Published online Feb 15, 2019. doi: 10.4239/wjd.v10.i2.87
Peer-review started: January 4, 2019
First decision: January 12, 2019
Revised: January 16, 2019
Accepted: February 11, 2019
Article in press: February 12, 2019
Published online: February 15, 2019
Processing time: 46 Days and 19.7 Hours
A large number of adults with long-term type 1 diabetes are affected by symmetrical peripheral neuropathy. These complications increase socioeconomic expenses and diminish the individual quality of life. The 36-Item Short Form Health Survey (SF-36) is a generic patient reported questionnaire, measuring mental and physical health related quality of life. We hypothesized that diabetic neuropathy would decrease physical and mental quality of life measured with SF-36, and that clinical appearance may be associated with the decline.
To investigate if diabetic neuropathy would decrease physical and mental quality of life measured with SF-36, and if clinical appearance may be associated with the decline.
Forty-eight adults [age 50 ± 9 years, 10 females, disease duration 32 (14-51) years] with verified diabetic symmetrical peripheral neuropathy and 21 healthy participants (age 51 ± 6 years, 6 females) underwent standardised nerve conduction testing and completed the SF-36 questionnaire. Furthermore, disease duration, number of comorbidities, both diabetes related and nondiabetes related, vibration perception threshold, number of hypoglycaemic events, HbA1c and administration way of insulin was notified.
In comparison to healthy subjects, patients’ mental composite score was not significantly diminished (51.9 ± 8.9 vs 53.1 ± 5.5, P = 0.558), while the physical composite score was (46.3 ± 11.7 vs 54.6 ± 3.3, P = 0.002). As expected, the overall physical health related symptoms in patients were associated to total number of comorbidities (P < 0.0001), comorbidities relation to diabetes (P = 0.0002) and HbA1c (P = 0.005) as well as comorbidities not related to diabetes (P = 0.0006).
The finding of this study emphasises the importance of focusing on quality of life in adults with diabetes and especially in those with multiple comorbidities as well as the possibility of HbA1c as a biomarker for severe complication.
Core tip: In this study, we found a diminishment of physical domains more so than mental components from the 36-Item Short Form Health Survey (SF-36), in 48 people with type 1 diabetes and verified diabetic symmetrical peripheral neuropathy when compared to 21 healthy controls. Additionally, this physical diminishment was associated with increases in number of comorbidities and HbA1c. To our knowledge a study of health related quality of life exclusively in people with type 1 diabetes and diabetic symmetrical peripheral neuropathy compared to healthy age-matched controls has not previously been performed, therefore these results are interesting for health care professionals interested in the connection between neuropathy and patient experience.
- Citation: Wegeberg AML, Meldgaard T, Hyldahl S, Jakobsen PE, Drewes AM, Brock B, Brock C. Quantities of comorbidities affects physical, but not mental health related quality of life in type 1 diabetes with confirmed polyneuropathy. World J Diabetes 2019; 10(2): 87-95
- URL: https://www.wjgnet.com/1948-9358/full/v10/i2/87.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i2.87
Diabetic symmetrical peripheral neuropathy is a frequent complication to type 1 diabetes, although the prevalence varies between countries[1]. The pathogenesis of diabetic neuropathy is not fully understood; however, it is generally accepted to be a consequence of hyperglycaemic exposure leading to activation of metabolic, biochemical, inflammatory and immune mediated pathways. Clinically, neuropathy can present itself both with and without symptoms, including decreased sensation (numbness) or pain[2,3]. The severity of peripheral neuropathy is associated with incapacitating complications and a shorter life expectancy for the individual. On top of this, research within the last years has focused increasingly on the physical as well as mental burden of diabetes on quality of life.
It has become more and more acknowledged in clinical practice, to evaluate patient centred outcomes in the measurement of disease burden, progression and treatment outcome and how these impacts fundamental health related quality of life (HRQoL). In an effort to standardise and examine HRQoL the development and validation of instruments, such as the 36-Item Short Form Health Survey (SF-36), enables comparisons of different conditions between diseases, populations and countries[4]. The SF-36 has proved valid and useful in surveys of general and specific populations comparing the relative burdens of diseases, and in differentiating the health benefits produced by a wide range of treatments. Previous trans-sectional studies have investigated the HRQoL status in diabetes; however, most studies were carried out in different types of diabetes and with mixed phenotypes. Thus, there is a knowledge gap in characterising the HRQoL in a population with type 1 diabetes and diabetic symmetrical peripheral neuropathy[5,6].
We hypothesized that the presence of diabetic symmetrical peripheral neuropathy would decrease the individual physical and mental quality of life. Thus, the aim of this study was to compare the HRQoL in adults with type 1 diabetes as compared with healthy age-matched controls using the SF-36 questionnaire. In addition, in patients we wanted to investigate associations between HRQoL and: (1) disease duration; (2) number of comorbidities, both diabetes related and non-diabetes related; (3) vibration thresholds; (4) nerve conduction velocity of the efferent median nerve; (5) nerve conduction velocity of the afferent sural nerve; (6) number of hypoglycaemic events; (7) glycaemic state; and (8) the use of insulin pen or pump.
The study comprised baseline observations of 48 people with long-term type 1 diabetes and verified diabetic symmetrical peripheral neuropathy recruited at the Department of Endocrinology, Aalborg University Hospital, Denmark from June 2014 to March 2016, as part of a clinical trial investigating the effect of liraglutide on neuropathy (TODINELI trial, (EUDRA CT 2013-004375-12). The local ethics committee approved the study protocols (N-20130077). Inclusion criteria were adults (> 18 years) with type 1 diabetes for a minimum duration of two years and diabetic sensory neuropathy verified by nerve conduction velocity testing. Additional criteria has been described elsewhere[7]. In comparison, 21 adult healthy, age-matched, participants were recruited for comparison. Informed consent was obtained from all individual participants included in the study.
To assess HRQoL, participants completed the SF-36[8-10]. Even though it is not specific for diabetes, it has been validated and the components are relevant to assess the symptom burden experienced in diabetes[11]. The SF-36 was developed as a multipurpose eight-scale profile of functional health and well-being scores (physical functioning, role limitation due to physical problems, bodily pain, general health, vitality, social functioning, role limitation due to emotional problems, and mental health), and two summary scores (physical component summary and mental component summary), to explain variations in patient outcomes, covering 4 wk prior to the test[8,12]. Scores from the 36 items are transformed to a 0-100 scale with higher scores equals better quality of life[6,12]. A greater than five point change on this scale is considered clinically significant[6].
Health care professionals obtained information about disease duration, comorbidities and way of insulin intake. Vibration perception threshold was measured using a biothesiometer (Bio-Medical Instruments, Newbury, OH, United States) on the distal plantar surface of the big toes. Peripheral nerve conduction testing of the efferent median and afferent sural nerves was evaluated at the elbow and the ankle, respectively, with plastic bar electrodes at skin temperatures above 32 °C. A blood sample was taken for measurement of HbA1c (IFCC) and number of hypoglycaemic events was registered in a patient diary two days prior to the study day.
The statistical methods of this study were reviewed by all the authors. Normally distributed data was reported as means and standard deviations, non-normally distributed data as median and interquartile range while categorical data is provided as a percentage. An independent-sample t-test was undertaken to determine differences in HRQoL between the two groups. For parametric data, a Pearson’s correlation tests were performed to investigate associations between HRQoL and disease duration, HbA1c, average vibration thresholds and nerve conduction velocity for the efferent median and sural nerves. For nonparametric data, a Spearman’s correlation tests were performed to investigate associations between HRQoL and number of hypoglycaemic events, total number of comorbidities, diabetes related and non diabetes related comorbidities. Diabetes related includes hypertension, retinopathy, pain, albuminuria, erectile dysfunction and cardiovascular diseases. Non-diabetes related includes hypercholesterolemia, thrombose prophylaxis, operations, reflux, arthritis and arthroses, asthma and allergies, metabolic diseases, vitamin deficiencies and more. Additionally, an independent samples t-test was run to determine if there was a difference between people with diabetes who used insulin pens or pump.
A total of 48 people with type 1 diabetes and 21 adult heathy volunteers were included in and completed the study. The demographic distribution is shown in Table 1 and displays no notable difference in demographic characteristic between the two groups.
| Type 1 diabetes (n = 48) | Healthy controls (n = 21) | P value | |
| Gender, male n (%) | 38 (79) | 15 (71) | 0.48 |
| Age | 50 ± 9 | 51 ± 6 | 0.53 |
| Height | 178.4 ± 1.2 | 179.9 ± 1.9 | 0.51 |
| Weight | 90.0 ± 2.3 | 87.3 ± 4.5 | 0.56 |
| Right handed n (%) | 41 (85) | 17 (81) | 0.64 |
| Smoking n (%) | 10 (21) | 4 (19) | 0.87 |
| Disease duration | 32 (14-51) | ||
| HbA1c (IFCC), mmol/mol | 65.5 ± 9.7 |
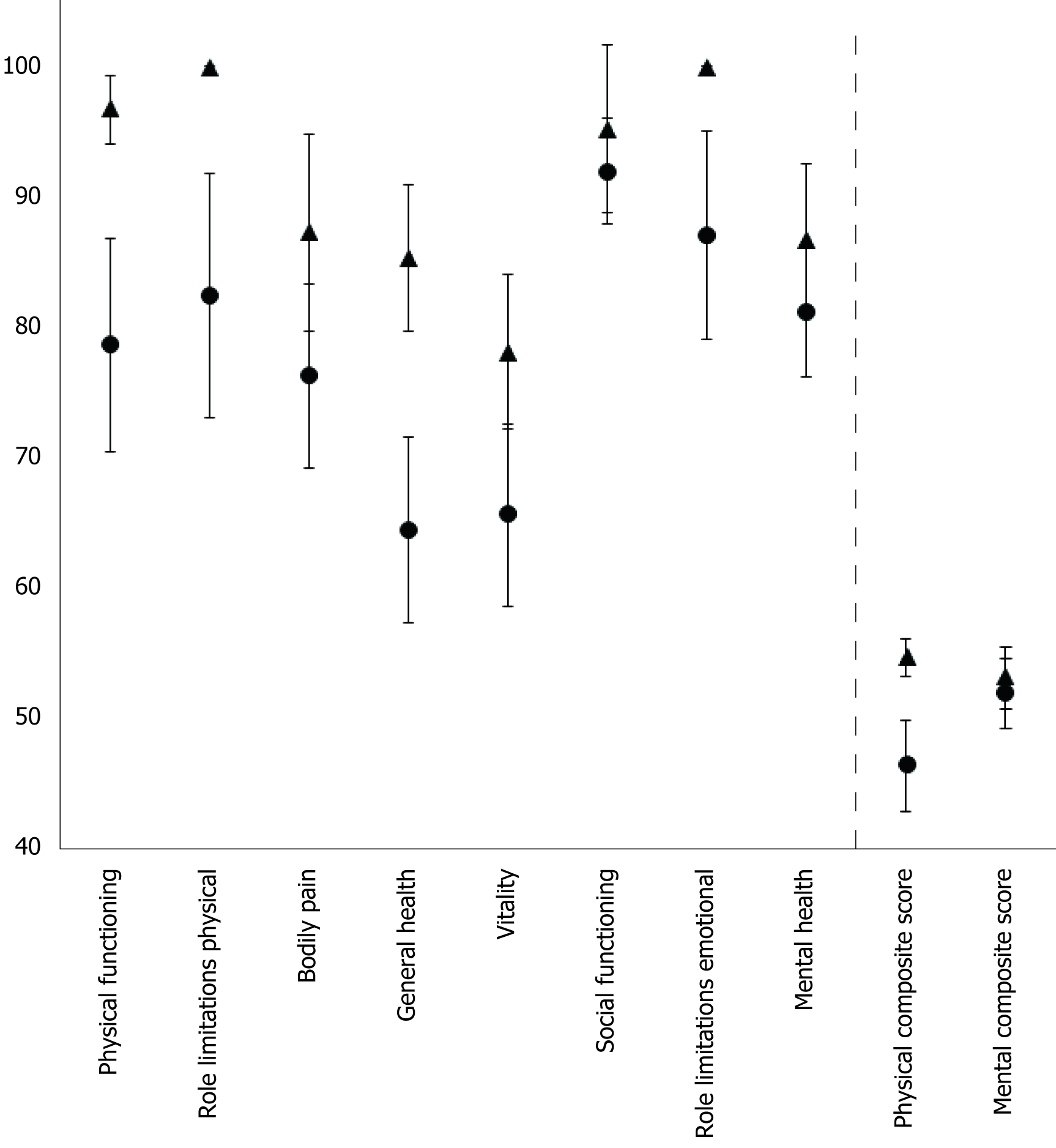
As seen in Figure 1, when diabetes was present, a numerical decline was observed in every SF-36 domain, compared to healthy subject. Significant differences were found on physical functioning (78.6 ± 27.7 vs 96.7 ± 6.2, P = 0.005), role limitation due to physical problems (82.4 ± 31.7 vs 100 ± 0, P = 0.01), general health (64.4 ± 24.5 vs 85.3 ± 13.1, P < 0.001), vitality (65.5 ± 23.9 vs 78.1 ± 13.9, P = 0.03), role limitations due to personal or emotional problems (87.0 ± 27.3 vs 100 ± 0, P = 0.03) and the physical composite score (46.3 ± 11.7 vs 54.6 ± 3.3, P = 0.002). However, no significance was found lookig at bodily pain (76.2 ± 24.34 vs 87.3 ± 17.7, P = 0.07), social functioning (91.9 ± 13.9 vs 95.2 ± 15.0, P = 0.39), mental health (81.2 ± 16.9 vs 86.7 ± 13.6, P = 0.20) and the mental composite score (51.9 ± 8.9 vs 53.1 ± 5.5, P = 0.56).
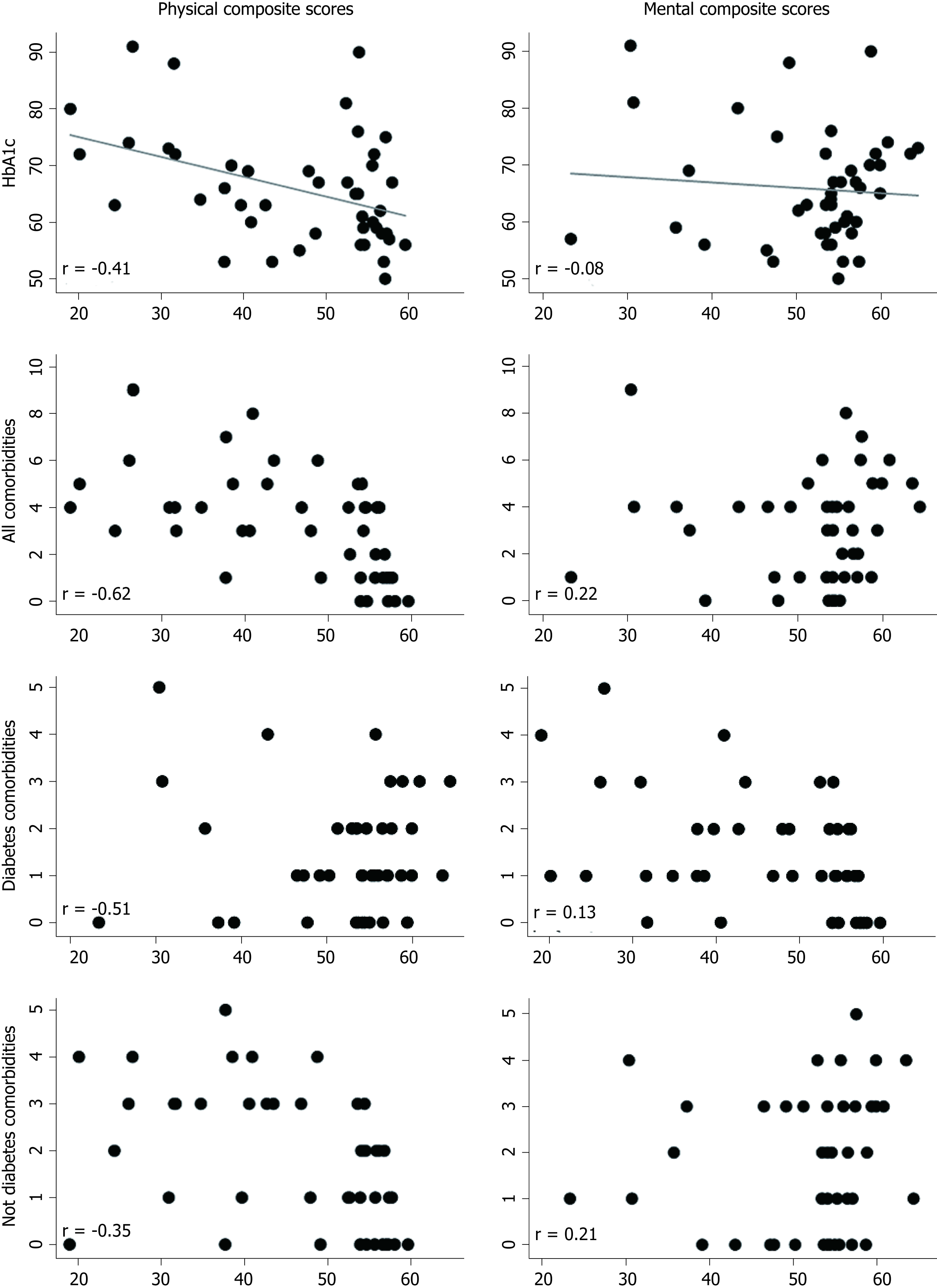
There was a negative association between the physical composite score of SF-36 and number of comorbidities (r = -0.62, P < 0.001), both diabetes (r = -0.53, P = 0.018) and non-diabetes related (r = -0.51, P < 0.001), and HbA1c level (r = -0.41, P = 0.005), as can be seen in Figure 2. However, one of these were associated with the mental composite score of SF-36 (P > 0.05).
Additionally, physical parameters of physical function, role limitation due to physical health, bodily aim and general health were all associated to and number of comorbidities (P < 0.01), both diabetes (P < 0.03) and non-diabetes related (P < 0.02), while only physical function and bodily pain were associated to HbA1c (P < 0.02). More detail can be found in Table 2.
| HbA1c | All omorbidities | Diabetes elated | Non-diabetes related | ||
| Physical functioning | r | -0.51 | -0.58 | -0.42 | -0.52 |
| p | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |
| Role limitation physical health | r | -0.44 | -0.33 | -0.38 | |
| p | < 0.01 | 0.03 | 0.01 | ||
| Bodily pain | r | -0.34 | -0.48 | -0.43 | -0.38 |
| p | 0.02 | < 0.01 | < 0.01 | 0.01 | |
| General health | r | -0.41 | -0.34 | -0.36 | |
| p | < 0.01 | 0.02 | 0.02 |
Disease duration, vibration threshold, nerve conduction velocity of the efferent median nerve and the afferent sural nerve, and number of hypoglycaemic events were not associated with HRQoL scores. Additionally, there was no difference in symptoms between people using standard insulin pens and people using insulin pumps.
This study partly confirms our hypothesis, as in particular physical domains and not mental domains were negatively affected in people with diabetes and diabetic symmetrical peripheral neuropathy, potentially limiting the patients in their daily work and social activities. This emphasizes the importance of assessing HRQoL in long-term diabetes. Additionally, increased numbers of comorbidities and high levels of HbA1c, were associated with decreased HRQoL scores.
Decreased HRQoL is of great importance. It has been shown that a 1 point decrease in physical functioning and physical composite scores equals an 9% increase in mortality risk, a 4% increase in the risk of hospitalization within six months, and a 12% increase in the risk of being unable to work[13]. As preventive medicine may be initiated in order to delay or reverse the negative impact on self-assessed health, the importance of assessing HRQoL is essential in monitoring the self-assessed burden of diabetes. Decreased HRQoL in people with diabetes has previously been shown in studies from Croatia, Norway and Australia[5,6,14,15]. However, the previously investigated cohorts consisted of both type 1 and type 2 diabetes. As patients with type 2 diabetes often appear with other comorbidities and stereotypical life style, this can have negative impact on the combined HRQoL[14]. In contrast, a study by Jacobson et al[16] studied HRQoL in people with type 1 diabetes over an average of 23 years and found no decrease in HRQoL scores over time. The present study we looked into HRQoL in patients with type 1 diabetes compared to healthy and we found numerically decreased and clinically relevant declines in HRQoL for all sub-scores, except social functioning and a most significant decrease in the physical components. This finding, related to the mixed cohorts, is possibly because people with type 1 diabetes are found in all social groups, and hence also in all social groups with larger psychological resources in comparison to people with type 2 diabetes. Contrary, compared with the study by Jacobson et al[16], we only had a cross-sectional look at HRQoL and therefore do not know the long-term ramifications for our patient group.
Mental health has received increased recognition in recent years. Studies have shown that people with type 1 diabetes have a three-fold rate of depression in comparison to the general population. However, in the current study we did not find a significant decreased mental composite score, nor in the mental domains of social functioning and mental health.
The presence of physical disabling diabetic complications such as cardiovascular events, gastrointestinal dysfunction and neuropathy with or without pain, have been shown to decrease HRQoL[6,14,17,18]. In a cohort of people with type 1 diabetes who were followed over 6 years, disease duration and the presence of complications convincingly decreased the physical composite scores[19], and the presence of neuropathy in type 1 diabetes negatively influenced the physical composite score[20], in line with the impact on the sensory and motor system. Additionally, a study over 17 years in people with type 1 diabetes showed that development of microvascular complications significantly decreased HRQoL[16]. We showed no association between the severity of neuropathy and the HRQoL scores, nor with disease severity and duration. These findings are plausibly biased by the fact that all patients were included based on severe polyneuropathy. In a study assessing HRQoL in chronic diseases with the presence of comorbidities, HRQoL was decreased due to the chronicity, but this was exaggerated as the number of comorbidities increased[21]. In particular, Bjorner et al[13] showed that number of comorbidities did not affect mental composite score. The current finding where HRQoL decreased with an increased number of comorbidities, needs to be considered when preventive medicine and adequate disease management is planned.
Even though HbA1c only indicates the preceding 3-mo’ glycaemic status, it provides patients and clinicians with an objective measure. Mellerio et al[22] studied the association between HbA1C and HRQoL in adult people with childhood onset type 1 diabetes. They concluded that no metabolic parameters, including HbA1c, was predictive of HRQoL, thereby indicating that social impact was more important than glycaemic control for the well-being[22]. In contrast, the mean disease duration in this cohort was 32 years and we showed that HRQoL decreased as HbA1c increased, which may reflect lack of life-long tight glucose control. On the other hand, this could also reflect the impact of neuropathy and its effect on the ability to tightly control glucose, thus pointing to HbA1c as a potential marker of complications and not solely of glucose control. The data on the association between HRQoL and hypoglycaemia is conflicting. Hypoglycaemic events affects negatively on the individual health, and quality of life[20]. However, in a larger cohorts of people with type 1 diabetes no association between hypoglycaemia and HRQoL was shown[20,23]. In contrast, in younger individuals an association between role limitations due to physical problems and hypoglycaemic events was reported[18]. Unfortunately, in the current study, hypoglycaemic episodes were only sparsely recorded (48 h), and therefore it was not surprisingly, that we did not find any association between the number of hypoglycaemic events and HRQoL.
Insulin treatment is the core of glycaemic control and management of type 1 diabetes and with the rise of new technologies such as continuous glucose measurements combined with improved treatment the last couple of years, this has become easier for the patients. Surprisingly, Hart et al[17] showed that continuous insulin treatment decreased the mental composite score, due to the stress of regular blood glucose monitoring. Such findings were not supported by this study, as no differences in HRQoL were shown between people using insulin pens or pump.
This study was not without limitations. Firstly, this study was conducted in a well-defined, middle-aged cohort with verified severe diabetic symmetrical peripheral neuropathy and thus the results cannot be directly generalised to other patient groups. Additionally, these were compared with healthy individuals, and therefore the effects measured may be skewed due to the effect of diabetes alone on SF-36. Secondly, we used the SF-36 to measure HRQoL assessments. Future studies may use the diabetes specific quality of life questionnaires and potentially add more insight into diabetes HRQoL. Lastly, it would have been interesting to study if hyperglycaemic events were associated to HRQoL in this cohort.
In summary, as hypothesised this study showed a decrease in the physical components of the HRQoL in a well-defined cohort of people with type 1 diabetes and severe diabetic symmetrical peripheral neuropathy. To our surprise, no associations were found in the mental components. Furthermore, decreased HRQoL was associated to number of co-morbidities and dysregulated glycaemic control, but not to the severity of neuropathy. This emphasises the importance of considering quality of life in people with diabetes, especially in those with multiple comorbidities. Furthermore, it is important to consider HbA1c as a biomarker for complication and thereby indirectly for quality of life.
Diabetic symmetrical peripheral neuropathy is a frequent complication to type 1 diabetes and is associated to incapacitating complication and decreased lifespan, possibly affecting health related quality of life (HRQoL). The 36-Item Short Form Health Survey (SF-36) is a generic patient reported questionnaire, which can be used to evaluate mental and physical HRQoL in patients with diabetes.
HRQoL is an increasingly acknowledged method in clinical practice, to evaluate patient centred outcomes in the measurement of disease burden, progression and treatment outcome.
To investigate if diabetic neuropathy would decrease physical and mental quality of life measured with SF-36, and if clinical appearance may be associated with the decline.
Baseline data of standardised nerve conduction and SF-36 questionnaire as well as information on disease duration, number of comorbidities, vibration perception threshold, number of hypoglycaemic events, HbA1c and administration way of insulin was collected from 48 adults with verified diabetic symmetrical peripheral neuropathy and 21 healthy participants as part of a clinical trial.
People with diabetic symmetrical peripheral neuropathy had a significantly decreased physical score, but not mental score compared with healthy. Furthermore, this decrease in physical score was associated with total number of comorbidities, comorbidities relation to diabetes and HbA1c as well as comorbidities not related to diabetes.
HRQoL is an important tool for evaluate patient centred outcomes in people with diabetes and is decreased with diabetic symmetrical peripheral neuropathy but also with increase in symptoms and suboptimal long-term glucose measures.
HRQoL is an informative measure for use in investigation of diabetes and related neuropathy or symptoms in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Denmark
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Karras SN S- Editor: Ji FF L- Editor: A E- Editor: Song H
| 1. | Tesfaye S, Stevens LK, Stephenson JM, Fuller JH, Plater M, Ionescu-Tirgoviste C, Nuber A, Pozza G, Ward JD. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia. 1996;39:1377-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 399] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 2. | Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P; Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1948] [Cited by in RCA: 1744] [Article Influence: 116.3] [Reference Citation Analysis (0)] |
| 3. | Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6:CD007543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 267] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 4. | Frendl DM, Ware JE. Patient-reported functional health and well-being outcomes with drug therapy: a systematic review of randomized trials using the SF-36 health survey. Med Care. 2014;52:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Dermanovic Dobrota V, Hrabac P, Skegro D, Smiljanic R, Dobrota S, Prkacin I, Brkljacic N, Peros K, Tomic M, Lukinovic-Skudar V, Basic Kes V. The impact of neuropathic pain and other comorbidities on the quality of life in patients with diabetes. Health Qual Life Outcomes. 2014;12:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Poljicanin T, Ajduković D, Sekerija M, Pibernik-Okanović M, Metelko Z, Vuletić Mavrinac G. Diabetes mellitus and hypertension have comparable adverse effects on health-related quality of life. BMC Public Health. 2010;10:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Brock C, Jessen N, Brock B, Jakobsen PE, Hansen TK, Rantanen JM, Riahi S, Dimitrova YK, Dons-Jensen A, Aziz Q, Drewes AM, Farmer AD. Cardiac vagal tone, a non-invasive measure of parasympathetic tone, is a clinically relevant tool in Type 1 diabetes mellitus. Diabet Med. 2017;34:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23352] [Cited by in RCA: 23969] [Article Influence: 726.3] [Reference Citation Analysis (0)] |
| 9. | McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4486] [Cited by in RCA: 4672] [Article Influence: 146.0] [Reference Citation Analysis (0)] |
| 10. | McHorney CA, Ware JE, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3052] [Cited by in RCA: 3201] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 11. | Polonsky WH. Emotional and quality-of-life aspects of diabetes management. Curr Diab Rep. 2002;2:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Quah JH, Luo N, Ng WY, How CH, Tay EG. Health-related quality of life is associated with diabetic complications, but not with short-term diabetic control in primary care. Ann Acad Med Singapore. 2011;40:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Bjorner JB, Lyng Wolden M, Gundgaard J, Miller KA. Benchmarks for interpretation of score differences on the SF-36 health survey for patients with diabetes. Value Health. 2013;16:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Hasler WL. Gastroparesis: symptoms, evaluation, and treatment. Gastroenterol Clin North Am. 2007;36:619-647, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Søfteland E, Brock C, Frøkjær JB, Brøgger J, Madácsy L, Gilja OH, Arendt-Nielsen L, Simrén M, Drewes AM, Dimcevski G. Association between visceral, cardiac and sensorimotor polyneuropathies in diabetes mellitus. J Diabetes Complications. 2014;28:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Jacobson AM, Braffett BH, Cleary PA, Gubitosi-Klug RA, Larkin ME; DCCT/EDIC Research Group. The long-term effects of type 1 diabetes treatment and complications on health-related quality of life: a 23-year follow-up of the Diabetes Control and Complications/Epidemiology of Diabetes Interventions and Complications cohort. Diabetes Care. 2013;36:3131-3138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Hart HE, Redekop WK, Bilo HJ, Berg M, Jong BM. Change in perceived health and functioning over time in patients with type I diabetes mellitus. Qual Life Res. 2005;14:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Klein BE, Klein R, Moss SE. Self-rated health and diabetes of long duration. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 1998;21:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Hart HE, Redekop WK, Berg M, Bilo HJ, Meyboom-de Jong B. Factors that predicted change in health-related quality of life were identified in a cohort of diabetes mellitus type 1 patients. J Clin Epidemiol. 2005;58:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Hirai FE, Tielsch JM, Klein BE, Klein R. Ten-year change in self-rated quality of life in a type 1 diabetes population: Wisconsin Epidemiologic Study of Diabetic Retinopathy. Qual Life Res. 2013;22:1245-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Hutchinson AF, Graco M, Rasekaba TM, Parikh S, Berlowitz DJ, Lim WK. Relationship between health-related quality of life, comorbidities and acute health care utilisation, in adults with chronic conditions. Health Qual Life Outcomes. 2015;13:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 22. | Mellerio H, Guilmin-Crépon S, Jacquin P, Labéguerie M, Lévy-Marchal C, Alberti C. Long-term impact of childhood-onset type 1 diabetes on social life, quality of life and sexuality. Diabetes Metab. 2015;41:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Hart HE, Bilo HJ, Redekop WK, Stolk RP, Assink JH, Meyboom-de Jong B. Quality of life of patients with type I diabetes mellitus. Qual Life Res. 2003;12:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |