression[117]. Despite no current consensus on the prophylactic use of anti-VEGF, their use for patients with more advanced NPDR or PDR and DME should be considered. Other ocular co-morbidities such as vitreous hemorrhage, epiretinal membranes, or TRD may benefit from a combined pars plana vitrectomy and cataract surgery[118].
As the number of people with DM is estimated to continue to increase, cataract surgery will remain important for diabetic patients. Patients with diabetes have multiple issues to be evaluated preoperatively, perioperatively, and in the postoperative period. With the advent of modern surgical and pharmacologic therapies, these patients can, like other cataract patients without diabetes, recover excellent vision. Postoperative monitoring and management of surgical complications will also help to alleviate the risk of vision loss in these patients.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dahiya K S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | International Diabetes Federation. IDF Diabetes Atlas 7th Edition. Brussels, Belgium. 2015; Available from: https://idf.org/e-library/epidemiology-research/diabetes-atlas/13-diabetes-atlas-seventh-edition.html. |
| 2. | Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr. 2010;8:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 868] [Cited by in RCA: 921] [Article Influence: 61.4] [Reference Citation Analysis (1)] |
| 3. | Skarbez K, Priestley Y, Hoepf M, Koevary SB. Comprehensive Review of the Effects of Diabetes on Ocular Health. Expert Rev Ophthalmol. 2010;5:557-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 4. | Drinkwater JJ, Davis WA, Davis TME. A systematic review of risk factors for cataract in type 2 diabetes. Diabetes Metab Res Rev. 2019;35:e3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Klein BE, Klein R, Wang Q, Moss SE. Older-onset diabetes and lens opacities. The Beaver Dam Eye Study. Ophthalmic Epidemiol. 1995;2:49-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Klein BE, Klein R, Lee KE. Diabetes, cardiovascular disease, selected cardiovascular disease risk factors, and the 5-year incidence of age-related cataract and progression of lens opacities: the Beaver Dam Eye Study. Am J Ophthalmol. 1998;126:782-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 173] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Klein BE, Klein R, Moss SE. Incidence of cataract surgery in the Wisconsin Epidemiologic Study of Diabetic Retinopathy. Am J Ophthalmol. 1995;119:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 130] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Saxena S, Mitchell P, Rochtchina E. Five-year incidence of cataract in older persons with diabetes and pre-diabetes. Ophthalmic Epidemiol. 2004;11:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Kador PF, Wyman M, Oates PJ. Aldose reductase, ocular diabetic complications and the development of topical Kinostat(®). Prog Retin Eye Res. 2016;54:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 10. | Pollreisz A, Schmidt-Erfurth U. Diabetic cataract-pathogenesis, epidemiology and treatment. J Ophthalmol. 2010;2010:608751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 244] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 11. | Snow A, Shieh B, Chang KC, Pal A, Lenhart P, Ammar D, Ruzycki P, Palla S, Reddy GB, Petrash JM. Aldose reductase expression as a risk factor for cataract. Chem Biol Interact. 2015;234:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Kinoshita JH. Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 1974;13:713-724. [PubMed] |
| 13. | Oishi N, Morikubo S, Takamura Y, Kubo E, Tsuzuki S, Tanimoto T, Akagi Y. Correlation between adult diabetic cataracts and red blood cell aldose reductase levels. Invest Ophthalmol Vis Sci. 2006;47:2061-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Šimunović M, Paradžik M, Škrabić R, Unić I, Bućan K, Škrabić V. Cataract as Early Ocular Complication in Children and Adolescents with Type 1 Diabetes Mellitus. Int J Endocrinol. 2018;2018:6763586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Esteves JF, Dal Pizzol MM, Sccoco CA, Roggia MF, Milano SB, Guarienti JA, Rodrigues TC, Canani LH. Cataract and type 1 diabetes mellitus. Diabetes Res Clin Pract. 