Copyright
©The Author(s) 2016.
World J Diabetes. May 10, 2016; 7(9): 189-197
Published online May 10, 2016. doi: 10.4239/wjd.v7.i9.189
Published online May 10, 2016. doi: 10.4239/wjd.v7.i9.189
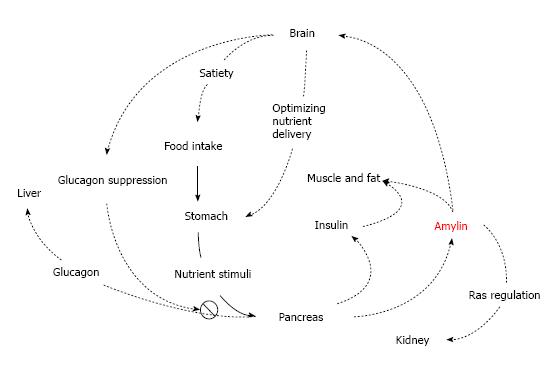
Figure 1 Overview of physiological actions of amylin.
(1) Amylin suppresses glucagon secretion from islet alpha cells at mealtime and thus, inhibits glucagons-induced glucose release from the liver; (2) Amylin delays nutrient delivery from the stomach to the small intestine for absorption; (3) Amylin reduces food intake by a signal mediated through the central nervous system; (4) Renal amylin may stimulate Renin-Angiotensin System; and (5) Amylin and insulin coordinate storage of carbohydrate.
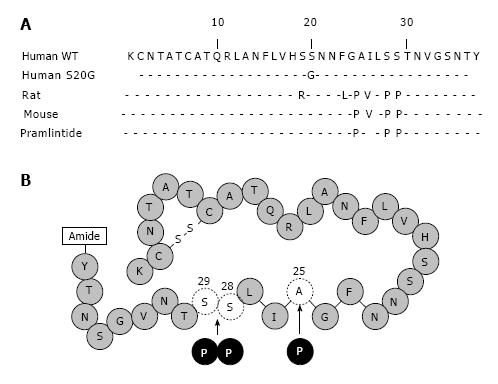
Figure 2 Amino acid sequence and diagrammatic representations of human amylin and pramlintide.
A: Amino acid sequence alignment of human (WT, S20G), rat, mouse amylin and pramlintide. Only the amino acids that differ are shown. The sequence between amino acids 20 to 29 represents the amyloidogenic domain; B: The synthetic amylin analog pramlintide differs from human amylin at three amino acid sites (proline at 25, 28, and 29) and this molecule overcomes these disadvantages of human amylin.
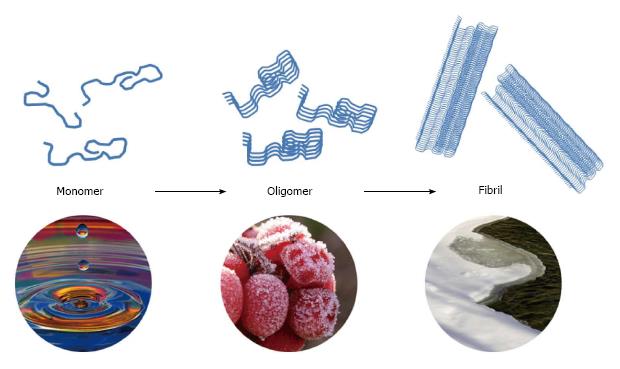
Figure 3 Three conformations of human amylin.
Monomers of amylin with physiological functions mainly contribute to glucose and lipid homeostasis and tend to misfold into the cytotoxic oligomers. A self-driven process is accumulating the misfolded oligomers into insoluble nontoxic amylin fibrils with a β-sheet structure.
- Citation: Zhang XX, Pan YH, Huang YM, Zhao HL. Neuroendocrine hormone amylin in diabetes. World J Diabetes 2016; 7(9): 189-197
- URL: https://www.wjgnet.com/1948-9358/full/v7/i9/189.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i9.189