Copyright
©The Author(s) 2017.
World J Diabetes. Mar 15, 2017; 8(3): 97-103
Published online Mar 15, 2017. doi: 10.4239/wjd.v8.i3.97
Published online Mar 15, 2017. doi: 10.4239/wjd.v8.i3.97
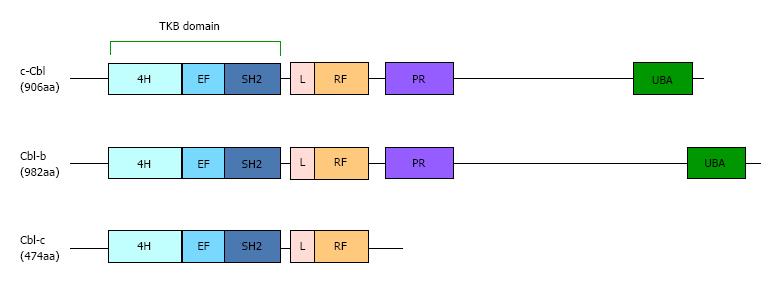
Figure 1 The primary structure and domain organization of human Casitas b-lineage lymphoma family proteins.
Cbl-b proteins contain highly conserved tyrosine kinase-binding (TKB), linker (L), RING finger (RF) and proline-rich (PR) domains. 4H: Four-helix bundle; SH2: Src-homology 2; UBA: Ubiquitin-associated domain.
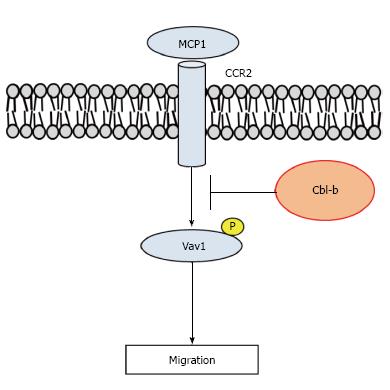
Figure 2 Casitas b-lineage lymphoma-b suppresses macrophage migration.
Monocyte chemoattractant protein (MCP)-1 causes macrophages to infiltrate adipose tissue via C-C chemokine receptor 2 (CCR2). Phosphorylation (P) of Vav1 mediates macrophage migration, and Cbl-b negatively regulates macrophage migration by suppressing Vav1 phosphorylation.
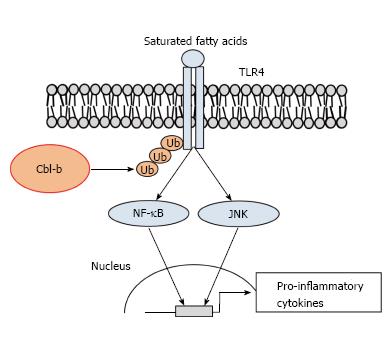
Figure 3 Casitas b-lineage lymphoma-b suppresses toll-like receptor 4 signaling in macrophages.
Cbl-b negatively regulates saturated fatty acid (SFA)-induced TLR4 signal transduction. SFA-triggered TLR4 signaling induces the expression of inflammatory cytokines via JNK and NF-κB. The released inflammatory cytokines cause insulin resistance in the liver, skeletal muscle and adipose tissue. In the presence of SFAs, Cbl-b induces the ubiquitination and degradation of TLR4 in macrophages. Ub: Ubiquitin; TLR4: Toll-like receptor 4; Cbl-b: Casitas b-lineage lymphoma-b; JNK: Jun N-terminal kinase.
- Citation: Abe T, Hirasaka K, Nikawa T. Involvement of Cbl-b-mediated macrophage inactivation in insulin resistance. World J Diabetes 2017; 8(3): 97-103
- URL: https://www.wjgnet.com/1948-9358/full/v8/i3/97.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i3.97