2008;82:324-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Wilson ME, Levin AV, Trivedi RH, Kruger SJ, Elliott LA, Ainsworth JR, Awner S, Cruz OA, Kivlin J, Vroman DT, Young WO. Cataract associated with type-1 diabetes mellitus in the pediatric population. J AAPOS. 2007;11:162-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Mulhern ML, Madson CJ, Kador PF, Randazzo J, Shinohara T. Cellular osmolytes reduce lens epithelial cell death and alleviate cataract formation in galactosemic rats. Mol Vis. 2007;13:1397-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Stitt AW. The maillard reaction in eye diseases. Ann N Y Acad Sci. 2005;1043:582-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Bron AJ, Sparrow J, Brown NA, Harding JJ, Blakytny R. The lens in diabetes. Eye (Lond). 1993;7:260-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 121] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Chiou SH, Chang CJ, Chou CK, Hsu WM, Liu JH, Chiang CH. Increased nitric oxide levels in aqueous humor of diabetic patients with neovascular glaucoma. Diabetes Care. 1999;22:861-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Ornek K, Karel F, Büyükbingöl Z. May nitric oxide molecule have a role in the pathogenesis of human cataract? Exp Eye Res. 2003;76:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Olofsson EM, Marklund SL, Behndig A. Enhanced diabetes-induced cataract in copper-zinc superoxide dismutase-null mice. Invest Ophthalmol Vis Sci. 2009;50:2913-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Olofsson EM, Marklund SL, Karlsson K, Brännström T, Behndig A. In vitro glucose-induced cataract in copper-zinc superoxide dismutase null mice. Exp Eye Res. 2005;81:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244:6049-6055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 25. | Li WC, Kuszak JR, Dunn K, Wang RR, Ma W, Wang GM, Spector A, Leib M, Cotliar AM, Weiss M. Lens epithelial cell apoptosis appears to be a common cellular basis for non-congenital cataract development in humans and animals. J Cell Biol. 1995;130:169-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 283] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Papadimitriou DT, Bothou C, Skarmoutsos F, Alexandrides TK, Papaevangelou V, Papadimitriou A. The autoimmune hypothesis for acute bilateral cataract in type 1 diabetes. Diabetes Metab. 2016;42:386-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Klein BE, Klein R, Moss SE. Prevalence of cataracts in a population-based study of persons with diabetes mellitus. Ophthalmology. 1985;92:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 178] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 28. | Nielsen NV, Vinding T. The prevalence of cataract in insulin-dependent and non-insulin-dependent-diabetes mellitus. Acta Ophthalmol (Copenh). 1984;62:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Javadi MA, Zarei-Ghanavati S. Cataracts in diabetic patients: a review article. J Ophthalmic Vis Res. 2008;3:52-65. [PubMed] |
| 30. | Ederer F, Hiller R, Taylor HR. Senile lens changes and diabetes in two population studies. Am J Ophthalmol. 1981;91:381-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 108] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 31. | Rowe NG, Mitchell PG, Cumming RG, Wans JJ. Diabetes, fasting blood glucose and age-related cataract: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2000;7:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Leske MC, Wu SY, Hennis A, Connell AM, Hyman L, Schachat A. Diabetes, hypertension, and central obesity as cataract risk factors in a black population. The Barbados Eye Study. Ophthalmology. 1999;106:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Srinivasan S, Raman R, Swaminathan G, Ganesan S, Kulothungan V, Sharma T. Incidence, Progression, and Risk Factors for Cataract in Type 2 Diabetes. Invest Ophthalmol Vis Sci. 2017;58:5921-5929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Pollack A, Leiba H, Bukelman A, Oliver M. Cystoid macular oedema following cataract extraction in patients with diabetes. Br J Ophthalmol. 1992;76:221-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 2.9] [Reference Citation Analysis (4)] |
| 35. | Schatz H, Atienza D, McDonald HR, Johnson RN. Severe diabetic retinopathy after cataract surgery. Am J Ophthalmol. 1994;117:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Chew EY, Benson WE, Remaley NA, Lindley AA, Burton TC, Csaky K, Williams GA, Ferris FL. Results after lens extraction in patients with diabetic retinopathy: early treatment diabetic retinopathy study report number 25. Arch Ophthalmol. 1999;117:1600-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Dowler JG, Sehmi KS, Hykin PG, Hamilton AM. The natural history of macular edema after cataract surgery in diabetes. Ophthalmology. 1999;106:663-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Saito Y, Ohmi G, Kinoshita S, Nakamura Y, Ogawa K, Harino S, Okada M. Transient hyperopia with lens swelling at initial therapy in diabetes. Br J Ophthalmol. 1993;77:145-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Sonmez B, Bozkurt B, Atmaca A, Irkec M, Orhan M, Aslan U. Effect of glycemic control on refractive changes in diabetic patients with hyperglycemia. Cornea. 2005;24:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Yoon KC, Im SK, Seo MS. Changes of tear film and ocular surface in diabetes mellitus. Korean J Ophthalmol. 2004;18:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 41. | Shah AS, Chen SH. Cataract surgery and diabetes. Curr Opin Ophthalmol. 2010;21:4-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Squirrell D, Bhola R, Bush J, Winder S, Talbot JF. A prospective, case controlled study of the natural history of diabetic retinopathy and maculopathy after uncomplicated phacoemulsification cataract surgery in patients with type 2 diabetes. Br J Ophthalmol. 2002;86:565-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Diabetic Retinopathy Clinical Research Network. Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL 3rd, Friedman SM, Glassman AR, Miller KM, Scott IU, Stockdale CR, Sun JK. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064-1077.e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1061] [Cited by in RCA: 1027] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 44. | Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1987;94:761-774. [PubMed] |
| 45. | Diabetic Retinopathy Clinical Research Network Authors/Writing Committee. Baker CW, Almukhtar T, Bressler NM, Glassman AR, Grover S, Kim SJ, Murtha TJ, Rauser ME, Stockdale C. Macular edema after cataract surgery in eyes without preoperative central-involved diabetic macular edema. JAMA Ophthalmol. 2013;131:870-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Kim SJ, Equi R, Bressler NM. Analysis of macular edema after cataract surgery in patients with diabetes using optical coherence tomography. Ophthalmology. 2007;114:881-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 47. | Yumuşak E, Örnek K. Comparison of Perioperative Ranibizumab Injections for Diabetic Macular Edema in Patients Undergoing Cataract Surgery. J Ophthalmol. 2016;2016:7945619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Danis RP, Sadda S, Li XY, Cui H, Hashad Y, Whitcup SM. Anatomical effects of dexamethasone intravitreal implant in diabetic macular oedema: a pooled analysis of 3-year phase III trials. Br J Ophthalmol. 2016;100:796-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Yang Y, Bailey C, Holz FG, Eter N, Weber M, Baker C, Kiss S, Menchini U, Ruiz Moreno JM, Dugel P, Lotery A; FAME study group. Long-term outcomes of phakic patients with diabetic macular oedema treated with intravitreal fluocinolone acetonide (FAc) implants. Eye (Lond). 2015;29:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 50. | Boyer DS, Yoon YH, Belfort R, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM; Ozurdex MEAD Study Group. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 763] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 51. | Medić A, Jukić T, Matas A, Vukojević K, Sapunar A, Znaor L. Effect of preoperative topical diclofenac on intraocular interleukin-12 concentration and macular edema after cataract surgery in patients with diabetic retinopathy: a randomized controlled trial. Croat Med J. 2017;58:49-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Yüksel B, Karti Ö, Kusbeci T. Topical nepafenac for prevention of post-cataract surgery macular edema in diabetic patients: patient selection and perspectives. Clin Ophthalmol. 2017;11:2183-2190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Miyake K, Ota I, Miyake G, Numaga J. Nepafenac 0.1% versus fluorometholone 0.1% for preventing cystoid macular edema after cataract surgery. J Cataract Refract Surg. 2011;37:1581-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Singh R, Alpern L, Jaffe GJ, Lehmann RP, Lim J, Reiser HJ, Sall K, Walters T, Sager D. Evaluation of nepafenac in prevention of macular edema following cataract surgery in patients with diabetic retinopathy. Clin Ophthalmol. 2012;6:1259-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Yazdani S, Hendi K, Pakravan M. Intravitreal bevacizumab (Avastin) injection for neovascular glaucoma. J Glaucoma. 2007;16:437-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 56. | Chilov MN, Grigg JR, Playfair TJ. Bevacizumab (Avastin) for the treatment of neovascular glaucoma. Clin Exp Ophthalmol. 2007;35:494-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Mozaffarieh M, Heinzl H, Sacu S, Wedrich A. Clinical outcomes of phacoemulsification cataract surgery in diabetes patients: visual function (VF-14), visual acuity and patient satisfaction. Acta Ophthalmol Scand. 2005;83:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Sadiq SA, Sleep T, Amoaku WM. The visual results and changes in retinopathy in diabetic patients following cataract surgery. Eur J Ophthalmol. 1999;9:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Takamura Y, Tomomatsu T, Yokota S, Matsumura T, Takihara Y, Inatani M. Large capsulorhexis with implantation of a 7.0 mm optic intraocular lens during cataract surgery in patients with diabetes mellitus. J Cataract Refract Surg. 2014;40:1850-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Ebihara Y, Kato S, Oshika T, Yoshizaki M, Sugita G. Posterior capsule opacification after cataract surgery in patients with diabetes mellitus. J Cataract Refract Surg. 2006;32:1184-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Kim EC, Hwang HS, Kim MS. Anterior capsular phimosis occluding the capsulorhexis opening after cataract surgery in a diabetic patient with high hemoglobin A1C. Semin Ophthalmol. 2013;28:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Henricsson M, Heijl A, Janzon L. Diabetic retinopathy before and after cataract surgery. Br J Ophthalmol. 1996;80:789-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Mittra RA, Borrillo JL, Dev S, Mieler WF, Koenig SB. Retinopathy progression and visual outcomes after phacoemulsification in patients with diabetes mellitus. Arch Ophthalmol. 2000;118:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 64. | Ferrari GL, Marques JL, Gandhi RA, Heller SR, Schneider FK, Tesfaye S, Gamba HR. Using dynamic pupillometry as a simple screening tool to detect autonomic neuropathy in patients with diabetes: a pilot study. Biomed Eng Online. 2010;9:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 65. | Cahill M, Eustace P, de Jesus V. Pupillary autonomic denervation with increasing duration of diabetes mellitus. Br J Ophthalmol. 2001;85:1225-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Cetinkaya A, Yilmaz G, Akova YA. Photic retinopathy after cataract surgery in diabetic patients. Retina. 2006;26:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Inoue K, Kato S, Ohara C, Numaga J, Amano S, Oshika T. Ocular and systemic factors relevant to diabetic keratoepitheliopathy. Cornea. 2001;20:798-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Shih KC, Lam KS, Tong L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr Diabetes. 2017;7:e251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 128] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 69. | Morikubo S, Takamura Y, Kubo E, Tsuzuki S, Akagi Y. Corneal changes after small-incision cataract surgery in patients with diabetes mellitus. Arch Ophthalmol. 2004;122:966-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 70. | Yang R, Sha X, Zeng M, Tan Y, Zheng Y, Fan F. The influence of phacoemulsification on corneal endothelial cells at varying blood glucose levels. Eye Sci. 2011;26:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 71. | Wormstone IM, Eldred JA. Experimental models for posterior capsule opacification research. Exp Eye Res. 2016;142:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 72. | Nishi O, Nishi K, Osakabe Y. Effect of intraocular lenses on preventing posterior capsule opacification: design versus material. J Cataract Refract Surg. 2004;30:2170-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Eaton AM, Jaffe GJ, McCuen BW 2nd, Mincey GJ. Condensation on the posterior surface of silicone intraocular lenses during fluid-air exchange. Ophthalmology. 1995;102:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Kim CJ, Choi SK. Analysis of aqueous humor calcium and phosphate from cataract eyes with and without diabetes mellitus. Korean J Ophthalmol. 2007;21:90-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Kugelberg M, Wejde G, Jayaram H, Zetterström C. Posterior capsule opacification after implantation of a hydrophilic or a hydrophobic acrylic intraocular lens: one-year follow-up. J Cataract Refract Surg. 2006;32:1627-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Lee DH, Seo Y, Joo CK. Progressive opacification of hydrophilic acrylic intraocular lenses in diabetic patients. J Cataract Refract Surg. 2002;28:1271-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Vasavada AR, Raj SM, Shah A, Shah G, Vasavada V, Vasavada V. Comparison of posterior capsule opacification with hydrophobic acrylic and hydrophilic acrylic intraocular lenses. J Cataract Refract Surg. 2011;37:1050-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Rodríguez-Galietero A, Montés-Micó R, Muñoz G, Albarrán-Diego C. Blue-light filtering intraocular lens in patients with diabetes: contrast sensitivity and chromatic discrimination. J Cataract Refract Surg. 2005;31:2088-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 79. | Braga-Mele R, Chang D, Dewey S, Foster G, Henderson BA, Hill W, Hoffman R, Little B, Mamalis N, Oetting T, Serafano D, Talley-Rostov A, Vasavada A, Yoo S; ASCRS Cataract Clinical Committee. Multifocal intraocular lenses: relative indications and contraindications for implantation. J Cataract Refract Surg. 2014;40:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 80. | Jaffe GJ, Burton TC, Kuhn E, Prescott A, Hartz A. Progression of nonproliferative diabetic retinopathy and visual outcome after extracapsular cataract extraction and intraocular lens implantation. Am J Ophthalmol. 1992;114:448-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 81. | Lee JS, Lee JE, Choi HY, Oum BS, Cho BM. Corneal endothelial cell change after phacoemulsification relative to the severity of diabetic retinopathy. J Cataract Refract Surg. 2005;31:742-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 82. | Hugod M, Storr-Paulsen A, Norregaard JC, Nicolini J, Larsen AB, Thulesen J. Corneal endothelial cell changes associated with cataract surgery in patients with type 2 diabetes mellitus. Cornea. 2011;30:749-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 83. | Ivancić D, Mandić Z, Barać J, Kopić M. Cataract surgery and postoperative complications in diabetic patients. Coll Antropol. 2005;29 Suppl 1:55-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 84. | Hong T, Mitchell P, de Loryn T, Rochtchina E, Cugati S, Wang JJ. Development and progression of diabetic retinopathy 12 months after phacoemulsification cataract surgery. Ophthalmology. 2009;116:1510-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 85. | Denniston AK, Lee AY, Lee CS, Crabb DP, Bailey C, Lip PL, Taylor P, Pikoula M, Cook E, Akerele T, Antcliff R, Brand C, Chakravarthy U, Chavan R, Dhingra N, Downey L, Eleftheriadis H, Ghanchi F, Khan R, Kumar V, Lobo A, Lotery A, Menon G, Mukherjee R, Palmer H, Patra S, Paul B, Sim DA, Talks JS, Wilkinson E, Tufail A, Egan CA. United Kingdom Diabetic Retinopathy Electronic Medical Record (UK DR EMR) Users Group: report 4, real-world data on the impact of deprivation on the presentation of diabetic eye disease at hospital services. Br J Ophthalmol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Liao SB, Ku WC. Progression of diabetic retinopathy after phacoemulsification in diabetic patients: a three-year analysis. Chang Gung Med J. 2003;26:829-834. [PubMed] |
| 87. | Greenberg PB, Tseng VL, Wu WC, Liu J, Jiang L, Chen CK, Scott IU, Friedmann PD. Prevalence and predictors of ocular complications associated with cataract surgery in United States veterans. Ophthalmology. 2011;118:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 88. | Nishi O, Nishi K, Fujiwara T, Shirasawa E, Ohmoto Y. Effects of the cytokines on the proliferation of and collagen synthesis by human cataract lens epithelial cells. Br J Ophthalmol. 1996;80:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Zaczek A, Olivestedt G, Zetterström C. Visual outcome after phacoemulsification and IOL implantation in diabetic patients. Br J Ophthalmol. 1999;83:1036-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Zaczek A, Zetterström C. Posterior capsule opacification after phacoemulsification in patients with diabetes mellitus. J Cataract Refract Surg. 1999;25:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 91. | Hayashi Y, Kato S, Fukushima H, Numaga J, Kaiya T, Tamaki Y, Oshika T. Relationship between anterior capsule contraction and posterior capsule opacification after cataract surgery in patients with diabetes mellitus. J Cataract Refract Surg. 2004;30:1517-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 92. | Praveen MR, Vasavada AR, Shah GD, Shah AR, Khamar BM, Dave KH. A prospective evaluation of posterior capsule opacification in eyes with diabetes mellitus: a case-control study. Eye (Lond). 2014;28:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Chu CJ, Johnston RL, Buscombe C, Sallam AB, Mohamed Q, Yang YC; United Kingdom Pseudophakic Macular Edema Study Group. Risk Factors and Incidence of Macular Edema after Cataract Surgery: A Database Study of 81984 Eyes. Ophthalmology. 2016;123:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 94. | Kim SJ, Schoenberger SD, Thorne JE, Ehlers JP, Yeh S, Bakri SJ. Topical Nonsteroidal Anti-inflammatory Drugs and Cataract Surgery: A Report by the American Academy of Ophthalmology. Ophthalmology. 2015;122:2159-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 95. | Patel JI, Hykin PG, Cree IA. Diabetic cataract removal: postoperative progression of maculopathy--growth factor and clinical analysis. Br J Ophthalmol. 2006;90:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 96. | Jurecka T, Bátková Z, Ventruba J, Synek S. [Macular edema after cataract surgery in diabetic patients without retinopathy]. Cesk Slov Oftalmol. 2007;63:274-284. [PubMed] |
| 97. | Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema. Curr Opin Ophthalmol. 2012;23:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 98. | Schmier JK, Covert DW, Hulme-Lowe CK, Mullins A, Mahlis EM. Treatment costs of cystoid macular edema among patients following cataract surgery. Clin Ophthalmol. 2016;10:477-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 99. | Schmier JK, Halpern MT, Covert DW, Matthews GP. Evaluation of costs for cystoid macular edema among patients after cataract surgery. Retina. 2007;27:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 100. | Kessel L, Tendal B, Jørgensen KJ, Erngaard D, Flesner P, Andresen JL, Hjortdal J. Post-cataract prevention of inflammation and macular edema by steroid and nonsteroidal anti-inflammatory eye drops: a systematic review. Ophthalmology. 2014;121:1915-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 101. | Modjtahedi BS, Paschal JF, Batech M, Luong TQ, Fong DS. Perioperative Topical Nonsteroidal Anti-inflammatory Drugs for Macular Edema Prophylaxis Following Cataract Surgery. Am J Ophthalmol. 2017;176:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 102. | Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796-1806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2067] [Cited by in RCA: 2009] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 103. | Writing Committee for the Diabetic Retinopathy Clinical Research Network. Fong DS, Strauber SF, Aiello LP, Beck RW, Callanan DG, Danis RP, Davis MD, Feman SS, Ferris F, Friedman SM, Garcia CA, Glassman AR, Han DP, Le D, Kollman C, Lauer AK, Recchia FM, Solomon SD. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 104. | Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, Rubio RG, Ehrlich JS; RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 1226] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 105. | Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, Boyer D, Heier JS, Abraham P, Thach AB, Lit ES, Foster BS, Kruger E, Dugel P, Chang T, Das A, Ciulla TA, Pollack JS, Lim JI, Eliott D, Campochiaro PA; READ-2 Study Group. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 385] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 106. | Do DV, Nguyen QD, Khwaja AA, Channa R, Sepah YJ, Sophie R, Hafiz G, Campochiaro PA; READ-2 Study Group. Ranibizumab for edema of the macula in diabetes study: 3-year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmol. 2013;131:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 107. | Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A; RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 991] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 108. | Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H, Marcus DM, Nguyen QD, Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Zeitz O, Metzig C, Brown DM. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247-2254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 593] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 109. | Heier JS, Korobelnik JF, Brown DM, Schmidt-Erfurth U, Do DV, Midena E, Boyer DS, Terasaki H, Kaiser PK, Marcus DM, Nguyen QD, Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Vitti R, Berliner AJ, Zeitz O, Metzig C, Holz FG. Intravitreal Aflibercept for Diabetic Macular Edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology. 2016;123:2376-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 302] [Article Influence: 33.6] [Reference Citation Analysis (2)] |
| 110. | Brown DM, Schmidt-Erfurth U, Do DV, Holz FG, Boyer DS, Midena E, Heier JS, Terasaki H, Kaiser PK, Marcus DM, Nguyen QD, Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Zeitz O, Metzig C, Korobelnik JF. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology. 2015;122:2044-2052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 417] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 111. | Takamura Y, Kubo E, Akagi Y. Analysis of the effect of intravitreal bevacizumab injection on diabetic macular edema after cataract surgery. Ophthalmology. 2009;116:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 112. | Sebestyen JG. Intraocular lenses and diabetes mellitus. Am J Ophthalmol. 1986;101:425-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 113. | Alpar JJ. Cataract extraction and diabetic retinopathy. J Am Intraocul Implant Soc. 1984;10:433-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 114. | Linebarger EJ, Hardten DR, Shah GK, Lindstrom RL. Phacoemulsification and modern cataract surgery. Surv Ophthalmol. 1999;44:123-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 115. | Krepler K, Biowski R, Schrey S, Jandrasits K, Wedrich A. Cataract surgery in patients with diabetic retinopathy: visual outcome, progression of diabetic retinopathy, and incidence of diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol. 2002;240:735-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 116. | Denniston AK, Chakravarthy U, Zhu H, Lee AY, Crabb DP, Tufail A, Bailey C, Akerele T, Al-Husainy S, Brand C, Downey L, Fitt A, Khan R, Kumar V, Lobo A, Mahmood S, Mandal K, Mckibbin M, Menon G, Natha S, Ong JM, Tsaloumas MD, Varma A, Wilkinson E, Johnston RL, Egan CA; UK DR EMR Users Group. The UK Diabetic Retinopathy Electronic Medical Record (UK DR EMR) Users Group, Report 2: real-world data for the impact of cataract surgery on diabetic macular oedema. Br J Ophthalmol. 2017;101:1673-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 117. | Writing Committee for the Diabetic Retinopathy Clinical Research Network.. Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, Elman MJ, Ferris FL 3rd, Friedman SM, Marcus DM, Melia M, Stockdale CR, Sun JK, Beck RW, Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA. 2015;314:2137-2146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 555] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 118. | Silva PS, Diala PA, Hamam RN, Arrigg PG, Shah ST, Murtha TL, Schlossman DK, Cavallerano JD, Sun JK, Aiello LP. Visual outcomes from pars plana vitrectomy versus combined pars plana vitrectomy, phacoemulsification, and intraocular lens implantation in patients with diabetes. Retina. 2014;34:1960-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |