Published online Oct 15, 2019. doi: 10.4239/wjd.v10.i10.511
Peer-review started: June 29, 2019
First decision: August 2, 2019
Revised: September 4, 2019
Accepted: September 22, 2019
Article in press: September 22, 2019
Published online: October 15, 2019
Processing time: 110 Days and 8.8 Hours
Most occurrences of type 1 diabetes cases in any population are sporadic rather than familial. Hence, type 1 diabetes among siblings is a rare occurrence. Even more rare is for three or more siblings to develop type 1 diabetes. In this report, we describe a case of a Nigerian family in which type 1 diabetes occurred in three siblings among four children with neither parent having diabetes. All three siblings are positive for glutamic acid decarboxylase and anti-islet cell antibodies.
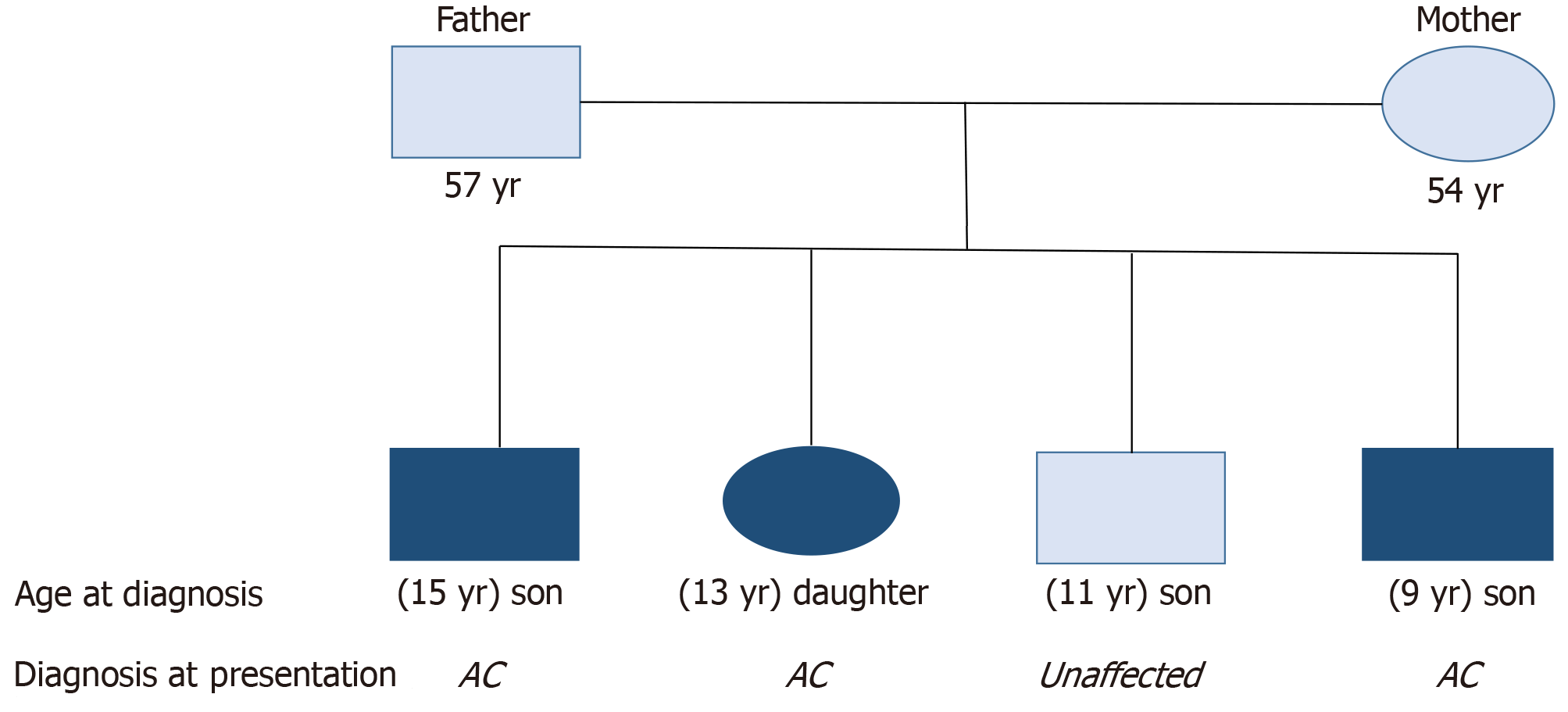
There were four siblings (three males and one female) born to a couple without a diagnosis of diabetes. The eldest child (male) was diagnosed with diabetes at the age of 15, the second child (female) was diagnosed at the age of 11 and the fourth child (male) was diagnosed at the age of 9. All the siblings presented with similar osmotic symptoms and were diagnosed of diabetic ketoacidosis. All of them had markedly reduced serum C-peptide levels with high levels of glutamic acid decarboxylase and insulinoma-associated protein-2 antibodies. We could not perform genetic analysis of HLA-DR, DQ and CTLA4 in the siblings as well as the parents; hence haplotypes could not be characterized. Both parents of the probands have no prior history of diabetes, and their blood glucose and glycated hemoglobin levels were within normal ranges. The third child (male) has no history suggestive of diabetes, and his blood glucose and glycated hemoglobin have remained within normal ranges.
Although the occurrence of type 1 diabetes in proband siblings is uncommon, screening for diabetes among siblings especially with islet autoantibodies should be encouraged.
Core tip: Although most occurrences of type 1 diabetes are sporadic, it can also be familial. Type 1 diabetes among siblings is a rare occurrence. Even more rare is for three or more siblings to develop type 1 diabetes. Hence due to the possibility of this familial occurrence, screening for diabetes among siblings should be encouraged. This report describes a case of a Nigerian family in which type 1 diabetes occurred in three siblings among four children with neither parent having diabetes.
- Citation: Olamoyegun MA, Ala OA. Type 1 diabetes in a Nigerian family - occurrence in three out of four siblings: A case report. World J Diabetes 2019; 10(10): 511-516
- URL: https://www.wjgnet.com/1948-9358/full/v10/i10/511.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i10.511
Type 1 diabetes (T1D) is a life-long medical condition that primarily affects young people. It is characterized by immune destruction of insulin-producing beta-cells in the pancreas resulting from the action of environmental factors in genetically predisposed individuals[1]. It usually begins in childhood or young adulthood but can develop at any age. The presence of any of the following antibodies increases the risk of T1D: Glutamic acid decarboxylase-65, islet cell, insulin autoantibody and insulinoma-associated protein-2[2]. In general, 70% of people with new-onset T1D have a positive antibody if only one antibody is measured, whereas 90% will have at least one antibody when all forms are measured[2]. Most occurrences of T1D cases in any population are sporadic; that is, first-degree relatives do not have diabetes at the time patient with diabetes is diagnosed. Nevertheless, siblings of childhood-onset T1D patients are at increased risk of developing the same disease compared with the general population[3,4].
Genetic susceptibility is important in the development of T1D. In Caucasian populations, the lifetime risk in siblings of type 1 diabetic probands has been reported to be much higher than that in the general population (6% vs 0.4%), indicating that T1D clusters in families[5]. This represent a 15-fold risk tendency in siblings compared to the risk in the general population[5]. This classic role of genetics in diabetes risk is demonstrated by comparing concordance rates in monozygotic versus dizygotic twins. In Finland, with the highest incidence of T1D, concordance rates for T1D were found to be 27% and 3.8% among monozygotic and dizygotic twins, respectively[6]. Familial clustering (which refers to the occurrence of a disorder at a higher frequency in first-degree relatives of an affected person compared to the general population)[7] of T1D is a rare occurrence. Even more rare is for three or more siblings to develop T1D.
It is estimated that HLA (a cluster of genes located within the major histocompatibility complex) on chromosome 6p21 accounts for 40%-50% of familial clustering and the strongest genetic association with T1D[8], in addition to other different genetic loci that contribute susceptibility to the development of T1D[9]. Although the risk of developing T1D is increased in relatives of individuals with the disease, the risk is relatively low. This risk depends on which HLA haplotypes are shared[8]. From multiple family pedigrees and HLA typing data, it is estimated that if a sibling shares both HLA-D haplotypes with an index patient, the risk for that individual is 12% to 20%. For a sibling sharing only one haplotype, the risk for T1D is 5% to 7%. For a sibling with no haplotype in common, the risk is only 1% to 2%[10]. According to Redondo et al[11], approximately 85% of new cases of T1D occur in persons without an affected first-degree relative. Approximately 13% of children and adolescents who develop T1D have a parent or sibling with diabetes. Dahiquist and Gothefors[12] reported that among children with newly diagnosed diabetes, 2%-3% had a mother with T1D, 5%-6% had a father with T1D and 4%-5% had a brother or a sister with T1D.
The study of familial clustering is an important concept in genetic epidemiology. The lifetime risk in siblings of type 1 diabetic probands in Nigerians is unknown (it has never been reported). Hence, the purpose of this paper is to report a case of a Nigerian family in which T1D occurred in three siblings among four children with neither of the parents having diabetes. This report will encourage clinicians to assess the possibilities of diabetes in siblings of children with T1D.
The three siblings presented with polyuria, polydipsia and weight loss of about 2 wk duration.
Case 1: First child, male, was diagnosed of acute-onset T1D at 15-years-old. He had presented with osmotic symptoms (polyuria and polyuria) and associated weight loss about 2 weeks before presentation. At presentation, he was drowsy and dehydrated.
Case 2: Second child, female, was diagnosed of acute-onset T1D at 11-years-old. She had presented with polyuria, polydipsia and weight loss.
Case 3: Fourth child, male, was delivered at estimated gestation age of 36 wk and birth weight was 4.65 kg. He presented with features of acute-onset osmotic symptoms at 9-years-old (Figure 1).
None of the siblings were previously diagnosed with diabetes.
Both parents of the probands have no prior history of diabetes. Their blood glucose and glycated hemoglobin (HbA1C) levels were within normal ranges. The third child (male) had no history suggestive of diabetes, and his blood glucose and HbA1C were within normal ranges. None of the siblings or parents were smokers or used alcohol. The father was overweight, and the mother was diagnosed with obesity.
Case 1: At presentation, he was drowsy and dehydrated. His plasma glucose and HbA1C levels were 403 mg/dL (22.4 mmol/L) and 10.6%, respectively. His urinalysis showed glucosuria (4+) and ketonuria (3+). His serum C-peptide level was markedly low, which showed that his insulin secretory capacity was reduced.
Case 2: Her blood glucose at diagnosis was 448 mg/dL (24.9 mmol/L), and HbA1C was 11.0%. Urinalysis showed ketonuria (3+) and glucose (4+) but no proteinuria.
Case 3: His blood glucose at diagnosis was 448 mg/dL (24.9 mmol/L) and HbA1C was 11.0%. Urinalysis showed ketonuria (3+) and glucose (4+) but no proteinuria.
Anti-glutamic acid decarboxylase, anti-insulinoma-associated protein-2 and anti-thyroid peroxidase antibodies were determined by radioimmunoassay. However, the genotype of the HLA could not be done due to lack of facility (Table 1).
| Sibling 1 | Sibling 2 | Sibling 3 | |
| Age of diagnosis, yr | 15 | 11 | 9 |
| BG at presentation, mg/dL BMI, kg/m2 | 403 23 | 448 28.4 | 337 22.4 |
| Mode of presentation of diagnosis | DKA | DKA | DKA |
| HbA1C, % | 10.7 | 11.0 | 10.7 |
| Urinalysis | |||
| Glucose | 4+ | 4+ | 3+ |
| Ketone | 3+ | 3+ | 2+ |
| Serum C-peptide levels, pmol/L | < 33.0 (364-1655) | Undetectable | Low |
| Anti-GAD antibodies, IU/mL (Normal range: 0.0-10.0) | 1561.0 | 1208.8 | 1054.2 |
| Anti-IA-2 Ab | Increased | Increased | Increased |
| TPO Ab, U/mL | Normal | Normal | Normal |
All were diagnosed of acute-onset diabetic ketoacidosis and T1D.
All were treated with intravenous fluid and multiple doses of insulin at admission.
All are currently being treated with multiple injections of insulin with total daily insulin dosage ranges of 90-122 U/d. They all have fair glycemic control with occasional hypoglycemia in the youngest sibling with diabetes.
The occurrence of T1D among three or more siblings is extremely rare. Although large families with T1D arising from a common ancestor have been reported among Arabs[13] and in the Netherlands[14]. However, none have been reported among Nigerians except a patient with T1D whose three siblings also had prediabetes[15]. An analysis of 767 multiplex Caucasian families showed that fifty-one families had three affected siblings, one family had four affected siblings and two families had five affected siblings[16]. There has also been a reported case of T1D in three sisters in a Japanese family, whose parents also had T1D[17]. To our knowledge, no case report has described T1D in three or more siblings in Nigerian patients.
In this case report, three out of four siblings (two males and one female) developed T1D, although neither of the parents had diabetes. Fathers have been known to transmit T1D to their offspring more than mothers[17,18]. At diagnosis, 4%-7% of children have a father with T1D whereas 1.5%-3% have an affected mother. This tendency of transmitting the disease is amplified if both parents have the disease. This familial T1D has been shown to be associated with increased genetic susceptibility attributed to a higher prevalence of HLA genes among family members[8,19]. Hence, the T1D risk for siblings depends on genetic background primarily in the HLA region on chromosome 6 in particular[8], and it depends on siblings sharing the HLA haplotype with the proband[18]. While HLA-B8 is strongly associated with T1D in Caucasians, the contrary was the case among Nigerians[20]. Another study[21] reported a low prevalence of HLA-DR4 among Nigerians with T1D. The incidence and prevalence of T1D is not known in Nigeria although the prevalence was said to be much lower than and occurred at later ages compared to Caucasians[22]. This rare occurrence makes it difficult to identify families with multiple siblings with T1D.
Regarding the age of diagnoses of our patients, there was a progressive decline in the age of diagnosis and reduced levels of glycemia at diagnosis as assessed by both plasma glucose and HbA1C with subsequent siblings with diabetes. The decreasing age of diagnosis of other siblings compared to the first affected sibling is in keeping with previous reports demonstrating a younger age at diagnosis among affected siblings[23-25]. The progressive reduction in the age of diagnosis might be due to modification of the genetic susceptibility by environmental factors such as diets, abnormal weight gain or sedentary lifestyles. Previous studies showed that HLA genes related to high disease susceptibility (HLA-DR and HLA-DG genotypes) were associated with earlier onset of diabetes[26,27]. Also, Harjutsalo et al[16] suggested that genetic effects in such families may be particularly strong. They proposed that a young age at onset in the first child diagnosed with T1D indicated an overall increased lifetime risk for T1D in siblings and that the process leading to diabetes seems more rapid in such siblings. Long-term follow-up studies corroborated this statement[28,29]. Another explanation that has been proposed is that genetic or environmental factors that precipitate an earlier onset of T1D in the proband are shared with the other siblings and may also increase their risk for T1D.
The mode of presentation at diagnosis in all these three siblings was quite similar. All of them presented with polyuria, polydipsia and weight loss over a similar mean period of time with consequent diagnosis of diabetic ketoacidosis. Although, there was a slight increase in blood sugar of the second sibling compared to the first, there was a reduction in the plasma glucose at presentation in the third sibling who had the lowest blood sugar among the three affected siblings. The first affected sibling presented with the worst clinical symptoms at presentation as he was drowsy. This observation was probably due to greater family awareness of diabetic symptoms, hence earlier presentation to the hospital of the younger siblings.
Although this report showed the possibility of familial clustering of T1D among siblings, our inability to do genetic analysis for proper characterization of the exact loci genes affected was a limitation of this study. Although, one sibling of the four children was not affected by T1D, we could not assess him for the presence of auto-antibodies, which may also predispose him to development of diabetes in the future.
This is the first report of familial clustering of T1D in three out of four children in a Nigerian family. This shows that the occurrence of T1D among siblings is possible even in areas where the incidence and prevalence of T1D is rare (as in Nigeria). This report is also unique because neither of the parents had either type 1 or type 2 diabetes. The finding of decreasing age at diagnosis and severity of symptoms at presentation with each subsequent sibling suggested a possible influence of environmental effects on genetic susceptibility and awareness of diabetes symptoms from prior diagnosis.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Nigeria
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosseinpour-Niazi S, Koch TR S-Editor: Ma RY L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Haller MJ, Atkinson MA, Schatz D. Type 1 diabetes mellitus: etiology, presentation, and management. Pediatr Clin North Am. 2005;52:1553-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 2. | Korhonen S, Knip MM, Kulmala P, Savola K, Akerblom HK, Knip M; Finnish Study Group for ICA Screening. Autoantibodies to GAD, IA-2 and insulin in ICA-positive first-degree relatives of children with type 1 diabetes: a comparison between parents and siblings. Diabetes Metab Res Rev. 2002;18:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Bloom A, Hayes TM, Gamble DR. Register of newly diagnosed diabetic children. Br Med J. 1975;3:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 108] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | West R, Belmonte MM, Colle E, Crepeau MP, Wilkins J, Poirier R. Epidemiologic survey of juvenile-onset diabetes in Montreal. Diabetes. 1979;28:690-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Steck AK, Rewers MJ. Genetics of type 1 diabetes. Clin Chem. 2011;57:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 6. | Redondo MJ, Yu L, Hawa M, Mackenzie T, Pyke DA, Eisenbarth GS, Leslie RD. Heterogeneity of type I diabetes: analysis of monozygotic twins in Great Britain and the United States. Diabetologia. 2001;44:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 209] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Harjustalo V. Familial aggregation of type 1 diabetes and diabetic nephropathy in Finland. Academic dissertation presented to the National Public Health Institute, Helsinki. Finland and the Department of Public Health, University of Helsinki, Finland. 2007;. |
| 8. | Pociot F, McDermott MF. Genetics of type 1 diabetes mellitus. Genes Immun. 2002;3:235-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 208] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Hakonarson H, Qu HQ, Bradfield JP, Marchand L, Kim CE, Glessner JT, Grabs R, Casalunovo T, Taback SP, Frackelton EC, Eckert AW, Annaiah K, Lawson ML, Otieno FG, Santa E, Shaner JL, Smith RM, Onyiah CC, Skraban R, Chiavacci RM, Robinson LJ, Stanley CA, Kirsch SE, Devoto M, Monos DS, Grant SF, Polychronakos C. A novel susceptibility locus for type 1 diabetes on Chr12q13 identified by a genome-wide association study. Diabetes. 2008;57:1143-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | Siljander HT, Veijola R, Reunanen A, Virtanen SM, Akerblom HK, Knip M. Prediction of type 1 diabetes among siblings of affected children and in the general population. Diabetologia. 2007;50:2272-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Redondo MJ, Eisenbarth GS. Genetic control of autoimmunity in Type I diabetes and associated disorders. Diabetologia. 2002;45:605-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 78] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Dahlquist G, Gothefors L. The cumulative incidence of childhood diabetes mellitus in Sweden unaffected by BCG-vaccination. Diabetologia. 1995;38:873-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Verge CF, Vardi P, Babu S, Bao F, Erlich HA, Bugawan T, Tiosano D, Yu L, Eisenbarth GS, Fain PR. Evidence for oligogenic inheritance of type 1 diabetes in a large Bedouin Arab family. J Clin Invest. 1998;102:1569-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Vaessen N, Heutink P, Houwing-Duistermaat JJ, Snijders PJ, Rademaker T, Testers L, Batstra MR, Sandkuijl LA, van Duijn CM, Oostra BA. A genome-wide search for linkage-disequilibrium with type 1 diabetes in a recent genetically isolated population from the Netherlands. Diabetes. 2002;51:856-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Onyiriuka AN, Ofoeze CA. Prediabetes in three siblings of a Nigerian boy with type 1 diabetes mellitus: Is this a case of familial clustering? Aristotle Univ Ed J. 2005;42:23-28. |
| 16. | Cox NJ, Wapelhorst B, Morrison VA, Johnson L, Pinchuk L, Spielman rS, Todd JA. Concannon P: Seven regions of the genome show evidence of linkage to type 1 diabetes in a consensus analysis of 767 multi-plex families. Am J Hum Genet. 2001;69:820-830. |
| 17. | Kishi A, Kawabata Y, Ugi S, Iwai T, Tanaka Y, Yoshizaki T, Uzu T, Nishio Y, Ikegami H, Kashiwagi A, Maegawa H. The onset of diabetes in three out of four sisters: a Japanese family with type 1 diabetes. A case report. Endocr J. 2009;56:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Warram JH, Krolewski AS, Gottlieb MS, Kahn CR. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 229] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Ilonen J, Reijonen H, Green A, Reunanen A, Knip M, Simell O, Akerblom HK. Geographical differences within Finland in the frequency of HLA-DQ genotypes associated with type 1 diabetes susceptibility. The Childhood Diabetes in Finland Study Group. Eur J Immunogenet. 2000;27:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Famuyiwa OO, Nwabuebo IE, Abioye AA. Pattern of histocompatibility (HLA) antigen distribution among Nigerian (West African black) diabetics. Diabetes. 1982;31:1119-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | MacDonald MJ, Famuyiwa OO, Nwabuebo IA, Bella AF, Junaid TA, Marrari M, Duquesnoy RJ. HLA-DR associations in black type I diabetics in Nigeria. Further support for models of inheritance. Diabetes. 1986;35:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Gill GV, Mbanya JC, Ramaiya KL, Tesfaye S. A sub-Saharan African perspective of diabetes. Diabetologia. 2009;52:8-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Dahlquist GG, Mustonen LR. Clinical onset characteristics of familial versus nonfamilial cases in a large population-based cohort of childhood-onset diabetes patients. Diabetes Care. 1995;18:852-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | O'Leary LA, Dorman JS, LaPorte RE, Orchard TJ, Becker DJ, Kuller LH. Familial and sporadic insulin-dependent diabetes: evidence for heterogeneous etiologies. Diabetes Res Clin Pr. 1991;14:183-190. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Pociot F, Nørgaard K, Hobolth N, Andersen O, Nerup J. A nationwide population-based study of the familial aggregation of type 1 (insulin-dependent) diabetes mellitus in Denmark. Danish Study Group of Diabetes in Childhood. Diabetologia. 1993;36:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Fennessy M, Metcalfe K, Hitman GA, Niven M, Biro PA, Tuomilehto J, Tuomilehto-Wolf E. A gene in the HLA class I region contributes to susceptibility to IDDM in the Finnish population. Childhood Diabetes in Finland (DiMe) Study Group. Diabetologia. 1994;37:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Caillat-Zucman S, Garchon HJ, Timsit J, Assan R, Boitard C, Djilali-Saiah I, Bougnères P, Bach JF. Age-dependent HLA genetic heterogeneity of type 1 insulin-dependent diabetes mellitus. J Clin Invest. 1992;90:2242-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 208] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Harjutsalo V, Podar T, Tuomilehto J. Cumulative incidence of type 1 diabetes in 10,168 siblings of Finnish young-onset type 1 diabetic patients. Diabetes. 2005;54:563-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Steck AK, Barriga KJ, Emery LM, Fiallo-Scharer RV, Gottlieb PA, Rewers MJ. Secondary attack rate of type 1 diabetes in Colorado families. Diabetes Care. 2005;28:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |