Published online Feb 15, 2019. doi: 10.4239/wjd.v10.i2.114
Peer-review started: October 6, 2018
First decision: November 15, 2018
Revised: December 14, 2018
Accepted: December 29, 2018
Article in press: December 30, 2018
Published online: February 15, 2019
Processing time: 136 Days and 14.8 Hours
Non-alcoholic fatty liver disease (NAFLD) is a common comorbidity with type 2 diabetes. The existing therapeutic options for NAFLD are not adequate. Hypocaloric diet and exercise is the cornerstone of therapy in NAFLD. Pioglitazone is the only drug recommended in diabetes patients with biopsy proven non-alcoholic steatohepatitis. The frequent coexistence of NAFLD and type 2 diabetes with their combined adverse health consequences and inadequate therapeutic options makes it necessary to search for newer alternatives.
To assess the effect of sodium glucose cotransporter-2 (SGLT-2) inhibitors on liver enzymes in type 2 diabetes patients with NAFLD.
We searched PubMed/MEDLINE, Cochrane library, Google scholar, and Clinicaltrials.gov for the relevant articles to be included in this systematic review. Human studies done in type 2 diabetes patients with NAFLD treated with SGLT-2 inhibitors for at least 12 wk were included. Data from eight studies (four randomised controlled trials and four observational studies) were extracted and a narrative synthesis was done. A total of 214 patients were treated with SGLT-2 inhibitors in these studies (94 in randomised controlled trials and 120 in observational studies).
The primary outcome measure was change in serum alanine aminotransferase level. Out of eight studies, seven studies showed a significant decrease in serum alanine aminotransferase level. Most of the studies revealed reduction in serum level of other liver enzymes like aspartate aminotransferase and gamma glutamyl transferase. Five studies that reported a change in hepatic fat exhibited a significant reduction in hepatic fat content in those treated with SGLT-2 inhibitors. Likewise, among the three studies that evaluated a change in indices of hepatic fibrosis, two studies revealed a significant improvement in liver fibrosis. Moreover, there was an improvement in obesity, insulin resistance, glycaemia, and lipid parameters in those subjects taking SGLT-2 inhibitors. The studies disclosed that about 17% (30/176) of the subjects taking SGLT-2 inhibitors developed adverse events and more than 40% (10/23) of them had genitourinary tract infections.
Based on low to moderate quality of evidence, SGLT-2 inhibitors improve the serum level of liver enzymes, decrease liver fat, and fibrosis with additional beneficial effects on various metabolic parameters in type 2 diabetes patients with NAFLD.
Core tip: The frequent coexistence of non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes, their adverse health consequences, and lack of adequate therapeutic options makes it necessary to search for newer alternatives. Currently, pioglitazone and vitamin E are recommended in addition to lifestyle modifications for the management of NAFLD. Animal studies have shown that sodium glucose cotransporter-2 inhibitors might be beneficial in NAFLD present in diabetes patients. The current systematic review shows that sodium glucose cotransporter-2 inhibitors improve the serum level of liver enzymes, liver fat, and liver fibrosis with additional beneficial effects on various metabolic parameters in type 2 diabetes patients with NAFLD.
- Citation: Raj H, Durgia H, Palui R, Kamalanathan S, Selvarajan S, Kar SS, Sahoo J. SGLT-2 inhibitors in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus: A systematic review. World J Diabetes 2019; 10(2): 114-132
- URL: https://www.wjgnet.com/1948-9358/full/v10/i2/114.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i2.114
Non-alcoholic fatty liver disease (NAFLD) is an emerging public health issue worldwide. The prevalence of NAFLD in type 2 diabetes mellitus patients is three times greater as compared to the general population. Its prevalence in diabetic subjects ranges from 69%-87% depending upon the imaging modality used[1]. The spectrum of NAFLD includes simple steatosis, steatohepatitis, and cirrhosis[2]. Besides NAFLD is a risk factor for extrahepatic complications like cardiovascular disease, chronic kidney disease, and type 2 diabetes. In addition, the prevalence of both microvascular and macrovascular complications is increased in patients with NAFLD and type 2 diabetes[3].
The existing therapeutic options for NAFLD are not adequate. Hypocaloric diet and exercise is the cornerstone of therapy in NAFLD. Pioglitazone and vitamin E are recommended only in biopsy-proven non-alcoholic steatohepatitis (NASH), but vitamin E is not recommended in diabetic patients due to inadequate evidence[4]. The frequent coexistence of NAFLD and type 2 diabetes with their combined adverse health consequences and inadequate therapeutic options makes it necessary to search for newer alternatives. Based on the information from animal studies, sodium glucose cotransporter-2 (SGLT-2) inhibitors appear promising in the management of NAFLD[5-7]. This systematic review is an effort to review the available literature on the effect of SGLT-2 inhibitors on NAFLD in type 2 diabetes patients.
This systematic review was performed according to the predefined protocol registered in PROSPERO (Registration ID: CRD42018104572). The protocol can be accessed at the website address https://http://www.crd.york.ac.uk/prospero. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2009 guidelines for reporting this systematic review[8]. Ethics committee approval was not required for this systematic review because it was done using published data found in the public domain.
All observational and randomised controlled trials (RCTs) done using SGLT-2 inhibitors among type 2 diabetes patients with NAFLD having both baseline and post-treatment serum alanine aminotransferase (ALT) level data with a minimum follow-up duration of 12 wk were included in this systematic review. The studies with concomitant pharmacological therapy like pioglitazone or α-tocopherol (vitamin E) for treating NAFLD were excluded to avoid the confounding effects of these drugs on liver function tests. Only those studies that were done in humans and published in English were considered for inclusion. We excluded abstract-only articles, case reports, conference presentations, editorials, reviews, expert opinions, and studies with five participants and less.
The primary outcome was the change in serum ALT levels in type 2 diabetes patients with NAFLD treated with SGLT-2 inhibitors. The secondary outcomes were change in serum aspartate aminotransferase (AST) and gamma-glutamyl transferase (GGT) levels, hepatic fat, hepatic fibrosis, metabolic profile, anthropometric parameters, and the adverse effects of SGLT-2 inhibitors.
PubMed/MEDLINE, Cochrane library, Google scholar, and Clinicaltrials.gov were searched from their date of inception until 31st August, 2018.
The search terms/MeSH terms used were “NAFLD”, “Nonalcoholic fatty liver disease”, “Non-alcoholic fatty liver disease”, “Non alcoholic fatty liver disease”, “NASH”, “Non-alcoholic steatohepatitis”, “Nonalcoholic steatohepatitis”, “Non alcoholic steatohepatitis”, “Fatty liver”, “Type 2 diabetes mellitus”, “Type 2 diabetes”, “Diabetes mellitus type 2”, “Diabetes type 2”, “SGLT-2 inhibitors”, “Sodium glucose cotransporter-2 inhibitors”, “SGLT-2”, “SGLT2”, “SGLT 2”, “Canagliflozin”, “Dapagliflozin”, “Empagliflozin”, “Ipragliflozin”, “Luseogliflozin”, “Tofogliflozin”, “Sotagliflozin”, “Remogliflozin”, “Ertugliflozin”, and “Sergliflozin”(Table 1). The references of the search articles were scrutinised for relevant articles.
| S. No | Search terms |
| 1 | NAFLD |
| 2 | Nonalcoholic fatty liver disease |
| 3 | Non-alcoholic fatty liver disease |
| 4 | Non alcoholic fatty liver disease |
| 5 | NASH |
| 6 | Non-alcoholic steatohepatitis |
| 7 | Nonalcoholic steatohepatitis |
| 8 | Non alcoholic steatohepatitis |
| 9 | Fatty liver |
| 10 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 |
| 11 | Type 2 diabetes mellitus |
| 12 | Type 2 diabetes |
| 13 | Diabetes mellitus type 2 |
| 14 | Diabetes type 2 |
| 15 | 11 OR 12 OR 13 OR 14 |
| 16 | SGLT-2 inhibitors |
| 17 | Sodium glucose cotransporter-2 inhibitors |
| 18 | SGLT-2 |
| 19 | SGLT2 |
| 20 | SGLT 2 |
| 21 | Canagliflozin |
| 22 | Dapagliflozin |
| 23 | Empagliflozin |
| 24 | Ipragliflozin |
| 25 | Luseogliflozin |
| 26 | Tofogliflozin |
| 27 | Sotagliflozin |
| 28 | Remogliflozin |
| 29 | Ertugliflozin |
| 30 | Sergliflozin |
| 31 | 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 OR 30 |
| 32 | 10 AND 15 AND 31 |
The titles and/or abstracts of studies were retrieved using the search strategy and those from additional sources were scrutinised independently by two review authors (HR and JPS) to identify studies that potentially met the inclusion criteria as outlined above. The full texts of these potentially eligible studies were retrieved and independently assessed for eligibility by three review team members (HD, SS, and RP). Any disagreements between the reviewers over the eligibility of particular studies were resolved through discussion with a fourth senior reviewer (SKK). A standardised, pre-formatted excel form was used to extract data from the included studies for the assessment of study quality.
The extracted data included the author of the study with year, the study methodology, the recruitment and study completion rates, the types of population, the exposure/intervention (dose of SGLT-2 inhibitor, duration), the results (outcome measures like change in serum ALT, AST, GGT, hepatic fat, markers of liver fibrosis, fasting plasma glucose (FPG), glycosylated haemoglobin (HbA1c), lipid profile, homeostasis model assessment-estimated insulin resistance (HOMA-IR), body mass index (BMI), any adverse effects, information for the assessment of the risk of bias, and sources of funding/support.
The statistical review of the study was performed by a biomedical statistician (SSK). A narrative synthesis of the results of individual studies was done. The change in the difference in means and difference in proportions and the respective P values as mentioned in the original manuscripts were tabulated and explained in our study.
The risk of bias of the RCTs was done using Cochrane risk of bias tool[9]. The studies were graded as “good quality” or “fair quality” or “poor quality” according to the level of risk. Methodological Index for Non-Randomized Studies (MINORS) scale was used to assess the risk of bias of observational studies[10]. A study was considered to be an ideal study if the score was 16 for single arm and 24 for comparative studies.
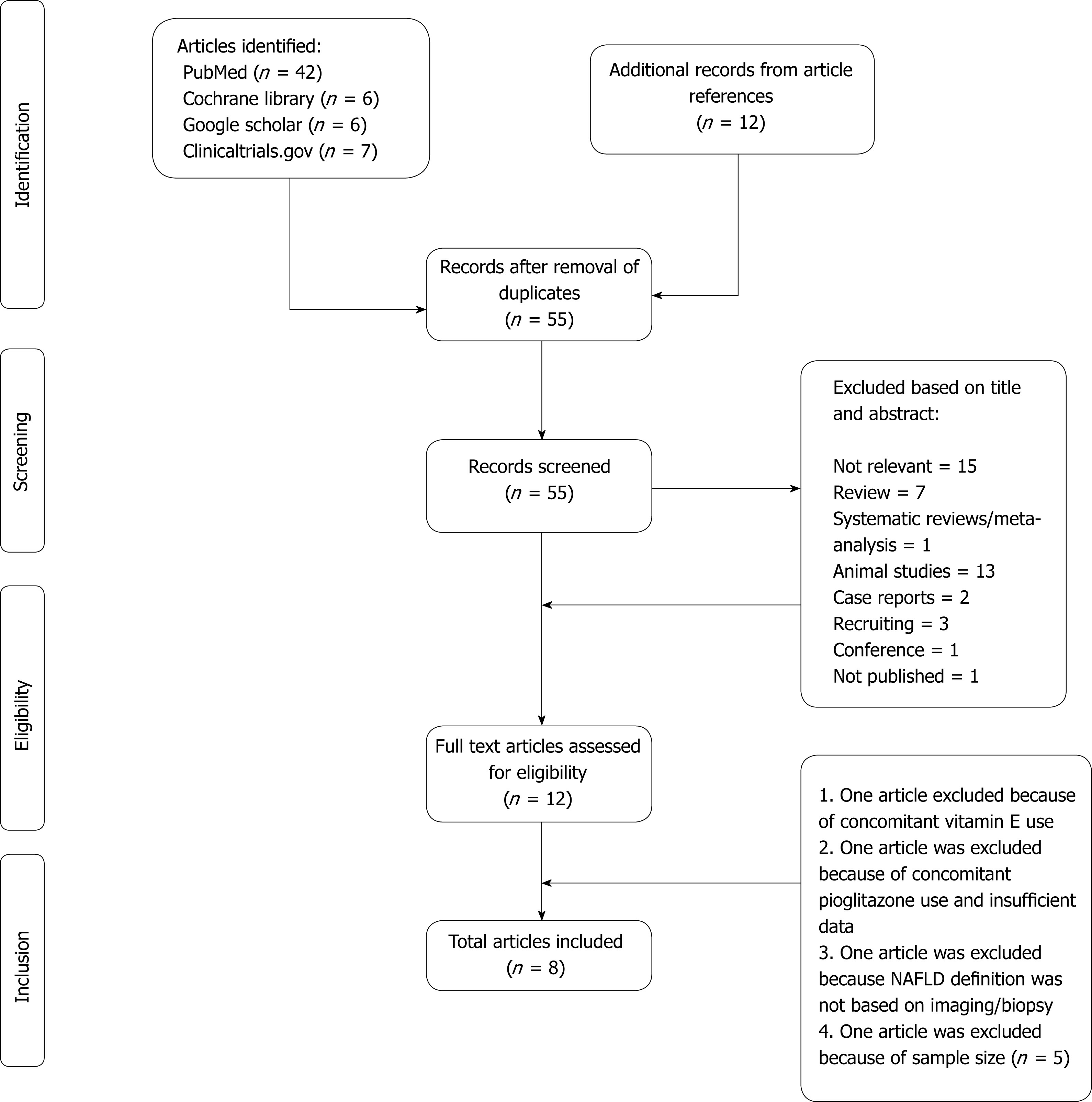
Our literature search from all the aforementioned databases yielded 73 articles (including references of the relevant articles). After eliminating duplicate articles, 55 articles were screened. Eight articles met all of the inclusion criteria (total 214 patients were on SGLT-2 inhibitors) (Figure 1).
The summary of all studies included in this systematic review is given in Tables 2 and 3. Out of the eight studies, four are RCTs[11-14] and four are observational[15-18]. Five studies were conducted amongst the Japanese population. Ipragliflozin was used in three studies whereas canagliflozin and luseogliflozin were used in two studies each, but dapagliflozin and empagliflozin were used in one study each. All studies used one type of SGLT-2 inhibitor except the one authored by Seko et al[16], where both canagliflozin and ipragliflozin were used. The change in serum ALT was a secondary outcome while the effect of SGLT- 2 inhibitors on liver fat was the primary outcome in all RCTs.
| S. No | Ref. | Inclusion criteria | Age (yr) | Male gender | Intervention arm | Control arm | Follow-up duration | Primary outcome |
| 1 | Kuchay et al[11], 2018 | Age > 20 yr, hepatic steatosis (MRI-PDFF > 6%), HbA1c > 7.0% to < 10.0% | Intervention arm: 50.7 (12.8) | Intervention arm: 16 (64%) | Standard treatment + Empagliflozin 10 mg daily (n = 25) | Standard treatment (n = 25) | 20 wk | Change in liver fat content by MRI-PDFF |
| Control arm: 49.1 (10.3) | Control arm: 17 (68%) | |||||||
| 2 | Ito et al[12], 2017 | Age 20-75 yr, HbA1c 7.0–11.0%, BMI < 45 kg/m2, On diet and exercise therapy alone or with oral hypoglycaemic agents other than SGLT-2 inhibitors and thiazolidinediones and/or insulin, NAFLD, findings suggesting hepatic steatosis and hepatic dysfunction on clinical laboratory tests or on imaging studies (e.g., computed tomography or ultrasound) | Pioglitazone arm: 59.1 (9.8) | Pioglitazone arm: 18 (53%) | Ipragliflozin 50 mg daily (n = 32) | Pioglitazone 15-30 mg daily (n = 34) | 24 wk | Change in L/S attenuation ratio |
| Ipragliflozin arm: 57.3 (12.1) | Ipragliflozin arm: 14 (44%) | |||||||
| 3 | Shibuya et al[13], 2018 | Fatty liver diagnosed on the basis of computed tomography or abdominal sonography, HbA1c 6.0%–10.0%, age 20–70 yr | Luseogliflozin arm: 51 (47-62) | Luseogliflozin arm: 10 (62.5%) | Luseogliflozin 2.5 mg daily (n = 16) | Metformin 1.5 g daily (n = 16) | 24 wk | Change in L/S attenuation ratio |
| Metformin arm: 60 (53-66) | Metformin arm: 8 (50%) | |||||||
| 4 | Eriksson et al[14], 2018 | Age 40–75 yr, treated with a stable dose of metformin or sulfonylurea alone or in combination for at least 3 mo, MRI-PDFF > 5.5%, BMI 25–40 kg/m2 | Dapagliflozin arm: 65 (6.5) | Dapagliflozin arm: 16 (76.2%) | Dapagliflozin 10 mg daily (n = 21) or Omega 3-carboxylic acid 4 g daily (n = 20) or Combination (n = 22) | Placebo (n = 21) | 12 wk | Change in liver fat content by MRI-PDFF |
| Omega 3-carboxylic acid arm: 66.2 (5.9) | Omega 3-carboxylic acid arm: 11 (55%) | |||||||
| O + D arm: 65(5.4) | O + D arm: 15 (68.2%) | |||||||
| Placebo arm: 65.6 (6.1) | Placebo arm: 17 (81%) |
| S. No | Ref. | Design | Inclusion criteria | Age (yr) | Male gender | Sample size | SGLT-2 inhibitor | Follow-up duration |
| 1 | Ohki et al[15], 2016 | Prospective study | Type 2 diabetes with NAFLD treated with GLP-1 analogues or DPP-4 inhibitors and failed to normalise serum ALT levels | 54.2 (49.3-60.1) | 19 (79.2%) | 24 | Ipragliflozin 25-50 mg daily | 320 d (302-329) |
| 2 | Seko et al[16], 2016 | Retrospective cohort study | Type 2 diabetes with NAFLD | SGLT-2 inhibitor arm: 60.3 (1.8) | SGLT-2 inhibitor arm: 9 (37.5%) | 24 (SGLT-2 inhibitor); 21 (Sitagliptin ) | Canagliflozin 100 mg (n = 18) or Ipragliflozin 50 mg daily (n = 6) | 24 wk |
| Sitagliptin arm: 59.4 (3.7) | Sitagliptin arm: 8 (38.1%) | |||||||
| 3 | Gautam et al[17], 2018 | Prospective study | Type 2 diabetes with NAFLD | - | - | 32 | Canagliflozin 100 mg daily | 24 wk |
| 4 | Sumida et al[18], 2018 | Prospective study | Age > 20 yr, HbA1c > 6.5% to < 8.5%, NAFLD | 55.4 (13.6) | 28 (70%) | 40 | Luseogliflozin 2.5 mg daily | 24 wk |
The risk of bias of RCTs was assessed using the Cochrane risk of bias tool. Among the four RCTs, the studies done by Kuchay et al[11] and Eriksson et al[14] were of good quality however those done by Ito et al[12] and Shibuya et al[13] were of fair quality (Table 4). The risk of bias of observational studies was assessed using the MINORS scale. All the observational studies were of less than ideal quality (Table 5).
| Study | Criteria | Risk of bias | Study quality |
| Kuchay et al[11] | Random sequence generation | Low risk | Good quality |
| Allocation concealment | Low risk | ||
| Selective reporting | Low risk | ||
| Other bias | Low risk | ||
| Blinding of participants and personnel | Low risk | ||
| Blinding of outcome assessment | Low risk | ||
| Incomplete outcome data | Low risk | ||
| Ito et al[12] | Random sequence generation | Low risk | Fair quality |
| Allocation concealment | Unclear risk | ||
| Selective reporting | Low risk | ||
| Other bias | Low risk | ||
| Blinding of participants and personnel | Low risk | ||
| Blinding of outcome assessment | Low risk | ||
| Incomplete outcome data | Low risk | ||
| Shibuya et al[13] | Random sequence generation | Unclear risk | Fair quality |
| Allocation concealment | Unclear risk | ||
| Selective reporting | Low risk | ||
| Other bias | Low risk | ||
| Blinding of participants and personnel | Low risk | ||
| Blinding of outcome assessment | Low risk | ||
| Incomplete outcome data | Low risk | ||
| Eriksson et al[14] | Random sequence generation | Low risk | Good quality |
| Allocation concealment | Low risk | ||
| Selective reporting | Low risk | ||
| Other bias | Low risk | ||
| Blinding of participants and personnel | Low risk | ||
| Blinding of outcome assessment | Low risk | ||
| Incomplete outcome data | Low risk |
| S. No | Criteria | Ohki et al[15] | Seko et al[16] | Gautam et al[17] | Sumida et al[18] |
| 1 | A clearly stated aim | 2 | 2 | 2 | 2 |
| 2 | Inclusion of consecutive patients | 0 | 2 | 2 | 1 |
| 3 | Prospective collection of data | 2 | 0 | 2 | 2 |
| 4 | Endpoints appropriate to the aim of the study | 2 | 2 | 2 | 2 |
| 5 | Unbiased assessment of the study endpoint | 0 | 0 | 0 | 0 |
| 6 | Follow-up period appropriate to the aim of the study | 2 | 2 | 2 | 2 |
| 7 | Loss to follow up less than 5% | 2 | 2 | 2 | 2 |
| 8 | Prospective calculation of the study size | 0 | 0 | 0 | 0 |
| 9 | An adequate control group | NA | 0 | NA | NA |
| 10 | Contemporary groups | NA | 2 | NA | NA |
| 11 | Baseline equivalence of groups | NA | 2 | NA | NA |
| 12 | Adequate statistical analyses | NA | 2 | NA | NA |
| 13 | Total score | 10/16 | 16/24 | 12/16 | 11/16 |
Change in serum ALT levels: In all of the studies, there was a decrease in serum ALT levels from the baseline in those treated with SGLT-2 inhibitors (Table 6) but in the study done by Shibuya et al[13] it did not reach statistical significance.
| Study | Serum ALT level (IU/L) | P value | P value between groups | ||
| Group | Baseline | Study completion | |||
| Kuchay et al[11] | Empagliflozin | 64.3 (20.2) | 49.7 (25.8) | 0.001 | 0.005 |
| Control | 65.3 (40.3) | 61.6 (38.4) | 0.422 | ||
| Ito et al[12] | Ipragliflozin | 57.4 (27.3) | 38.2 (20.5) | < 0.05 | 0.642 |
| Pioglitazone | 53.1 (26.6) | 36.8 (15.1) | < 0.05 | ||
| Shibuya et al[13] | Luseogliflozin | 49.5 (31.0, 70.0) | 31 (26.0, 55.0) | 0.057 | 0.064 |
| Metformin | 39 (23.0, 56.0) | 39 (27.0, 51.0) | 0.518 | ||
| Eriksson et al[14] | Placebo | 33.53 (12.4) | -0.2 (8.8)1 | - | - |
| Omega-3 CA | 37.65 (14.7) | +5.9 (16.5)1 | - | Non-significant2 | |
| Dapagliflozin | 39.41 (14.7) | -8.2 (8.2)1 | - | < 0.052 | |
| O + D | 35.88 (17.1) | +0.1 (12.9)1 | - | Non-significant2 | |
| Ohki et al[15] | Ipragliflozin | 62 (43.0-75.0) | 38.0 (31.0-65.0) | 0.01 | - |
| Seko et al[16] | SGLT-2 inhibitor | 70.8 (8.1) | 48.8 (5.5) | 0.002 | 0.039 |
| Sitagliptin | 92.4 (11.2) | 71.1 (10.0) | 0.012 | ||
| Gautam et al[17] | Canagliflozin | 96 (18.7) | 60.0 (17.6) | < 0.00001 | - |
| Sumida et al[18] | Luseogliflozin | 54.7 (28.2) | 42.4 (26.5) | < 0.001 | - |
Kuchay et al[11] found a significant decrease in serum ALT levels in the empagliflozin arm compared to the control arm at the end of the study (difference between the two arms was -10.9 IU/L, P = 0.005). In the study done by Ito et al[12] ALT levels decreased equally in both the groups [Change from baseline in ipragliflozin group: -17.5 (4) and pioglitazone group: -20 (3.4), P = 0.642]. Similar results were found in the study by Shibuya et al[13] [ΔALT in luseogliflozin arm was 9 (-20, 1) and in metformin arm was 4.5 (-5, 9), P = 0.064]. Eriksson et al[14] found that the ALT reduction in the dapagliflozin arm was more compared to placebo [ΔALT in dapagliflozin arm was -8.24 (8.24) and in the placebo arm was -0.18 (8.82), P < 0.05]. Seko et al[16] demonstrated that the serum ALT levels in SGLT-2 inhibitor arm was lower compared to the sitagliptin arm at the end of the study [48.8 (5.5) vs 71.1 (10), P = 0.039]
Change in serum AST levels: Seven of the included studies had data regarding change in serum AST levels (Table 7). The study done by Shibuya et al[13] did not have data on AST levels. All the studies showed a significant reduction in serum AST levels in those treated with SGLT-2 inhibitors. The decrease in AST with empagliflozin and ipragliflozin was similar compared to placebo and pioglitazone respectively whereas dapagliflozin was better than placebo.
| Study | Serum AST levels (IU/L) | P value | P value between groups | ||
| Group | Baseline | Study completion | |||
| Kuchay et al[11] | Empagliflozin | 44.6 (23.5) | 36.2 (9.0) | 0.04 | 0.212 |
| Control | 45.3 (24.3) | 44.6 (23.8) | 0.931 | ||
| Ito et al[12] | Ipragliflozin | 39.7 (16.7) | 27.3 (8.9) | < 0.05 | 0.802 |
| Pioglitazone | 43.3 (20.5) | 32.4 (15.4) | < 0.05 | ||
| Eriksson et al[14] | Placebo | 29.4 (13.2) | -1.2 (7.2)1 | - | - |
| Omega-3 CA | 30.6 (10.2) | +4.8 (9.0)1 | - | Non-significant2 | |
| Dapagliflozin | 31.2 (11.4) | -4.2 (5.4)1 | - | < 0.052 | |
| O + D | 30 (10.2) | +1.2 (5.4)1 | - | Non-significant2 | |
| Ohki et al[15] | Ipragliflozin | 37 (29.0-52.0) | 28 (23.0-31.0) | 0.03 | - |
| Seko et al[16] | SGLT-2 inhibitor | 54.4 (5.6) | 38 (3.1) | 0.001 | - |
| Sitagliptin | 67 (7.7) | 52.5 (7.7) | 0.016 | ||
| Gautam et al[17] | Canagliflozin | 72 (16.7) | 53 (10.3) | < 0.00001 | - |
| Sumida et al[18] | Luseogliflozin | 40.7 (22.2) | 31.9 (18.2) | < 0.001 | - |
Change in serum GGT levels: Seven studies had data regarding GGT levels. Six studies reported a significant decrease in serum GGT levels in those treated with SGLT-2 inhibitors (Table 8). In the study done by Seko et al[16], there was an insignificant decrease in both the SGLT-2 inhibitor and DPP-4 inhibitor groups. The decrease in GGT with empagliflozin and ipragliflozin was similar compared to placebo and pioglitazone correspondingly while dapagliflozin was better than placebo.
| Study | Serum GGT (IU/L ) | P value | P value between groups | ||
| Group | Baseline | Study completion | |||
| Kuchay et al[11] | Empagliflozin | 65.8 (36.1) | 50.9 (24.6) | 0.002 | 0.057 |
| Control | 63.9 (45.3) | 60.0 (39.0) | 0.421 | ||
| Ito et al[12] | Ipragliflozin | 62.8 (58.3) | 44.0 (38.3) | < 0.05 | 0.642 |
| Pioglitazone | 71.6 (54.1) | 48.8 (61.2) | < 0.05 | ||
| Eriksson et al[14] | Placebo | 32.4 (17.4) | +2.4 (9.6)1 | - | - |
| Omega-3 CA | 54.0 (57.6) | +2.4 (12.0)1 | - | Non-significant2 | |
| Dapagliflozin | 58.2 (43.2) | -4.8 (13.8)1 | - | < 0.052 | |
| O + D | 40.2 (14.4) | -0.6 (13.8)1 | - | Non-significant2 | |
| Ohki et al[15] | Ipragliflozin | 75.0 (47.0-105.0) | 60.0 (40.0-101.0) | 0.03 | - |
| Seko et al[16] | SGLT-2 inhibitor | 61.7 (9.1) | 58.7 (11.5) | 0.051 | - |
| Sitagliptin | 89.2 (11.8) | 82.4 (11.9) | 0.36 | ||
| Gautam et al[17] | Canagliflozin | 75.1 (31.8) | 69.2 (26.2) | 0.003 | - |
| Sumida et al[18] | Luseogliflozin | 62.4 (77.1) | 48.2 (56.3) | 0.003 | - |
Change in hepatic fat: Kuchay et al[11] and Eriksson et al[14] evaluated hepatic fat using magnetic resonance imaging- derived proton density fat fraction (Table 9). It was found that there was a significant reduction in hepatic fat in the empagliflozin arm compared to the control arm in the study done by Kuchay et al[11]. In the study done by Eriksson et al[14], dapagliflozin or omega-3 carboxylic acid when administered alone or in combination reduced hepatic fat fraction significantly. When compared with placebo, only the combination of both drugs reduced hepatic fat fraction significantly. Sumida et al[18] showed that luseogliflozin significantly reduced hepatic fat fraction using magnetic resonance imaging-hepatic fat fraction. Ito et al[12] and Shibuya et al[13] used liver/spleen attenuation ratio for measuring hepatic fat. They found that ipragliflozin was equivalent to pioglitazone in improving liver/spleen attenuation ratio while luseogliflozin was found to be superior to metformin in the same aspect.
| Study | Parameter | Group | Baseline | Study completion | P value | P value between groups |
| Kuchay et al[11] | MRI-PDFF | Empagliflozin | 16.2 (7) | 11.3 (5.3) | < 0.0001 | < 0.0001 |
| Control | 16.4 (7.3) | 15.5 (6.7) | 0.054 | |||
| Ito et al[12] | L/S ratio | Ipragliflozin | 0.8 (0.2) | 1.0 (0.2) | < 0.05 | 0.90 |
| Pioglitazone | 0.8 (0.3) | 1.0 (0.2) | < 0.05 | |||
| Shibuya et al[13] | L/S ratio | Luseogliflozin | 0.9 (0.6-1.0) | 1.0 (0.8-1.2) | 0.0008 | 0.00002 |
| Metformin | 1.0 (0.8-1.1) | 0.9 (0.7-1.0) | 0.017 | |||
| Eriksson et al[14] | MRI-PDFF | Placebo | 15.1 (6.5) | -0.6 (1.9)1 | - | - |
| Omega-3 CA | 22.2 (11.0) | -3.2 (2.9)1 | - | Non-significant2 | ||
| Dapagliflozin | 17.3 (9.1) | -2.2 (3.3)1 | - | Non-significant2 | ||
| O + D | 17.8 (9.2) | -3.2 (3.5)1 | - | < 0.052 | ||
| Sumida et al[18] | MRI-HFF | Luseogliflozin | 21.5 (7.2) | 15.7 (6.8) | < 0.001 | - |
Ito et al[12] and Ohki et al[15] evaluated liver fibrosis using the FIB-4 index (Table 10). There was a significant decrease in the FIB-4 index in the ipragliflozin arms compared to baseline. Ipragliflozin was similar to pioglitazone in decreasing the FIB-4 index. Sumida et al[18] used both the FIB-4 index and NAFLD fibrosis score. There was no significant change in either indices.
| Study | Parameter | Group | Baseline | Study completion | P value | P value between groups |
| Ito et al[12] | FIB-4 index | Ipragliflozin | 1.44 (0.64) | 1.22 (0.55) | < 0.05 | 0.596 |
| Pioglitazone | 1.84 (1.13) | 1.71 (1.19) | Non-significant | |||
| Ohki et al[15] | FIB-4 index | Ipragliflozin | 1.75 (0.82-1.93) | 1.39 (0.77-1.99) | 0.04 | - |
| Sumida et al[18] | FIB-4 index | Luseogliflozin | 1.63 (1.19) | 1.52 (0.92) | 0.17 | - |
| NAFLD fibrosis score | Luseogliflozin | 1.61 (0.71) | 1.62 (0.88) | 0.86 | - |
Seven studies reported changes in FPG and HbA1c (Tables 11 and 12). The majority of the studies showed a decrease in FPG and HbA1c.
| Study | Fasting plasma glucose (mg/dL) | P value | P value between groups | ||
| Group | Baseline | Study completion | |||
| Kuchay et al[11] | Empagliflozin | 173.0 (44.0) | 124.0 (17.0) | < 0.001 | 0.85 |
| Control | 176.0 (57.0) | 120.0 (19.0) | < 0.0001 | ||
| Ito et al[12] | Ipragliflozin | 160.1 (38.7) | 136.5 (26.7) | < 0.05 | 0.785 |
| Pioglitazone | 169.4 (50.9) | 139.0 (26.6) | < 0.05 | ||
| Shibuya et al[13] | Luseogliflozin | 127.0 (116.0, 136.0) | 125.0 (113.0, 138.0) | 0.87 | 0.583 |
| Metformin | 147.0 (126.0, 161.0) | 134.0 (122.0, 145.0) | 0.32 | ||
| Eriksson et al[14] | Placebo | 169.2 (29.7) | +6.7 (14.8)1 | - | - |
| Omega-3 CA | 162.4 (26.6) | +3.8 (19.3)1 | - | Non-significant2 | |
| Dapagliflozin | 161.8 (33.3) | -17.6 (26.8)1 | - | < 0.052 | |
| O + D | 168.8 (35.5) | -16.4 (36.0)1 | - | < 0.052 | |
| Ohki et al[15] | Ipragliflozin | 162.0 (135.0-189.0) | 135.0 (120.0-166.0) | 0.3 | - |
| Seko et al[16] | SGLT-2 inhibitor | 125.0 (6.0) | 116.6 (4.2) | 0.07 | Non-significant |
| Sitagliptin | 114.6 (7.0) | 134.0 (10.5) | 0.067 | ||
| Sumida et al[18] | Luseogliflozin | 142.0 (30.3) | 135.4 (25.6) | 0.04 | - |
| Study | Glycosylated haemoglobin (%) | P value | P value between groups | ||
| Group | Baseline | Study completion | |||
| Kuchay et al[11] | Empagliflozin | 9.0 (1.0) | 7.2 (0.6) | < 0.001 | 0.88 |
| Control | 9.1 (1.4) | 7.1 (0.9) | < 0.0001 | ||
| Ito et al[12] | Ipragliflozin | 8.5 (1.5) | 7.6 (1.0) | < 0.05 | 0.522 |
| Pioglitazone | 8.3 (1.4) | 7.1 (0.9) | < 0.05 | ||
| Shibuya et al[13] | Luseogliflozin | 7.8 (7.2, 7.9) | 6.5 (6.4, 7.0) | 0.002 | 0.023 |
| Metformin | 7.4 (6.9, 7.7) | 7.3 (6.7, 7.6) | 0.362 | ||
| Eriksson et al[14] | Placebo | 7.4 (0.8) | -0.1 (0.4)1 | - | - |
| Omega-3 CA | 7.4 (0.7) | +0.1 (0.4)1 | - | Non-significant2 | |
| Dapagliflozin | 7.4 (0.6) | -0.6 (0.7)1 | - | < 0.052 | |
| O + D | 7.5 (0.8) | -0.5 (0.5)1 | - | Non-significant2 | |
| Ohki et al[15] | Ipragliflozin | 8.4 (7.8-8.9) | 7.6 (6.9-8.2) | < 0.01 | - |
| Seko et al[16] | SGLT-2 inhibitor | 6.7 (0.1) | 6.5 (0.1) | 0.055 | Non-significant |
| Sitagliptin | 7.0 (0.3) | 6.9 (0.3) | 0.331 | ||
| Sumida et al[18] | Luseogliflozin | 7.3 (0.7) | 7.0 (0.7) | 0.002 | - |
In the study done by Ito et al[12] there was no difference in the change in HOMA-IR in those treated with either ipragliflozin or pioglitazone (P = 0.401) (Table 13). There was a significant decrease in HOMA-IR in those treated with dapagliflozin compared to placebo in the study done by Eriksson et al[14]. Surprisingly there was an insignificant increase in HOMA-IR in those treated with either a SGLT-2 inhibitor or a gliptin in the study done by Seko et al[16].
| Study | HOMA-IR | P value | P value between groups | ||
| Group | Baseline | Study completion | |||
| Ito et al[12] | Ipragliflozin | 5.2 (2.5) | 4.8 (5.5) | Non-significant | 0.401 |
| Pioglitazone | 5.7 (3.4) | 4.5 (2.7) | < 0.05 | ||
| Eriksson et al[14] | Placebo | 4.2 (2.4) | -0.2 (1.4)1 | - | - |
| Omega 3-CA | 5.4 (2.9) | +0.3 (2.4)1 | - | Non-significant2 | |
| Dapagliflozin | 4.3 (1.9) | -1.1 (1.4)1 | - | < 0.052 | |
| O + D | 4.4 (1.7) | -0.9 (1.6)1 | - | < 0.052 | |
| Seko et al[16] | SGLT-2 inhibitor | 4.5 (0.5) | 7.9 (2.3) | 0.955 | - |
| Sitagliptin | 4.4 (0.5) | 6.5 (0.8) | 0.163 | ||
Six studies included data on the changes in lipid profile (Tables 14, 15, and 16). There was a significant decrease in serum triglycerides in two studies (Kuchay et al[11] and Ito et al[12]). Three studies exhibited an increase in high-density lipoprotein cholesterol levels (Ito et al[12], Ohki et al[15], and Seko et al[16]). Most of the studies (Ito et al[12], Eriksson et al[14], Ohki et al[15], Seko et al[16], and Sumida et al[18]) showed no change in serum LDL levels.
| Study | Serum triglycerides (mg/dL) | P value | P value between groups | ||
| Group | Baseline | Study completion | |||
| Kuchay et al[11] | Empagliflozin | 201.0 (124.0) | 155.0 (52.0) | 0.01 | 0.678 |
| Control | 212.0 (115.0) | 175.0 (43.0) | 0.019 | ||
| Ito et al[12] | Ipragliflozin | 166.9 (76.4) | 143.4 (81.4) | < 0.05 | 0.938 |
| Pioglitazone | 188.4 (148.8) | 169.3 (131.3) | Non-significant | ||
| Eriksson et al[14] | Placebo | 169.2 (84.1) | -11.5 (45.6)1 | - | - |
| Omega-3 CA | 186.9 (81.5) | -15.9 (47.4)1 | - | Non-significant2 | |
| Dapagliflozin | 178.0 (103.6) | +14.2 (40.5)1 | - | Non-significant2 | |
| O + D | 168.3 (72.6) | -25.7 (57.1)1 | - | Non-significant2 | |
| Ohki et al[15] | Ipragliflozin | 148.0 (107.0, 222.) | 145.0 (114.0, 172.0) | 0.75 | - |
| Seko et al[16] | SGLT-2 inhibitor | 153.8 (15.9) | 137.8 (10.5) | 0.236 | - |
| Sitagliptin | 193.4 (25.2) | 191.1 (23.8) | 0.986 | ||
| Sumida et al[18] | Luseogliflozin | 158.1 (110.5) | 129.4 (59.5) | 0.062 | - |
| Study | Serum low-density lipoprotein cholesterol (mg/dL) | P value | P value between groups | ||
| Group | Baseline | Study completion | |||
| Kuchay et al[11] | Empagliflozin | 112.0 (35.0) | 95.0 (22.0) | 0.018 | 0.512 |
| Control | 114.0 (30.0) | 96.0 (17.0) | 0.001 | ||
| Ito et al[12] | Ipragliflozin | 108.3 (36.2) | 110.7 (40.1) | Non-significant | 0.057 |
| Pioglitazone | 104.0 (27.9) | 114.6 (29.5) | < 0.05 | ||
| Eriksson et al[14] | Placebo | 98.2 (34.4) | +1.6 (15.5)1 | - | - |
| Omega-3 CA | 111.8 (34.4) | +2.3 (17.4)1 | - | Non-significant2 | |
| Dapagliflozin | 109.4 (34.8) | +7.7 (20.5)1 | - | Non-significant2 | |
| O + D | 88.9 (23.2) | +5.8 (21.7)1 | - | Non-significant2 | |
| Ohki et al[15] | Ipragliflozin | 113.0 (89.0-142.0) | 103.0 (92.0-122.0) | 0.08 | - |
| Seko et al[16] | SGLT-2 inhibitor | 119.2 (5.8) | 119.8 (5.7) | 0.943 | - |
| Sitagliptin | 112.9 (4.9) | 127.1 (8.8) | 0.063 | ||
| Sumida et al[18] | Luseogliflozin | 101.0 (22.4) | 105.0 (24.4) | 0.11 | - |
| Study | Serum high-density lipoprotein cholesterol (mg/dL) | P value | P value between groups | ||
| Group | Baseline | Study completion | |||
| Kuchay et al[11] | Empagliflozin | 42.0 (12.0) | 45.0 (12.0) | 0.087 | 0.752 |
| Control | 45.0 (15.0) | 47.0 (12.0) | 0.097 | ||
| Ito et al[12] | Ipragliflozin | 48.9 (9.3) | 54.7 (10.4) | < 0.05 | 0.82 |
| Pioglitazone | 47.4 (11.6) | 52.7 (13.5) | < 0.05 | ||
| Eriksson et al[14] | Placebo | 51.4 (14.9) | -0.4 (5.0)1 | - | - |
| Omega-3 CA | 49.9 (14.1) | +0.4 (3.2)1 | - | Non-significant2 | |
| Dapagliflozin | 49.9 (9.5) | +0.4 (4.8)1 | - | Non-significant2 | |
| O + D | 51.4 (10.2) | +1.6 (5.0)1 | - | Non-significant2 | |
| Ohki et al[15] | Ipragliflozin | 42.0 (40.0-50.0) | 44.0 (42.0-59.0) | 0.01 | - |
| Seko et al[16] | SGLT-2 inhibitor | 53.9 (2.5) | 55.4 (2.6) | 0.043 | - |
| Sitagliptin | 54.8 (3.3) | 55.6 (2.3) | 0.531 | ||
| Sumida et al[18] | Luseogliflozin | 55.6 (11.7) | 57.5 (13.4) | 0.062 | - |
Five studies included BMI change (Table 17). There was a reduction in BMI in the SGLT-2 inhibitor arms in all the studies. Empagliflozin was similar to placebo in reducing BMI whereas luseogliflozin was superior to metformin in reducing BMI.
| Study | Body mass index (kg/m2) | P value | P value between groups | ||
| Group | Baseline | Study completion | |||
| Kuchay et al[11] | Empagliflozin | 30.0 (3.8) | 28.7 (3.5) | 0.001 | 0.124 |
| Control | 29.4 (3.1) | 28.8 (2.8) | 0.019 | ||
| Shibuya et al[13] | Luseogliflozin | 27.9 (26.2, 28.7) | 27.0 (25.6, 28.3) | 0.002 | 0.031 |
| Metformin | 27.2 (24.8, 32.1) | 27.3 (24.3, 31.6) | 0.646 | ||
| Ohki et al[15] | Ipragliflozin | 30.1 (26.1-31.4) | 27.6 (25.3-30.2) | < 0.01 | - |
| Seko et al[16] | SGLT-2 inhibitor | 29.6 (0.7) | 28.3 (0.7) | < 0.001 | - |
| Sitagliptin | 29.2 (1.5) | 28.9 (1.4) | 0.295 | ||
| Sumida et al[18] | Luseogliflozin | 27.8 (3.6) | 27.2 (1.0) | < 0.001 | - |
Out of the eight studies, six studies reported the adverse effects of SGLT-2 inhibitors. There were a total of 30 reported adverse events in 176 patients taking SGLT-2 inhibitors (Table 18). The most common adverse event was genitourinary tract infection (10 events).
| Study | No. of adverse events | No. of patients | Types of adverse events |
| Kuchay et al[11] | 3 | 25 | Nonspecific fatigue: 1 |
| Arthralgia: 1 | |||
| Balanoposthitis: 1 | |||
| Ito et al[12] | 9 | 32 | UTI: 3 |
| Increased appetite: 2 | |||
| Nausea: 1 | |||
| Headache: 1 | |||
| Diarrhoea: 1 | |||
| Vaginal candidiasis: 1 | |||
| Eriksson et al[14] | 7 | 21 | - |
| Seko et al[16] | 2 | 26 | UTI: 2 |
| Gautam et al[17] | 1 | 32 | Recurrent UTI with genital candidiasis: 1 |
| Sumida et al[18] | 8 | 40 | Low blood pressure: 3 |
| Vaginal itching: 2 | |||
| Constipation: 1 | |||
| Vertigo: 1 | |||
| Dehydration: 1 | |||
| Total | 30 | 176 | Most common adverse event: Genitourinary tract infections-10 |
Type 2 diabetes is commonly associated with NAFLD. Serum ALT levels are commonly above the upper limit of normal with AST levels lesser than ALT levels[19]. Animal studies have shown that SGLT-2 inhibitors decrease liver enzymes (ALT, AST), liver weight, and hepatic steatosis[20-23]. There are several mechanisms for improvement in serum liver enzymes in the patients taking SGLT-2 inhibitors. These drugs cause hyperglucagonemia by increasing glucagon secretion from the pancreatic α cells. Glucagon stimulates gluconeogenesis and β-oxidation of fatty acids in the liver via stimulation of peroxisome proliferator-activated receptor alpha and carnitine palmitoyl transferase-1[13]. Thus SGLT-2 inhibitors help to reduce hepatic fat. They reduce collagen deposition and inflammatory cytokine expression in liver[5,22]. They decrease liver enzymes by additionally improving glycaemic parameters and insulin resistance. Out of eight studies, seven showed a decrease in serum ALT and AST levels in our systematic review. Shibuya et al[13] observed a decrease in ALT that almost reached statistical significance, however data regarding AST was unavailable[13]. Out of seven studies, six illustrated a significant decrease in GGT levels while in the study by Seko et al[16] the change in serum GGT level almost reached statistical significance.
Liver enzymes are surrogate markers of liver histological response, but an improvement in liver histology is not always associated with a decrease in serum liver enzymes[11]. The five studies that evaluated changes in hepatic fat showed a decrease in hepatic fat. There was no correlation of a change in ALT with a change in hepatic fat in the study by Shibuya et al[13], however there was a correlation between these two parameters in the study by Sumida et al[18]. The decrease in hepatic fat in the SGLT-2 inhibitor arm was comparable to pioglitazone, which is an approved drug for treatment of NAFLD irrespective of the presence of diabetes. Eriksson et al[14] observed that although the hepatic fat content decreased in the dapagliflozin arm it did not reach statistical significance compared to placebo. The lesser duration of this study (12 wk) compared to other studies may have contributed to this difference.
The progression of NAFLD to cirrhosis is determined to a large extent by the liver histology. Studies with up to 20 years follow-up have shown that the risk of progression to cirrhosis for simple steatosis, NASH, and NASH with fibrosis are 0%-4%, 25%, and 38%, respectively[24]. The FIB-4 index is a non-invasive tool to assess liver fibrosis[25]. It is calculated from the patient’s age, platelet count, ALT levels, and AST levels. The FIB-4 index was decreased with SGLT-2 inhibitor therapy in two out of three studies. Sumida et al[18] used the NAFLD fibrosis score in addition to the FIB-4 index to assess liver fibrosis. The NAFLD fibrosis score is a composite score of six variables (age, BMI, hyperglycaemia, platelet count, albumin, and AST/ALT ratio)[26]. There was no significant change in either indices in this study.
It has been shown that NAFLD is more common in those with poor glycaemic control than those with good glycaemic control[27]. SGLT-2 inhibitors promote glycosuria by inhibiting SGLT-2 in the proximal convoluted tubule. Therefore their action is dependent on blood glucose levels but insulin independent[28]. They cause a significant reduction in FPG[29]. A meta-analysis of RCTs has concluded that the average HbA1c reduction at 52 wk of SGLT-2 inhibitor therapy to be 0.6%[30]. Another meta-analysis has shown that SGLT-2 inhibitor monotherapy is equivalent to metformin monotherapy in reducing HbA1c levels[31]. However, the decrease in HbA1c was more in the luseogliflozin arm compared to the metformin arm in the study by Shibuya et al[13]. Four out of seven studies and six out of seven studies showed a decrease in FPG and HbA1c, respectively, in the SGLT-2 inhibitor arm. Thus, the improved glycaemic status is one of the mechanisms by which SGLT-2 inhibitors ameliorate NAFLD.
SGLT-2 inhibitors ameliorate insulin resistance in numerous ways. SGLT-2 inhibitors improve obesity associated insulin resistance by regulating macrophage recruitment and altering the proportion of pro-inflammatory and anti-inflammatory macrophages. They enhance fat utilization by promoting β-oxidation of fatty acids and browning of white adipose tissue by inducing the expression of thermogenin leading to an improvement in the lipid profile. Similar to other antidiabetic drugs, SGLT-2 inhibitors reduce insulin resistance by decreasing glucotoxicity. Dapagliflozin has been shown to improve insulin sensitivity by increasing adiponectin and zinc-A2-glycoprotein levels[32]. Only dapagliflozin was shown to decrease insulin resistance in the study by Eriksson et al[14].
SGLT-2 inhibitors cause weight reduction. The major mechanism that causes weight reduction is the decrease in fat mass. The decrease in fat mass is due to the shift in substrate utilization to lipids instead of carbohydrates[33,34]. Ito et al[12] and Shibuya et al[13] demonstrated that SGLT-2 inhibitors caused a significant reduction in abdominal visceral and subcutaneous fat area as measured by computed tomography scan. Similarly, Eriksson et al[14] showed that dapagliflozin significantly reduced abdominal visceral and subcutaneous adipose tissue volume as assessed by magnetic resonance imaging. The other mechanisms of weight loss are the urinary glucose loss which amounts to approximately 200 Kcal/d and osmotic diuresis[33,35]. Unlike the other weight-reducing effects of SGLT-2 inhibitors, which are potentially beneficial, osmotic diuresis is clearly an adverse effect. Seko et al[16] showed that ipragliflozin and canagliflozin significantly reduced total body water in addition to body fat mass as measured by bioelectrical impedance analysis. Five studies showed a significant decrease in BMI in patients on SGLT-2 inhibitor therapy. Thus, the major beneficial effects of SGLT-2 inhibitors on NAFLD are exerted via reduction in hepatic fat and fibrosis, improved glycaemic control, decrease in insulin resistance, and weight loss.
The most common adverse effects of SGLT-2 inhibitors are genitourinary tract infections. In addition, they may cause diabetic ketoacidosis, dizziness, acute kidney injury, lower limb amputations, and bone fractures[36,37]. A meta-analysis concluded that there was no difference between placebo and SGLT-2 inhibitors for serious adverse events[38]. Among the 30 adverse events reported in all the studies, the most common was genitourinary tract infections (10 out of 23 characterised events).
The major strength of this systematic review was that the effect of five SGLT-2 inhibitors on NAFLD in patients with type 2 diabetes was evaluated in both RCTs and observational studies. Moreover, liver fat, liver fibrosis, metabolic, and anthropometric parameters in addition to liver enzymes were assessed as outcome variables following SGLT-2 inhibitor therapy. Yet this systematic review has a few limitations. First, most of the studies were done amongst the Japanese population. As a result, the study findings may not be applicable to patients from other ethnicities. Second, the sample size was considerably small and the duration of follow-up was of limited period in most of the studies. Third, the confounding effect of concomitant anti-diabetes drugs like metformin, DPP- 4 inhibitors, and glucagon like peptide-1 analogues on NAFLD cannot be ruled out, particularly in observational studies. Fourth, two studies (Eriksson et al[14] and Sumida et al[18]) were funded by pharmaceutical companies, which is a source of potential conflicts of interest.
In conclusion based on the available evidence, SGLT-2 inhibitors were found to improve serum levels of liver enzymes, liver fibrosis indices, and liver fat without significant side effects in type 2 diabetes patients with NAFLD. They showed additional beneficial effects on obesity, glycaemic parameters, insulin resistance, and dyslipidaemia in these subjects. However, the quality of evidence was low to moderate. Prospective studies, preferably RCTs, comparing different SGLT-2 inhibitors with standard treatments of NAFLD in multi-ethnic populations with a longer follow-up period are needed in the future.
Non-alcoholic fatty liver disease (NAFLD) is a common comorbidity with type 2 diabetes. The existing therapeutic options for NAFLD are not adequate. Hypocaloric diet and exercise is the cornerstone of therapy in NAFLD. Pioglitazone is the only drug recommended in diabetes patients with biopsy proven non-alcoholic steatohepatitis. The frequent coexistence of NAFLD and type 2 diabetes along with their combined adverse health consequences and inadequate therapeutic options makes it necessary to search for newer alternatives. This systematic review is an effort to review the available literature on the effect of sodium glucose cotransporter-2 (SGLT-2) inhibitors on NAFLD in type 2 diabetes patients.
Because the existing therapeutic options are not adequate for NAFLD patients, there is a need for finding newer alternatives. SGLT-2 inhibitors have shown promise in the management of NAFLD in animals. Hence, we reviewed the available literature on the effect of SGLT-2 inhibitors in NAFLD in type 2 diabetes patients. This will promote further high quality research on the effect of SGLT-2 inhibitors in NAFLD.
The primary outcome was the change in serum alanine aminotransferase levels in type 2 diabetes patients with NAFLD treated with SGLT-2 inhibitors. The secondary outcomes were change in serum aspartate aminotransferase and gamma-glutamyl transferase levels, hepatic fat, hepatic fibrosis, metabolic profile, anthropometric parameters, and the adverse effects of SGLT-2 inhibitors.
This systematic review was registered in PROSPERO and performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. We searched PubMed/MEDLINE, Cochrane library, Google scholar, and Clinicaltrials.gov for the relevant articles to be included in this systematic review. A narrative synthesis of the results of individual studies was done. The change in the difference in means and difference in proportions and the respective P values as mentioned in the original manuscripts were tabulated and explained. The quality of the randomised controlled trials and observational studies was analysed using the Cochrane risk of bias tool and MINORS scale, respectively.
Eight articles (four randomised controlled trials and four observational studies) were included in this systematic review. A total of 214 patients were treated with SGLT-2 inhibitors. SGLT-2 inhibitors caused a significant improvement in liver enzymes, hepatic fat, hepatic fibrosis, glycaemia, insulin resistance, obesity, and lipid parameters with minimal adverse effects. However, the quality of evidence is low to moderate.
We found that SGLT-2 inhibitors improved the serum levels of liver enzymes, liver fat, and liver fibrosis with additional beneficial effects on various metabolic and anthropometric parameters in type 2 diabetes patients with NAFLD. However, the number of patients treated with SGLT-2 inhibitors was small. The findings of this systematic review will have impact in choosing anti-diabetes medication like SGLT-2 inhibitors to treat NAFLD associated with type 2 diabetes.
The studies included in this systematic review were heterogeneous with regard to study design and intervention drugs. Most of the studies were done amongst the Japanese population. Prospective studies, preferably randomised controlled trials, comparing different SGLT-2 inhibitors with standard treatments of NAFLD in multi-ethnic populations with a longer follow-up period are needed in the future.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Joseph PM, Serhiyenko VA, Tzamaloukas AHH S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Song H
| 1. | Saponaro C, Gaggini M, Gastaldelli A. Nonalcoholic fatty liver disease and type 2 diabetes: common pathophysiologic mechanisms. Curr Diab Rep. 2015;15:607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 2. | Burt AD, Lackner C, Tiniakos DG. Diagnosis and Assessment of NAFLD: Definitions and Histopathological Classification. Semin Liver Dis. 2015;35:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Williams KH, Shackel NA, Gorrell MD, McLennan SV, Twigg SM. Diabetes and nonalcoholic Fatty liver disease: a pathogenic duo. Endocr Rev. 2013;34:84-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 4. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4954] [Article Influence: 707.7] [Reference Citation Analysis (9)] |
| 5. | Qiang S, Nakatsu Y, Seno Y, Fujishiro M, Sakoda H, Kushiyama A, Mori K, Matsunaga Y, Yamamotoya T, Kamata H, Asano T. Treatment with the SGLT2 inhibitor luseogliflozin improves nonalcoholic steatohepatitis in a rodent model with diabetes mellitus. Diabetol Metab Syndr. 2015;7:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Tahara A, Kurosaki E, Yokono M, Yamajuku D, Kihara R, Hayashizaki Y, Takasu T, Imamura M, Li Q, Tomiyama H, Kobayashi Y, Noda A, Sasamata M, Shibasaki M. Effects of SGLT2 selective inhibitor ipragliflozin on hyperglycemia, hyperlipidemia, hepatic steatosis, oxidative stress, inflammation, and obesity in type 2 diabetic mice. Eur J Pharmacol. 2013;715:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 7. | Yokono M, Takasu T, Hayashizaki Y, Mitsuoka K, Kihara R, Muramatsu Y, Miyoshi S, Tahara A, Kurosaki E, Li Q, Tomiyama H, Sasamata M, Shibasaki M, Uchiyama Y. SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur J Pharmacol. 2014;727:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 159] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47200] [Article Influence: 2950.0] [Reference Citation Analysis (0)] |
| 9. | Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18487] [Cited by in RCA: 24865] [Article Influence: 1776.1] [Reference Citation Analysis (3)] |
| 10. | Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3743] [Cited by in RCA: 5642] [Article Influence: 256.5] [Reference Citation Analysis (0)] |
| 11. | Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, Bansal B, Kaur P, Jevalikar G, Gill HK, Choudhary NS, Mithal A. Effect of Empagliflozin on Liver Fat in Patients With Type 2 Diabetes and Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial (E-LIFT Trial). Diabetes Care. 2018;41:1801-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 431] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 12. | Ito D, Shimizu S, Inoue K, Saito D, Yanagisawa M, Inukai K, Akiyama Y, Morimoto Y, Noda M, Shimada A. Comparison of Ipragliflozin and Pioglitazone Effects on Nonalcoholic Fatty Liver Disease in Patients With Type 2 Diabetes: A Randomized, 24-Week, Open-Label, Active-Controlled Trial. Diabetes Care. 2017;40:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 13. | Shibuya T, Fushimi N, Kawai M, Yoshida Y, Hachiya H, Ito S, Kawai H, Ohashi N, Mori A. Luseogliflozin improves liver fat deposition compared to metformin in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective randomized controlled pilot study. Diabetes Obes Metab. 2018;20:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 156] [Article Influence: 22.3] [Reference Citation Analysis (1)] |
| 14. | Eriksson JW, Lundkvist P, Jansson PA, Johansson L, Kvarnström M, Moris L, Miliotis T, Forsberg GB, Risérus U, Lind L, Oscarsson J. Effects of dapagliflozin and n-3 carboxylic acids on non-alcoholic fatty liver disease in people with type 2 diabetes: a double-blind randomised placebo-controlled study. Diabetologia. 2018;61:1923-1934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 15. | Ohki T, Isogawa A, Toda N, Tagawa K. Effectiveness of Ipragliflozin, a Sodium-Glucose Co-transporter 2 Inhibitor, as a Second-line Treatment for Non-Alcoholic Fatty Liver Disease Patients with Type 2 Diabetes Mellitus Who Do Not Respond to Incretin-Based Therapies Including Glucagon-like Peptide-1 Analogs and Dipeptidyl Peptidase-4 Inhibitors. Clin Drug Investig. 2016;36:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Seko Y, Sumida Y, Tanaka S, Mori K, Taketani H, Ishiba H, Hara T, Okajima A, Umemura A, Nishikawa T, Yamaguchi K, Moriguchi M, Kanemasa K, Yasui K, Imai S, Shimada K, Itoh Y. Effect of sodium glucose cotransporter 2 inhibitor on liver function tests in Japanese patients with non-alcoholic fatty liver disease and type 2 diabetes mellitus. Hepatol Res. 2017;47:1072-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Gautam A, Agrawal PK, Doneria J, Nigam A. Effects of Canagliflozin on Abnormal Liver Function Tests in Patients of Type 2 Diabetes with Non-Alcoholic Fatty Liver Disease. JAPI. 2018;66:62-66. |
| 18. | Sumida Y, Murotani K, Saito M, Tamasawa A, Osonoi Y, Yoneda M, Osonoi T. Effect of luseogliflozin on hepatic fat content in type 2 diabetes patients with non-alcoholic fatty liver disease: A prospective, single-arm trial (LEAD trial). Hepatol Res. 2019;49:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 19. | Sattar N, Fitchett D, Hantel S, George JT, Zinman B. Empagliflozin is associated with improvements in liver enzymes potentially consistent with reductions in liver fat: results from randomised trials including the EMPA-REG OUTCOME® trial. Diabetologia. 2018;61:2155-2163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 20. | Nakano S, Katsuno K, Isaji M, Nagasawa T, Buehrer B, Walker S, Wilkison WO, Cheatham B. Remogliflozin Etabonate Improves Fatty Liver Disease in Diet-Induced Obese Male Mice. J Clin Exp Hepatol. 2015;5:190-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 21. | Komiya C, Tsuchiya K, Shiba K, Miyachi Y, Furuke S, Shimazu N, Yamaguchi S, Kanno K, Ogawa Y. Ipragliflozin Improves Hepatic Steatosis in Obese Mice and Liver Dysfunction in Type 2 Diabetic Patients Irrespective of Body Weight Reduction. PLoS One. 2016;11:e0151511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 22. | Jojima T, Tomotsune T, Iijima T, Akimoto K, Suzuki K, Aso Y. Empagliflozin (an SGLT2 inhibitor), alone or in combination with linagliptin (a DPP-4 inhibitor), prevents steatohepatitis in a novel mouse model of non-alcoholic steatohepatitis and diabetes. Diabetol Metab Syndr. 2016;8:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 23. | Wang D, Luo Y, Wang X, Orlicky DJ, Myakala K, Yang P, Levi M. The Sodium-Glucose Cotransporter 2 Inhibitor Dapagliflozin Prevents Renal and Liver Disease in Western Diet Induced Obesity Mice. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Calzadilla Bertot L, Adams LA. The Natural Course of Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 339] [Cited by in RCA: 457] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 25. | Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104-1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1205] [Cited by in RCA: 1168] [Article Influence: 73.0] [Reference Citation Analysis (1)] |
| 26. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, Lindor K, Sanderson SO, Lenzi M, Adams LA, Kench J, Therneau TM, Day CP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1917] [Cited by in RCA: 2285] [Article Influence: 126.9] [Reference Citation Analysis (1)] |
| 27. | Afolabi BI, Ibitoye BO, Ikem RT, Omisore AD, Idowu BM, Soyoye DO. The Relationship Between Glycaemic Control and Non-Alcoholic Fatty Liver Disease in Nigerian Type 2 Diabetic Patients. J Natl Med Assoc. 2018;110:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Kalra S. Sodium Glucose Co-Transporter-2 (SGLT2) Inhibitors: A Review of Their Basic and Clinical Pharmacology. Diabetes Ther. 2014;5:355-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 29. | Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 332] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 30. | Monami M, Nardini C, Mannucci E. Efficacy and safety of sodium glucose co-transport-2 inhibitors in type 2 diabetes: a meta-analysis of randomized clinical trials. Diabetes Obes Metab. 2014;16:457-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 31. | Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, Maggo J, Gray V, De Berardis G, Ruospo M, Natale P, Saglimbene V, Badve SV, Cho Y, Nadeau-Fredette AC, Burke M, Faruque L, Lloyd A, Ahmad N, Liu Y, Tiv S, Wiebe N, Strippoli GF. Comparison of Clinical Outcomes and Adverse Events Associated With Glucose-Lowering Drugs in Patients With Type 2 Diabetes: A Meta-analysis. JAMA. 2016;316:313-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 32. | Mohammad SH, Fadhil NN, Mahmood MD. Effects of metformin and dapagliflozin on glycemic indices and HOMA-IR in type 2 diabetes mellitus patients. Int J Pharm Biol Sci. 2018;8:66-73. |
| 33. | Trujillo JM, Nuffer WA. Impact of Sodium-Glucose Cotransporter 2 Inhibitors on Nonglycemic Outcomes in Patients with Type 2 Diabetes. Pharmacotherapy. 2017;37:481-491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 34. | Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to Fatty Substrate Utilization in Response to Sodium-Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes. 2016;65:1190-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 535] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 35. | Ferrannini G, Hach T, Crowe S, Sanghvi A, Hall KD, Ferrannini E. Energy Balance After Sodium-Glucose Cotransporter 2 Inhibition. Diabetes Care. 2015;38:1730-1735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 275] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 36. | Esteban-Jiménez O, Navarro-Pemán C, Urieta-González L. Seguridad de los iSGLT-2. Revisión de las reacciones adversas notificadas a nivel nacional. Med Fam SEMERGEN. 2018;44:23–29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 37. | Blau JE, Taylor SI. Adverse effects of SGLT2 inhibitors on bone health. Nat Rev Nephrol. 2018;14:473–474. [RCA] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 38. | Storgaard H, Gluud LL, Bennett C, Grøndahl MF, Christensen MB, Knop FK, Vilsbøll T. Benefits and Harms of Sodium-Glucose Co-Transporter 2 Inhibitors in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0166125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |