INTRODUCTION
During the past decade, it has become clear that inflammation is a key feature in diabetes that leads to long-term complications in specific organs, in particular the eye and kidney. In the eye, the major complication is diabetic retinopathy, a leading cause of blindness in the Western world affecting three-fourths of diabetic patients within 15 years after onset of the disease[1,2]. Many diabetic patients are referred to an ophthalmologist for evaluation and treatment only after visual complications have already occurred. The recommended treatment for diabetic retinopathy has been laser photo-coagulation but the procedure also destroys neural tissues. Therefore, there is a great need for the development of new non-invasive therapies. These visual complications are most likely associated with oxidative stress and inflammation. Our research in diabetic retinopathy has focused on delineating the inflammatory and neurodegenerative processes involved. We have identified new non-invasive receptor-based therapies for mitigating the retinal damage associated with diabetes. This review is focused on the therapeutic effects of cannabidiol (CBD) on animal models of diabetic retinopathy. Special emphasis is placed on novel mechanisms described in recent studies of retinal models which help to explain some of the pharmacological effects observed with CBD.
DIABETIC RETINOPATHY (DR)
DR is a chronic ocular disorder that, if untreated, will lead to legal blindness. In the United States, over 20 million adults (or 10% of the total population) currently have diabetes. Of this group, over 12 000 patients will be diagnosed with new-onset blindness annually, making it one of the leading causes of legal blindness in Americans within the age group of 20-74[3]. Type I diabetics usually have high incidence of retinopathy although retinopathy occurs in almost all patients with diabetes for 20 years or more[1]. The earliest detectable signs of retinopathy are categorized as nonproliferative diabetic retinopathy (NPDR). NPDR is clinically subdivided into mild, moderate and severe categories. Loss of retinal pericytes and alterations in retinal blood flow are preclinical changes that are often non-detectable by physical exam[4,5]. Retinal venous dilation and microaneurysms are the first alterations detectable by ophthalmoscopy. Following these alterations, intraretinal hemorrhage and exudation may occur. These may then lead to macular edema, which, if untreated may lead to irreversible vision loss and blindness. As hyperglycemia persists, the disease progresses to moderate and severe NPDR which presents with hemorrhages and venous beading, suggesting decreased retinal circulation and dilated capillaries[6].
Proliferative diabetic retinopathy (PDR) is the next stage when proliferation of new blood vessels begins. Approximately 50 percent of patients with severe NPDR progress to PDR within one year[7]. This stage is characterized by the onset of ischemia-induced new vessel proliferation from the optic nerve head as well as in the retina. These new vessels are fragile and tend to bleed easily resulting in vitreous hemorrhage. If untreated, the neovascularization will undergo fibrosis and contraction leading to traction retinal detachments. Additional complications may include neovascular glaucoma due to sprouting of new vessels on the iris and in the trabecular meshwork of the anterior chamber[8].
DR is a vascular-neuroinflammatory disease
The early signs of diabetic retinopathy in experimental diabetic models include vascular inflammatory reactions due to oxidative stress, pro-inflammatory cytokines, and the consequent binding of leukocyte adhesion molecules CD18 and intercellular adhesion molecule 1 (ICAM-1)[9]. These reactions lead to breakdown of the blood-retinal barrier (BRB) function, vascular occlusion and tissue ischemia, which in turn leads to neuronal cell death[9-14]. However, diabetes could also directly affect metabolism within the neural retina leading to neuronal cell death. Whether diabetes affects vascular or neural retina first, both microglial and macroglial cells are activated[15]. The function of activated macroglia in transporting[16] and metabolizing glutamate may be impaired[17] (unpublished observations). This leads to glutamate accumulation[18-20]. Glutamate excitotoxicity occurs via activation of N-methyl-D-aspartic acid (NMDA) and non-NMDA receptors, to directly or indirectly induce calcium influx and the release of superoxides, leading to neuronal cell death[21]. This is followed by neuro-inflammation, during which activated microglial cells migrate toward dying neurons and release inflammatory cytokines to further exacerbate the damage[22]. These findings suggest that pharmacological interventions that reduce oxidative stress and inflammation might be effective neuroprotectants for diabetic retinopathy[20,23].
Microglia in DR
Normally quiescent microglia become activated during early diabetes[24-27]. Cytokines such as interleukin (IL)-1β, IL-6, γ-interferon, and tumor necrosis factor-α (TNF-α) have been shown to directly activate microglia[28,29]. Activated microglia release (or promote the release of) glutamate, reactive oxygen species (ROS), IL-1β, IL-3, IL-6, TNF-α, vascular endothelial growth factor (VEGF), lymphotoxin, matrix metalloproteinases (MMPs) and nitric oxide (NO)[15,30]. The cytokines IL-1β, IL-6, TNF-α, and lymphotoxin alter expression of vascular cell adhesion molecules to recruit lymphocytes and macrophages to injury sites[31]. Lymphotoxin, TNF-α, NO and ROS can directly kill cells[32,33]. VEGF, NO and MMPs can weaken the BRB, thus enhancing the infiltration of leukocytes into the retina. It remains unclear why diabetes would incite microglia activation in the retina but research on retinal microglia activation may provide substantial insights into the pathogenesis of DR[34]. Cultured microglia have been used extensively to study microglial behavior. Treatment of microglia or macrophage-like cells with advanced glycation end-products (AGE) or Amadori-albumin[35,36], high glucose[37] or with endotoxins such as lipopolysaccharide (LPS) has been used as a model to simulate inflammation[38-40].
ROLES OF ADENOSINE RECEPTORS (ARs) AND NUCLEOSIDE TRANSPORTERS IN INFLAMMATION
Adenosine, an endogenous purine nucleoside, has been proposed to modulate a variety of physiological responses by stimulating specific extracellular receptors[41-43]. ARs have been classified as A1, A2A, A2B, and A3 receptors[44]. Under stress and ischemia conditions, the local tissue concentrations of extracellular adenosine are increased due to the release of adenosine itself, or of AMP, which is metabolized extracellularly to adenosine. This increased adenosine can protect against excessive cellular damage via a negative feedback mechanism[45] (unpublished observations). Adenosine released at inflamed sites exhibits anti-inflammatory effects through A2AAR[46]. Sub-threshold doses of an inflammatory stimulus that caused minimal tissue damage in wild-type mice were sufficient to induce extensive tissue damage and more prolonged and higher levels of pro-inflammatory cytokines in knock-out mice that lacked the A2AAR (A2AAR -/- mice)[47]. A2AAR agonist treatment blocked the inflammation, functional and histological changes associated with diabetic nephropathy in wild-type diabetic mice, whereas it had no effect on the A2AAR -/- diabetic mice[48]. A2AAR, a Gs-protein-coupled receptor, can increase levels of immunosuppressive cAMP in microglia or other immune cells[49]. Stimulation of the A2AAR decreases leukocyte adhesion and blocks the associated release of oxygen free radicals[50]. Adenosine released can activate endothelial adenosine receptors, leading to increases in intracellular cAMP and resealing of the endothelial junctions thereby promoting vascular barrier function[51]. Moreover, A2AAR activation induces the synthesis and release of nerve growth factor thereby is neuroprotective[52].
Although adenosine and its agonists are protective in animal models of inflammation, their therapeutic application has been limited by systemic side effects such as hypotension, bradycardia, and sedation[53]. Moreover, adenosine usually disappears very rapidly in physiological or inflammatory conditions due to rapid reuptake and subsequent intracellular metabolism[54]. Endogenous adenosine levels at inflamed sites are reported to increase further because of the increased need for energy supplied by ATP, which is metabolized to AMP and adenosine ultimately[55]. In addition, the activity of 5’-nucleotidase, which metabolizes AMP to adenosine, is reported to increase in inflammatory conditions[56]. It is therefore assumed that prevention of adenosine uptake into the cells and its subsequent metabolism can selectively enhance extracellular concentrations of adenosine at inflamed sites, resulting in an anti-inflammatory effect[57]. Protective or ameliorating effects of adenosine uptake inhibitors in ischemic cardiac and cerebral injury, organ transplantation, seizures, thrombosis, insomnia, pain and inflammatory diseases have been reported[58]. Preclinical and clinical results indicate the possibility of therapeutic application of adenosine uptake inhibitors[58,59].
Adenosine reuptake and degradation
Adenosine disappears rapidly in physiological or inflammatory conditions due to rapid reuptake via nucleoside transporters (NTs) and subsequent intracellular metabolism[54]. There are two subtypes of NTs: Concentrative NTs which are dependent on the presence of extracellular sodium, and equilibrative NT (ENTs). In the microglial cells, the majority of adenosine transport is not affected by sodium removal suggesting ENTs are the primary transporters functioning in these cells[60]. ENTs are further classified into two subtypes on the basis of their sensitivities to inhibition by the drug S-(4-nitrobenzyl)-6-thioinosine [nitrobenzylmercaptopurine riboside (NBMPR)]. NBMPR-sensitive ENTs bind NBMPR with high affinity and have the functional designation equilibrative sensitive (ENT1). NBMPR-insensitive transporters are designated ENT2. Dipyridamole, an inhibitor for both ENT1 and ENT2[61], is used clinically as a coronary vasodilator and a platelet aggregation inhibitor[62,63]. Dipyridamole plus aspirin improves retinal vasculature patterns in experimental diabetes[64].
Role of ENT1 in adenosine function in diabetes
ENT1 plays an integral role in adenosine function in diabetes by regulating adenosine levels in the vicinity of adenosine receptors. It was reported that adenosine uptake by ENT1 in human aortic smooth muscle cells (HASMCs) was increased by hyperglycemia[65]. To provide insight into mechanisms by which ENT1 was modulated by hyperglycemia, kinetic studies of adenosine transport and [3H]NBMPR binding were performed[65]. The results show that Vmax (representing the number of ENT1) of adenosine transport in high glucose (HG)-treated HASMCs was increased without affecting Km (representing the affinity of ENT1). Similarly, Bmax (representing the number of ENT1) of the high-affinity [3H]NBMPR binding was increased without affecting Kd (representing the affinity of ENT1). Consistent with these observations, HG increased mRNA and protein expression of ENT1. Pathologically, the increase in ENT1 activity in diabetes may affect the availability of adenosine in the vicinity of adenosine receptors and, thus, alter vascular functions in diabetes. Pharmacological intervention of ENT1 activity may prove to be effective therapeutics in diabetes. Current studies are in progress to elucidate the effect of hyperglycemia on the function and expression of ENT1 in the retinal microglial and vascular endothelial cells.
CANNABINOIDS AND CANNABINOID RECEPTORS
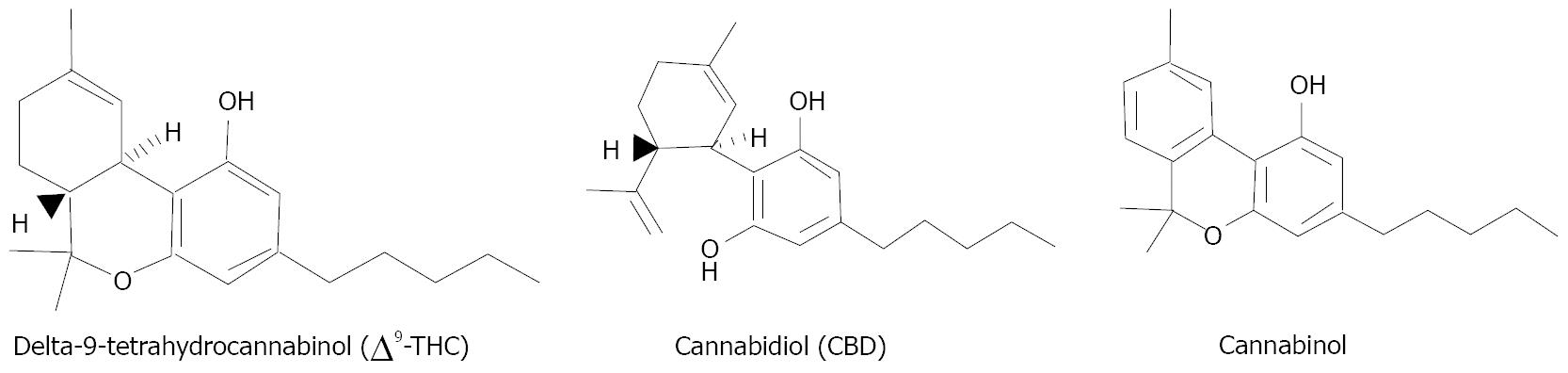
The best-known cannabinoids from marijuana are (-)-Δ9-tetrahydrocannabinol (THC), cannabinol (CBN), and (-)-cannabidiol (CBD) (Figure 1)[66]. THC, but not CBN or CBD, is known to exert psychotropic effects[67,68]. Cannabinoids are also known to be therapeutic with properties of anti-inflammation[69,70] and anti-oxidation[71]. Cannabinoids produce their biological effects by acting through at least two receptors. Receptor CB1 (cloned) is responsible for psychoactivity and is expressed in the brain[72] and retinal neurons[73,74]. Receptor CB2 (cloned) is expressed in immune cells[75] and cerebral microglial cells[76], but also in the retina[77]. These receptors are coupled to Gi/o proteins to inhibit adenylyl cyclase activity and immediate early gene signaling pathway(s)[78]. Receptor CB1 is also coupled through Gi/o proteins to inhibit voltage-sensitive calcium channels[79] and activate potassium channels[80].
Figure 1 The best-known cannabinoids from marijuana.
(-)-Δ9-tetrahydrocannabinol (THC), but not cannabinol or (-)-cannabidiol (CBD), is known to exert psychotropic effects.
CBD has very low affinity to either CB1 or CB2[81,82]. This low affinity of CBD for CB1 accounts for its inability to produce the subjective “high” and cognitive effects that are characteristic of marijuana and THC. CBD is very effective as a scavenger of ROS. The antioxidative effect of CBD is superior to α-tocopherol and ascorbate in vitro and in vivo[71] due to its ability to scavenge ROS and block NADPH oxidase[40]. CBD also has potent anti-inflammatory actions and have been shown to decrease inflammatory cytokines in arthritis[83] and in diabetes[12], prevent cerebral damage during ischemia[84] and to prevent cerebral infarction[85]. CBD is well tolerated when chronically administered to humans[86] and has been approved for the treatment of inflammation, pain and spasticity associated with multiple sclerosis in patients since 2005[87]. CBD attenuates high glucose-induced endothelial cell inflammatory response and barrier disruption in human coronary endothelial cells[88]. It also decreases the incidence of diabetes in non-obese diabetic mice[89] and is neuroprotective and BRB-preserving in streptozotocin-induced diabetes[12]. Most recently, CBD has been shown to decrease retinal inflammation by blocking ROS and TNF-α formation, p38 MAP kinase activation and microglial activation[40]. Current data in the effects of intraocularly introduced CBD in diabetic animal model are consistent with its anti-inflammatory activity (unpublished observations).
CBD enhances AR-mediated anti-inflammation
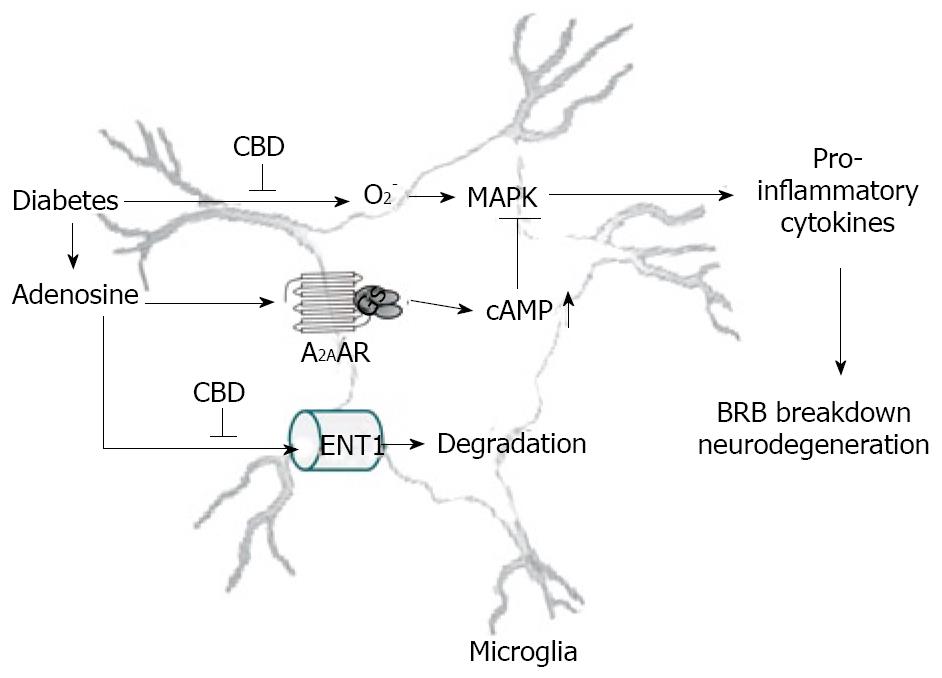
It has recently been shown that nanomolar concentrations of CBD or THC could inhibit uptake of adenosine by ENT1 in murine microglia, RAW264.7 macrophages[60] and in rat retinal microglia[39]. CBD synergistically enhances adenosine’s TNF-α suppression upon LPS treatment. Moreover, in vivo treatment with a low dose of CBD decreases TNF-α production in serum in the LPS-treated mice; this effect is reversed by treatment with an A2AAR antagonist and abolished in A2AAR -/- mice[60]. Similar results are observed in the rat retina[39]. These studies demonstrate that CBD has the ability to enhance adenosine signaling through inhibition of uptake and provide a non-cannabinoid receptor mechanism by which CBD can decrease endotoxin-induced inflammation. Current data suggest that CBD inhibits diabetes-induced retinal inflammation by the same mechanism (unpublished observations). A hypothetical pathway illustrating how CBD works to reduce retinal inflammation in diabetes is shown in Figure 2.
Figure 2 The hypothetical mechanism of anti-inflammation effect CBD in diabetic retinopathy.
Diabetes causes release of adenosine and pro-inflammatory cytokines via superoxide formation and MAPK activation, leading to DR. Adenosine-initiated anti-inflammation via A2AAR-Gs-cAMP signaling is terminated rapidly due to adenosine reuptake by equilibrative nucleoside transporter (ENT) and subsequent metabolism. CBD blocks superoxide formation and inhibits adenosine reuptake via inhibiting ENT1, thereby activating A2AAR-Gs-cAMP signaling.
CONCLUSION
Recent evidence suggests that local inflammation plays a major role in the pathogenesis of diabetic retinopathy. The function of CBD as an antioxidant to block oxidative stress and as an inhibitor of adenosine reuptake to enhance a self-defense mechanism against retinal inflammation represents a novel therapeutic approach to the treatment of ophthalmic complications associated with diabetes. This study is important for the development of adenosine reuptake inhibitors as a potentially novel and effective therapy for diabetic retinopathy. However, the therapeutic values of these agents should be confirmed by clinical trials. Furthermore, depending on the difference in the genetic make-ups for the metabolism and pharmacological target of CBD, it may be important to consider CBD as a personalized medicine, i.e. adjusted dosages according to individual’s genetic make-ups, to offer significant advantages over traditional clinical approaches[90].
Supported in part by grants from American Diabetes Association and Knights Templar Educational Foundation (GIL)
Peer reviewer: Ugur Cavlak, Professor, Pamukkale Universitesi Fizik Tedavi ve Rehab, Yuksekokulu Kinikli Kampusu Yeni Rektorluk Binasi B Kati 20070 Denizli, Turkey
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N