Published online Jan 15, 2019. doi: 10.4239/wjd.v10.i1.47
Peer-review started: September 3, 2018
First decision: November 8, 2018
Revised: December 29, 2018
Accepted: January 3, 2019
Article in press: January 3, 2019
Published online: January 15, 2019
Processing time: 138 Days and 19.2 Hours
Neuropathy is a common complication of diabetes mellitus resulting from direct damage by hyperglycemia to the nerves and/or ischemia by microvascular injury to the endoneurial vessels which supply the nerves. Median nerve is one of the peripheral nerves commonly affected in diabetic neuropathy. The median nerve size has been studied in non-Nigerian diabetic populations. In attempt to contribute to existing literature, a study in a Nigerian population is needed.
To evaluate the cross-sectional area (CSA) of the median nerve using B-mode ultrasonography (USS) and the presence of peripheral neuropathy (PN) in a cohort of adult diabetic Nigerians.
Demographic and anthropometric data of 85 adult diabetes mellitus (DM) and 85 age- and sex-matched apparently healthy control (HC) subjects were taken. A complete physical examination was performed on all study subjects to determine the presence of PN and modified Michigan Neuropathy Screening Instrument (MNSI) was used to grade its severity. Venous blood was taken from the study subjects for fasting lipid profile (FLP), fasting blood glucose (FBG) and glycated haemoglobin (HbA1c) while their MN CSA was evaluated at a point 5 cm proximal to (5cmCATL) and at the carpal tunnel (CATL) by high-resolution B-mode USS. Data was analysed using SPSS version 22.
The mean MN CSA was significantly thicker in DM subjects compared to the HC at 5cmCATL (P < 0.01) and at the CATL (P < 0.01) on both sides. The presence of diabetic peripheral neuropathy (DPN) further increased the MN CSA at the CATL (P < 0.05) but not at 5cmCATL (P > 0.05). However, the severity of DPN had no additional effect on MN CSA 5 cm proximal to and at the CATL. There was no significant association between MN CSA and duration of DM and glycemic control.
Thickening of the MN CSA at 5cmCATL and CATL is seen in DM. Presence of DPN is associated with worse thickening of the MN CSA at the CATL but not at 5cmCATL. Severity of DPN, duration of DM, and glycemic control had no additional effect on the MN CSA.
Core tip: We report median nerve cross-sectional area findings in diabetics of Nigerian origin. This study demonstrates that the median nerve is thicker at the carpal tunnel and 5 cm proximal to the carpal tunnel in diabetic subjects than age- and sex-matched healthy controls. Further thickening in the median nerve size is seen in the presence of diabetic peripheral neuropathy at the carpal tunnel but not at a point 5 cm proximal to it. Median nerve size has no significant relationship with age, gender, severity of diabetic peripheral neuropathy, duration of diabetes mellitus or glycemic control in diabetic subjects.
- Citation: Attah FA, Asaleye CM, Omisore AD, Kolawole BA, Aderibigbe AS, Alo M. Relationship between sonographically measured median nerve cross-sectional area and presence of peripheral neuropathy in diabetic subjects. World J Diabetes 2019; 10(1): 47-56
- URL: https://www.wjgnet.com/1948-9358/full/v10/i1/47.htm
- DOI: https://dx.doi.org/10.4239/wjd.v10.i1.47
Diabetes mellitus (DM) is used to describe several diseases where there is a persistent increase in blood sugar level due to deficiency in the production and/or action of insulin[1]. Broadly, diabetes mellitus can be classified into two major forms, type 1 or insulin-dependent and type 2 or non-insulin dependent DM according to insulin secretion or action respectively[2]. The prevalence of DM is on the increase worldwide in both developed and developing countries[1,2]. Diabetic peripheral neuropathy (DPN) is the most common complication of DM and is seen in patients with types 1 and 2 DM. DPN and peripheral nerve dysfunction have common signs and symptoms in people with diabetes when other aetiological factors of the defect are not considered[3].
After a long-term persistent hyperglycemia, DPN usually becomes symptomatic in type 1 DM while it is obvious in type 2 DM at detection or after a period of insufficient blood sugar level control[4].
Organ impairment or failure is related with prolonged hyperglycemia of diabetes, and some of the organs that can be affected include the eyes, kidneys, nerves, heart, and blood vessels[5]. Damage to peripheral nerves can occur directly from elevated blood sugar level or indirectly from reduced blood flow to nerves[1].
Diabetic neuropathy is responsible for substantial morbidity, increased mortality and impaired quality of life of diabetic patients[6]. Therefore, early detection of nerve dysfunction is important to appropriately care for patients with diabetic neuropathy[7].
The characteristic signs and symptoms of diabetic neuropathy basically suggest its diagnosis and confirmatory neurophysiological tests are required[8].
Although electroneuromyography and nerve conduction studies (NCS) are the major electro-neurophysiological methods for diagnosing pathology associated with the median nerve and other peripheral nerves, they only allow assessment of peripheral nerve function, but fail to provide any data on their morphology or the possible visible pathomorphology of the surrounding structures and tissues[9,10].
Magnetic resonance imaging (MRI) of the median nerve provides excellent morphological details of the nerve. Magnetic resonance imaging of peripheral nerves is known as Magnetic Resonance Neurography (MRN)[11,12]. It is used to assess peripheral nerve entrapments and impingements as well as localization and grading of nerve injuries and lesions[11,12]. Magnetic Resonance Neurography could be morphological or functional MRN[11]. The morphological MRN is based on 3D MRI sequences with or without fat suppression while the functional MRN is based on Diffusion-weighted imaging (DWI). DWI, an MR Neurographic technique used to measure the limited random movement of water molecules within tissues depends on the Brownian motion of water[13,14]. Diffusion within nerve fibres or white matter of the brain is usually high and tends to be directed towards the path of minimum opposition to the moving molecules and this is used to generate the final image in DWI. Diffusion tensor imaging (DTI) is an extension of DWI that enables measurement of Brownian movement of water molecules in nerves[11-15]. However, MRI is not widely available, patient selective, expensive, and involves the use of time-consuming techniques.
Ultrasonography (USS) is preferred to MRI as a diagnostic method for a variety of reasons such as noninvasiveness, low cost, accessibility, approach, etc[15]. The assessment of extremely small peripheral nerves via ultrasound has been made possible by employing Diagnostic high-resolution USS[8]. The median nerve can be examined as it courses from the arm to the hand using high-resolution USS. Ultrasound can be used to evaluate the shape, size, and echo-texture of the MN. The major disadvantage of ultrasound is that it is operator dependent as it requires trained experienced hands with appropriate high resolution equipment[15].
This study aimed to compare the MN cross-sectional area (CSA) measured on USS between DM subjects and age- and sex-matched apparently healthy controls (HC), and evaluate the relationship between MN CSA and presence and/ or severity of DPN.
The study was approved by the Ethics and Research Committee of our hospital. Eighty-five consenting DM patients aged between 18 and 80 years and an equal number of age- and sex-matched HC subjects were randomly recruited from the Endocrinology Unit of the Department of Medicine of our hospital. Hypertensives, current smokers and alcohol consumers, subjects with thyroid disease, liver disease, previous history of carpal tunnel operation, inflammation, malignancy, and elevated total cholesterol after serum fasting lipid profile (FLP) were excluded from the study. Our hospital is a tertiary hospital and one of the major referral centers for diabetes care in the Southwestern zone of Nigeria. It serves a catchment area of about 170000 people.
Physical examination was carried out on all prospective study subjects by the managing endocrinologist for the presence and severity of peripheral neuropathy. The modified clinical history part of Michigan Neuropathy Screening Instrument (MNSI) questionnaire[11] was administered to all study participants and scored over 15 to determine and grade peripheral neuropathy (PN). A total “Yes” score of 1-5 represented mild PN, a score of 6-10 moderate and 11-15 represented severe PN as done previously by Moghtaderi et al[16].
Phalen test[12] was performed to rule out carpal tunnel syndrome.
In the erect position with the acantho-meatal line set parallel to the floor, subjects had their weight in kilogram (Kg) and height in meters (M) measured without their shoes on. Their body weight and height were measured to the nearest 0.1 kg and 0.1 meter respectively using a mechanical physician weighing scale attached with a height gauge (model ZT-160, China).
Body mass index (BMI) was determined for all study participants using the formula: BMI = Weight/height2
Venipuncture of the antecubital veins in the left arm was done under sterile conditions for all study participants after an overnight fast of at least 8-12 h. 5 mL of venous blood was taken from each study subject for the assessment of fasting lipid profile (FLP) and glycated hemoglobin (Hb1Ac). The sample was emptied into an EDTA (Ethylene diamine tetra acetic acid) bottle and sent to chemical pathology laboratory for FLP using bioassay systems EnzyChrom cholesterol assay kit (E2CH-100) and Hb1Ac by chromatography using Siemens Hb1Ac machine (model SEMDIA-10311134, United States). Chromatography was either done immediately or sample stored in the refrigerator at a temperature of 2-8 ºC and test done within 7 d.
Fasting Blood Glucose (FBG) was determined from finger prick specimen using a glucometer (Accu-check, Roche 365702101104) based on the glucose oxidase method.
All sonographic examinations were performed using the MINDRAY Real-time ultrasound machine (Model DC-7) equipped with a linear array probe, with a transducer frequency of 6MHz-12MHz. Each participant was seated on examination couch with a pillow on his/her lap. The forearm was placed supine on the pillow with elbow and fingers semi-flexed during the examination of the median nerve. The patient was instructed not to move the fingers during the examination period.
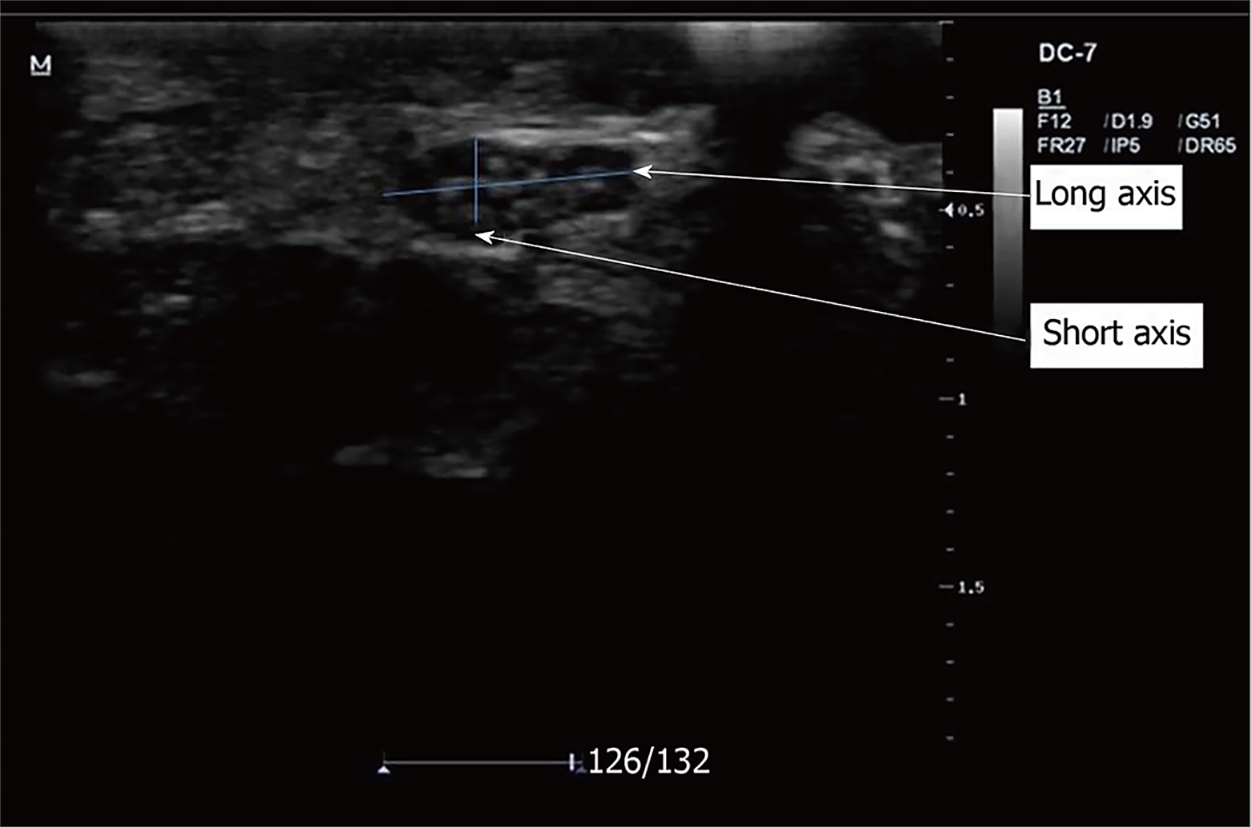
Following adequate positioning, coupling gel was applied to the anterior part of the wrist joint, over the carpal tunnel (CATL) and 5 cm proximal to it (5cmCATL). The volar wrist crease and pisiform bone were used as external reference points and landmarks during scanning. The transducer was positioned at right angles to the distal wrist crease and longitudinal axis of the forearm within the carpal tunnel inlet. The MN was identified and its major and minor axes were taken (Figure 1). Intra-observer variability was minimized by taking three measurements and recording the mean value. The CSA was calculated by the indirect method using the formula: CSA = major axis × minor axis × π × 1/4 (mm2)[13]; where π is a mathematical constant and is equal to 3.142.
All data were entered into the computer spreadsheet using Statistical Package for Scientific Solutions (SPSS) version 22.0 for Windows (SPSS, Chicago, IL, United States). Quantitative variables were indicated as mean ± SD, while qualitative variables were indicated as frequencies and percentages.
Independent Sample t-test was used to compare MN CSA between DM and apparently HC subjects. A subgroup analysis amongst the DM subjects was done between those with and those without PN using independent t-test. Median nerve CSA was further compared between DM without PN, with mild PN and moderate/severe PN using Analysis of Variance (ANOVA).
Pearson Correlation was done to determine the relationship between MN CSA, clinical and laboratory parameters of the DM and HC subjects.
A level of P ≤ 0.05 was considered as statistically significant for all tests.
The statistical review of this study was done by a biomedical statistician[17-20].
There were significant differences in mean weight (P = 0.003), BMI (P = 0.010), DBP (P = 0.001), MAP (P = 0.023) and FBG (P = 0.001) between the diabetic and control groups while mean age (P = 0.602), total cholesterol (P = 0.622), height (P = 0.473) and SBP (P = 0.557) were similar in them. Both groups were well matched for Gender (Table 1).
| Variables | DM, n = 85 | HC, n = 85 | P-value |
| Age (yr) | |||
| mean ± SD1 | 61.7 ± 11.1 | 60.9 ± 10.3 | 0.620 |
| < 50 yr | 11 (12.9) | 11 (12.9) | 0.965 |
| 50-59 yr | 25 (29.4) | 27 (31.8) | |
| 60-69 yr | 22 (25.9) | 23 (27.1) | |
| ≥ 70 yr | 27 (31.8) | 24 (28.2) | |
| Gender | |||
| Male | 44 (51.8) | 44 (51.8) | 1.000 |
| Female | 41 (48.2) | 41 (48.2) | |
| Height (m)1 | 1.66 ± 0.08 | 1.65 ± 0.08 | 0.473 |
| Weight (Kg)1 | 71.3 ± 13.6 | 65.4 ± 11.5 | 0.003 |
| BMI (Kg/m2)1 | |||
| mean ± SD1 | 26.0 ± 5.3 | 24.1 ± 4.3 | 0.010 |
| Underweight | 3 (3.5) | 4 (4.7) | 0.072 |
| Normal BMI | 35 (41.2) | 51 (60.0) | |
| Overweight | 31 (36.5) | 21 (24.7) | |
| Obese | 16 (18.8) | 9 (10.6) | |
| Systolic BP (mmHg)1 | 129.8 ± 20.7 | 131.9 ± 24.3 | 0.557 |
| Diastolic BP (mmHg)1 | 84.2 ± 11.5 | 82.8 ± 13.1 | 0.404 |
| Total cholesterol (mmHg)1 | 4.90 | 4.78 | 0.622 |
| FBG (mmol/L)1 | 7.00 ± 2.16 | 5.15 ± 0.06 | 0.001 |
| HbA1C in % (mean ± SD) | 8.69 ± 2.46 | - | - |
| Duration of DM in months median (range) | 48.0 (0.3-312) | - | - |
| Peripheral neuropathy | 51 (60) | - | - |
Diabetic subjects had significantly higher median nerve CSA than their age- and sex-matched apparently healthy controls at the CATL level (12.5 ± 2.5 mm2vs 8.8 ± 1.7 mm2 (P < 0.01) on the right and 12.3 ± 2.5 mm2vs 8.6 ± 1.7 mm2 (P < 0.01) on the left) and at 5cmCATL (8.0 ± 2.0 mm2vs 5.3 ± 1.2 mm2 (P < 0.01) on the right and 7.9 ± 1.9 mm2vs 5.4 ± 1.4 mm2 (P < 0.01) on the left) (Table 2).
| Median nerve CSA (mm2) | DM, n = 85 | HC, n = 85 | P-value | |
| Right | Carpal tunnel | 12.5 ± 2.5 | 8.8 ± 1.7 | < 0.01 |
| 5 cm proximal | 8.0 ± 2.0 | 5.3 ± 1.2 | < 0.01 | |
| Left | Carpal tunnel | 12.3 ± 2.5 | 8.6 ± 1.7 | < 0.01 |
| 5 cm proximal | 7.9 ± 1.9 | 5.4 ± 1.4 | < 0.001 | |
The median nerve CSA was significantly higher in diabetics with PN compared to diabetics without PN (12.9 ± 2.5 mm2vs 11.8 ± 2.4 mm2, P = 0.049) at the level of the CATL but not at 5cmCATL (8.0 ± 2.2 mm2vs 8.0 ± 2.0 mm2, P = 0.856) (Table 3).
| Median nerve CSA (mm2) | Diabetic peripheral neuropathy | P-value | |
| Absent n = 34 | Present n = 51 | ||
| Carpal tunnel | 11.8 ± 2.4 | 12.9 ± 2.5 | 0.049 |
| 5cm Proximal | 8.0 ± 2.2 | 8.0 ± 2.0 | 0.856 |
There was no association between median nerve CSA and severity of PN as median nerve CSA did not significantly differ between the absent, mild, and moderate/severe PN categories of diabetic subjects at the CATL (P = 0.062) and at 5cmCATL (P = 0.145) even after post-hoc Scheffe analysis for intergroup differences (Table 4).
| Median nerve CSA (mm2) | Peripheral neuropathy | P-value | ||
| Absent (n = 34) | Mild (n = 32) | Moderate/severe (n = 19) | ||
| Carpal tunnel | 11.8 ± 2.4a | 13.2 ± 2.6a | 12.3 ± 2.1a | 0.062 |
| 5 cm Proximal | 7.9 ± 2.2b | 8.3 ± 2.2b | 7.6 ± 1.4b | 0.145 |
Median nerve CSA at 5cmCATL and at the CATL did not show significant association with age greater than 60 years, duration of DM and glycemic control in both HC and DM subjects. However, MN CSA was significantly more thickened in males compared to females among the HC at both points of measurement. The association of MN CSA with gender was not found among DM subjects even after subgroup analysis of only those with DPN (Table 5).
| Median nerve CSA (mm2) at CATL | |||||||
| DM_nonDPN | P-value | DM_DPN | P-value | HC | P-value | ||
| Duration of DM | ≤ 5 yr | 11.4 ± 1.8 | 0.65 | 12.9 ± 2.4 | 0.99 | - | - |
| > 5 yr | 11.8 ± 3.3 | 12.9 ± 2.7 | - | ||||
| FBG | ≤ 7 mmol/L | 11.6 ± 2.3 | 0.79 | 13.2 ± 2.9 | 0.34 | - | - |
| > 7 mmol/L | 11.4 ± 2.0 | 12.5 ± 1.8 | - | ||||
| HBA1c | ≤ 7% | 11.0 ± 1.8 | 0.45 | 12.3 ± 2.4 | 0.20 | - | - |
| > 7% | 11.7 ± 2.3 | 13.2 ± 2.6 | - | ||||
| Age | ≤ 60 yr | 11.1 ± 1.9 | 0.25 | 12.9 ± 2.3 | 1.00 | 8.7 ± 1.5 | 0.37 |
| > 60 yr | 12.0 ± 2.4 | 12.9 ± 2.7 | 8.4 ± 1.9 | ||||
| Sex | Female | 11.3 ± 1.9 | 0.62 | 13.2 ± 2.6 | 0.42 | 8.1 ± 1.2 | 0.02 |
| Male | 11.7 ± 2.4 | 12.6 ± 2.4 | 9.0 ± 2.0 | ||||
| Median nerve CSA (mm2) at 5cmCATL | |||||||
| DM_nonDPN | P-value | DM_DPN | P-value | HC | P-value | ||
| Duration of DM | ≤ 5 yr | 7.6 ± 1.9 | 0.87 | 7.7 ± 1.7 | 0.13 | - | - |
| > 5 yr | 7.5 ± 1.5 | 8.5 ± 2.2 | - | ||||
| FBG | ≤ 7 mmol/L | 7.5 ± 1.3 | 0.74 | 8.2 ± 1.9 | 0.57 | - | - |
| > 7 mmol/L | 7.7 ± 2.5 | 7.9 ± 2.0 | - | ||||
| HBA1c | ≤ 7% | 7.5 ± 1.4 | 0.86 | 7.7 ± 2.2 | 0.29 | - | - |
| > 7% | 7.6 ± 1.9 | 8.3 ± 1.8 | - | ||||
| Age | ≤ 60 yr | 7.3 ± 1.5 | 0.32 | 8.4 ± 2.1 | 0.25 | 5.5 ± 1.2 | 0.79 |
| > 60 yr | 7.9 ± 2.2 | 7.8 ± 1.8 | 5.4 ± 1.5 | ||||
| Sex | Female | 7.0 ± 1.4 | 0.10 | 7.8 ± 1.7 | 0.37 | 4.9 ± 0.7 | 0.00 |
| Male | 8.0 ± 2.0 | 8.3 ± 2.2 | 5.9 ± 1.6 | ||||
Duration of DM, FBG and, HbA1c did not show any significant correlation with MN CSA in the diabetic subjects (Table 6).
| Variables | CATL | 5cmCATL | ||
| r | P-value | r | P-value | |
| Age | -0.041 | 0.898 | -0.050 | 0.650 |
| Sex | -0.0581 | 0.601 | -0.0501 | 0.651 |
| Weight | 0.094 | 0.390 | -0.041 | 0.708 |
| Height | 0.042 | 0.703 | 0.025 | 0.817 |
| BMI | 0.091 | 0.407 | -0.056 | 0.611 |
| SBP | -0.126 | 0.251 | 0.050 | 0.648 |
| DBP | 0.043 | 0.693 | 0.050 | 0.648 |
| TC | 0.092 | 0.402 | 0.092 | 0.056 |
| Duration of DM | 0.1102 | 0.318 | 0.1512 | 0.167 |
| FBG | -0.059 | 0.591 | 0.026 | 0.811 |
| HbA1c | -0.012 | 0.916 | 0.034 | 0.758 |
Diabetic neuropathy is a relatively early and common complication affecting approximately 30% of DM patients[14]. The prevalence of DPN from clinical assessment using the MNSI questionnaire was 60% in this study. This is at best an estimate since the gold standard for assessing PN, the nerve conduction test was not employed in our study.
We found higher MN CSA in the diabetic cohort which consisted of both types 1 and 2 DM subjects compared to non-diabetic HC at the 2 points of measurement. The finding of increased MN CSA in the diabetics relative to HC in our study agrees with those of 2 other hospital-based studies by Watanabe et al[8] in 30 type 2 DM subjects aged 36 to 83 years with mean age of 59.8 ± 10.2 years and 32 healthy volunteers aged 24-72 years with mean age of 53.7 ± 13.9 years in Japan, and Agirman et al[21] in 63 Type 2 DM subjects and 14 controls with mean age 47.6 ± 13.1 years in Turkey even though they were conducted in type 2 DM subjects only in racially and geographically different settings.
We also observed a significant additional increase in MN CSA in diabetics in the presence of DPN at the CATL similar to the findings of Watanabe et al[8] and Zaidman et al[22] which suggests that DPN can cause an increase in MN CSA apart from the hyperglycemic effect of DM. This statistical difference was however low (P = 049). The low statistical difference of MN CSA between subjects with and without PN in our study may be due to the fact that we did not diagnose PN using electrophysiological tests like NCS and as such, we may have misclassified some of the subjects with PN as those without PN. The statistically low significant difference between DM subjects with and without PN seen at the CATL was however not significant at 5cmCATL[23].
Furthermore, this study failed to establish a significant association between MN CSA and severity of DPN. It can, therefore, be inferred from the index study that the presence of DPN probably increases MN CSA to a given threshold beyond which no further increase is possible. Therefore, our study shows that the presence and not severity of DPN can give an additional thickening of the MN CSA at the CATL.
Long-term hyperglycemic state has been implicated in the occurrence of DPN[14,18,19]. Both FBG and HbA1c are short-term and long-term monitors of glycemic control respectively. The long-term monitor (HbA1c) gives a good estimate of glycemic control over a period of 3 months duration. Diabetic subjects were dichotomised into those with good and poor glycemic control based on their HbA1c levels in this study. Glycemic control did not show significant association with the MN CSA at the 2 points of measurements among the DM subjects, even after a subgroup analysis of only those with DPN. There was also no significant correlation between HbA1c levels and MN CSA at both points of measurement (5cmCATL: r = -0.012, P = 0.916 and CATL: r = 0.034, P = 0.758). This observation contrasts the findings of Watanabe et al[8] in 32 DM subjects who reported significant correlation between MN CSA and HbA1c levels despite fewer sample size in their study relative to ours. Genetic and racial differences may have contributed to this.
Since long-term hyperglycaemic state has been implicated in the occurrence of DPN[14,18,19], and MN CSA further thickened in the presence of PN in diabetics in this study, it would have been expected that a poor glycaemic state would be associated with a further thickening of the MN CSA in the diabetics with PN and show positive correlation with HbA1c. Factors that were not explored in this study such as impaired insulin signalling, insulin growth factor and C-peptide that mediate DPN as suggested by Dobretsov et al[24] and /or genetic factors may have contributed to the contrasting findings in our study.
Duration of DM of more than 5 years had no additional effect on the MN CSA at the 2 points of measurement. The duration of diabetes was estimated from the time of diagnosis in a hospital in this study. This obviously is a conservative estimation as patients would have had the disease before presenting to the hospital. This may be responsible for the insignificant association between MN CSA and duration of DM seen in our study even among DM subjects with PN.
In this study, we used a modified clinical history part of Michigan Neuropathy Screening Instrument (MNSI) questionnaire to identify subjects with peripheral neuropathy. Peripheral neuropathy could be large fiber mono-neuropathy/polyneuropathy or isolated small fiber neuropathy. We could only have been sure of MN neuropathy in our subjects if we had performed neurophysiological study of the MN. Also, we only assessed MN in this study on ultrasound. It is possible that our study participants may have had mono-neuropathy not affecting the MN or have isolated small fibre neuropathy. This could have accounted for the lack of correlation between MN CSA and disease duration, FBG, HBA1c levels in the index study.
DPN symptoms are induced by factors such as total hyperglycemic exposure, high lipid levels, blood pressure, increased height, exposure to high concentrations of ethanol. Also, hereditary factors are considered. In addition to the matching of our DM and HC subjects for age and sex, the results of their anthropometric and laboratory parameters showed that there was no significant differences in their height, BP and TC. Confounders like hypertension, smoking, and alcohol consumption were also eliminated by excluding subjects with a history of these risk factors from the two study groups.
Evidence from this study may have been limited by the selection bias of our hospital-based setting as subjects enrolled were only those that presented in the teaching hospital. We, however, minimised this by recruiting consecutive consenting subjects into the study. Another limitation is the fact that we did not confirm neuropathy using neurophysiological tests like NCS which uses supramaximal stimuli and recruits non-selectively all available fibres (both large and small fibres) and involves the proximal and distal parts of the nerve trunks. NCS would have picked abnormality in the function of the nerves even in the absence of clinical symptoms and as such we would have been able to diagnose subclinical peripheral neuropathy in our study subjects.
However, our findings are unique in that we report findings from diabetics of Nigerian origin. To the best of our knowledge, MN CSA measured on ultrasound in diabetic neuropathy has not been reported in Nigeria prior to this study.
We conclude from our study that DM subjects had thicker MN CSA at 5cmCATL and at CATL compared to their age- and sex-matched HC, Diabetics with PN had thicker MN CSA at the CATL but not at 5cmCATL compared with those without PN and MN CSA had no significant relationship with age, gender, severity of DPN, duration of DM or glycemic control in diabetics.
We recommend the CATL over 5cmCATL as the point of measurement for MN CSA when evaluating the MN in diabetics as both the increase in the MN CSA secondary to DM and additional thickening in presence of DPN seen in this study occurred at the CATL while only the increase secondary to DM occurred at 5cmCATL. Evaluation of the MN CSA in DM subjects is only recommended before DPN sets in as no additional thickening of the MN CSA with a worsening grade of DPN was seen in this study.
Peripheral neuropathy (PN) is a common complication of diabetes mellitus. High-resolution ultrasonography gives good morphological detail in the peripheral nerves.
Sonographic measurement of the Median nerve cross-sectional area may be a valuable tool in addition to clinical examination in identifying subjects with peripheral neuropathy in regions where standard electrophysiological studies like nerve conduction test are not available.
We evaluated the relationship between median nerve cross-sectional area (CSA) and the presence of PN in a cohort of adult diabetic Nigerians.
A one-year cross-sectional study carried out in diabetic subjects recruited in the endocrinology unit of a Nigerian tertiary hospital and age- and sex-matched controls.
This study demonstrates that the median nerve is thicker in CSA at the carpal tunnel (CATL) and 5 cm proximal to the carpal tunnel (5cmCATL) in diabetic subjects than in age- and sex-matched healthy controls. Further thickening in the median nerve CSA is seen in the presence of diabetic peripheral neuropathy at the carpal tunnel but not at a point 5 cm proximal to it. Median nerve size has no significant relationship with age, gender, severity of DPN, duration of DM or glycemic control in our diabetic subjects.
This study done in Diabetics of Nigerian origin adds to the current literature that Diabetic subjects have thicker MN CSA compared to their age- and sex-matched controls. Median nerve CSA was also thicker at the CATL in Diabetics with PN than in those without PN.
We suggest the need for longitudinal studies in diabetic subjects who have median nerve neuropathy confirmed with nerve conduction test to elucidate the progressive effect of diabetic peripheral neuropathy on MN CSA.
STROBE guidelines statement: The guidelines of the STROBE Statement have been adopted
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Nigeria
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Razek AAKA, Senol MG S- Editor: Cui LJ L- Editor: A E- Editor: Wu YXJ
| 1. | Ogbera AO, Ekpebegh C. Diabetes mellitus in Nigeria: The past, present and future. World J Diabetes. 2014;5:905-911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (2)] |
| 2. | American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care. 2014;37 Suppl 1:S14-S80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2830] [Cited by in RCA: 3016] [Article Influence: 274.2] [Reference Citation Analysis (0)] |
| 3. | Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4438] [Cited by in RCA: 4379] [Article Influence: 291.9] [Reference Citation Analysis (4)] |
| 4. | Kasper DL, Fauci AS, Hauser SL, Longo DL, Jameson JL and Loscalzo J. Endocrinology and Metabolism. In: Harrison’s Principles of Internal Medicine. 19th ed. New York: McGraw-Hill Education 2015; 2152-2165. |
| 5. | American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41:S13-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1853] [Cited by in RCA: 2255] [Article Influence: 322.1] [Reference Citation Analysis (0)] |
| 6. | Chudzik W, Kaczorowska B, Przybyła M, Chudzik B, Gałka M. [Diabetic neuropathy]. Pol Merkur Lekarski. 2007;22:66-69. [PubMed] |
| 7. | Ogawa K, Sasaki H, Yamasaki H, Okamoto K, Matsuno S, Shono T, Doi T, Arimoto K, Furuta H, Nishi M, Nakao T, Nanjo K. Peripheral nerve functions may deteriorate parallel to the progression of microangiopathy in diabetic patients. Nutr Metab Cardiovasc Dis. 2006;16:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Watanabe T, Ito H, Morita A, Uno Y, Nishimura T, Kawase H, Kato Y, Matsuoka T, Takeda J, Seishima M. Sonographic evaluation of the median nerve in diabetic patients: comparison with nerve conduction studies. J Ultrasound Med. 2009;28:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Tagliafico A, Martinoli C. Reliability of side-to-side sonographic cross-sectional area measurements of upper extremity nerves in healthy volunteers. J Ultrasound Med. 2013;32:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Azman D, Bosnjak J, Strineka M, Béné R, Budisić M, Lovrencić-Huzjan A, Demarin V. Median nerve imaging using high-resolution ultrasound in healthy subjects. Acta Clin Croat. 2009;48:265-269. [PubMed] |
| 11. | Du R, Auguste KI, Chin CT, Engstrom JW, Weinstein PR. Magnetic resonance neurography for the evaluation of peripheral nerve, brachial plexus, and nerve root disorders. J Neurosurg. 2010;112:362-371. [PubMed] |
| 12. | Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;65:1532-1556. [PubMed] |
| 13. | Razek AAKA, Shabana AAE, El Saied TO, Alrefey N. Diffusion tensor imaging of mild-moderate carpal tunnel syndrome: correlation with nerve conduction study and clinical tests. Clin Rheumatol. 2017;36:2319-2324. [PubMed] |
| 14. | Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL. Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fiber tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol. 2004;25:356-369. [PubMed] |
| 15. | Razek AA, Fouda NS, Elmetwaley N, Elbogdady E. Sonography of the knee joint. J Ultrasound. 2009;12:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. 2006;108:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 234] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Almasi-Doghaee M, Boostani R, Saeedi M, Ebrahimzadeh S, Moghadam-Ahmadi A, Saeedi-Borujeni MJ. Carpal compression, Phalen’s and Tinel’s test: Which one is more suitable for carpal tunnel syndrome? Iran J Neurol. 2016;15:173-174. [PubMed] |
| 18. | Suk JI, Walker FO, Cartwright MS. Ultrasonography of peripheral nerves. Curr Neurol Neurosci Rep. 2013;13:328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 19. | Christopher H, Edwin R, John AAH, Nicholas AB. Davidson’s Principles and Practice of Medicine. 18th ed. Edinburgh. Churchill Livingstone; Chapter 7. 471-509. |
| 20. | Watanabe T, Ito H, Sekine A, Katano Y, Nishimura T, Kato Y, Takeda J, Seishima M, Matsuoka T. Sonographic evaluation of the peripheral nerve in diabetic patients: the relationship between nerve conduction studies, echo intensity, and cross-sectional area. J Ultrasound Med. 2010;29:697-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Agirman M, Yagci I, Leblebicier MA, Ozturk D, Akyuz GD. Is ultrasonography useful in the diagnosis of the polyneuropathy in diabetic patients? J Phys Ther Sci. 2016;28:2620-2624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Zaidman CM, Al-Lozi M, Pestronk A. Peripheral nerve size in normals and patients with polyneuropathy: an ultrasound study. Muscle Nerve. 2009;40:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 252] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 23. | World Health Organization. Definition and diagnosis of Diabetes Mellitus and intermediate hyperglycemia; report of a WHO/IDF consultation. Available from: http://who.int/diabetes/publications/diagnosis. |
| 24. | Dobretsov M, Romanovsky D, Stimers JR. Early diabetic neuropathy: triggers and mechanisms. World J Gastroenterol. 2007;13:175-191. [PubMed] |