Copyright
©The Author(s) 2016.
World J Diabetes. Jan 25, 2016; 7(2): 14-26
Published online Jan 25, 2016. doi: 10.4239/wjd.v7.i2.14
Published online Jan 25, 2016. doi: 10.4239/wjd.v7.i2.14
Figure 1 Hyperglycaemia leads to increased hexosamines, polyols and diacylglycerol within the cell, which can cause oxidative stress leading to cell damage.
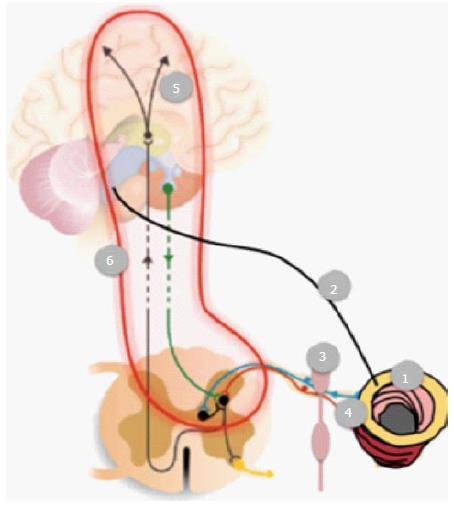
Figure 2 Schematic representation of the possible nerve pathways and mechanisms that theoretical can contribute to gastrointestinal symptoms in diabetics.
(1) Vascular and degenerative changes in the enteric nervous system; Autonomic neuropathy affecting (2) the vagal nerve and (3) sympathetic pathways; (4) Affection of visceral (and somatic in case the peritoneum is involved) afferents mediating sensations such as pain; (5) Structural and functional changes in the brain (and spinal cord), together with (6) affection of spino-bulbo-spinal loops.
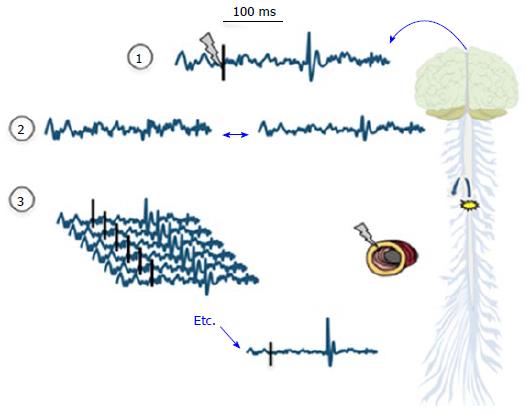
Figure 3 Evoked potentials are recorded following a peripheral stimulus as indicated with the grey “lightning” and ideally a corresponding activity can be seen following 80-90 ms as an evoked potential.
However, as illustrated in (2) the amplitudes of the evoked potential tend to be low and often comparable to the amplitudes of spontaneous electroencephalogram. In order to decode the evoked potentials from the background electroencephalographic activity and noise, signal averaging is necessary as illustrated in (3). Provided enough of recorded trials, the evoked potentials become bigger in amplitude and therefore visible and the random background activity cancel out. Then, the EP latencies and amplitudes of the peaks can be analyzed by visual inspection. When many (64-128) electrodes are used the corresponding brain sources can be computed based on the surface electroencephalographic recordings. EP: Evoked brain potential.
- Citation: Drewes AM, Søfteland E, Dimcevski G, Farmer AD, Brock C, Frøkjær JB, Krogh K, Drewes AM. Brain changes in diabetes mellitus patients with gastrointestinal symptoms. World J Diabetes 2016; 7(2): 14-26
- URL: https://www.wjgnet.com/1948-9358/full/v7/i2/14.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i2.14