Copyright
©The Author(s) 2016.
World J Diabetes. Nov 15, 2016; 7(19): 483-514
Published online Nov 15, 2016. doi: 10.4239/wjd.v7.i19.483
Published online Nov 15, 2016. doi: 10.4239/wjd.v7.i19.483
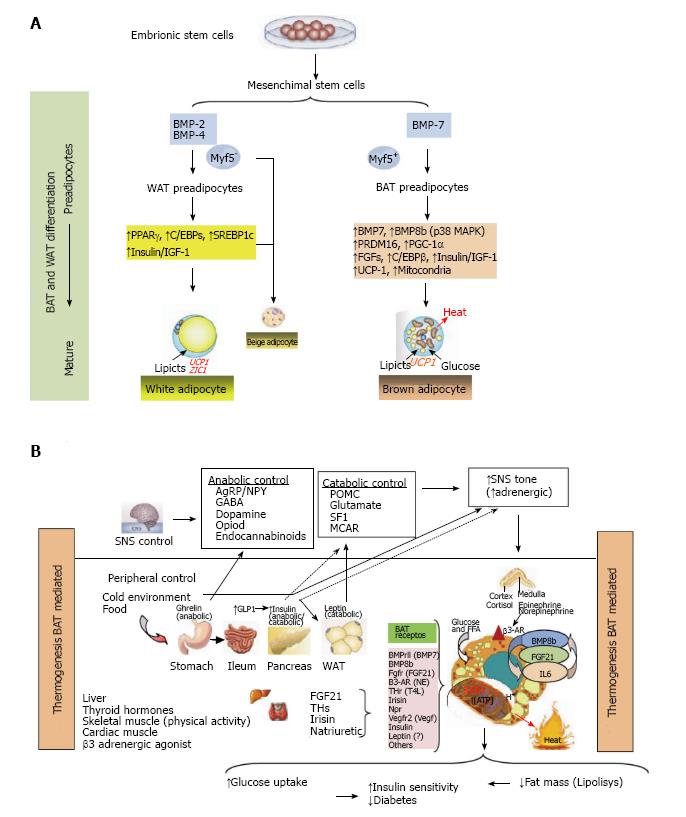
Figure 1 Thermogenesis brown adipose tissue (Bat) mediated.
A: Adipocytes were developed because non adipocytes cells are unable to store calories as fat to meet fuel needs during long periods without eating. If the energy intake is more than energy expenditure, WAT is expanded and leads to obesity. However, a second type of adipose tissue, called BAT was developed especially for energy expenditure (thermogenesis). Today, research in identifying the main genes that control differentiation, development and activation of BAT is highly active, because, activation of BAT, in detriment of WAT, could have anti-obesity effects, which can be utilized to keep the system of fat deposit balanced. In this research, PRDM16, PPAR-γ and PGC-1α, have been identified as the key nodes in the regulation of inducible BAT; B: The thermogenic potential of BAT is controlled by the SNS, which densely innervates brown fat depots. In addition, BAT is activated in response to cold temperatures, hormones and possibly diet. BAT content and activation is highest in children and decreases with age. BAT activation is decreased in fatness, and BAT activity has been inversely correlated to BMI, body fat, and visceral obesity. In humans, BAT amount and activation is higher in women than in men. Of clinical relevance, BAT activation is very low in diabetic patients in comparison with non-diabetic subjects. Thyroid hormones play a main role in control of BAT activation, therefore the cold-induced enhancement of the enzyme 5’-deiodinase type II activity, which deiodinates thyroxine (T4) to T3. Catecholamines such as norepinephrine binds to β-ARs and induce PGC1α through p38 MAPK and finally triggers expression of UCP1. Whereas β1-AR is considered important for proliferation of classical brown adipocyte precursors in response to norepinephrine, β3-AR plays a major role in thermogenic function of mature brown adipocytes. Another signal, Irisin hormone which comes from muscle to fat tissue, is able to induce a robust browning programme, and mediates the beneficial effects of exercise and could reduce diet-induced obesity and insulin resistance. A more generalized program in the control of adipose tissue is conducted by FGF21 through regulating lipolysis in WAT as well as increasing substrate utilization by increasing fatty acid oxidation in the liver. Last, beige fat cell functions include either a like to “WAT” when energy balance is exceeded, or a like to “BAT” in response to many stimuli similar to BAT activation. WAT: White adipose tissue; BAT: Brown adipose tissue; PRDM16: PR domain containing 16; PPAR-γ: Peroxisome proliferator-activated receptor-γ; PGC-1α: Peroxisome proliferator-activated receptor γ coactivator 1α; SNS: Sympathetic nervous system; BMI: Body mass index; FGF21: Fibroblast growth factor 21.
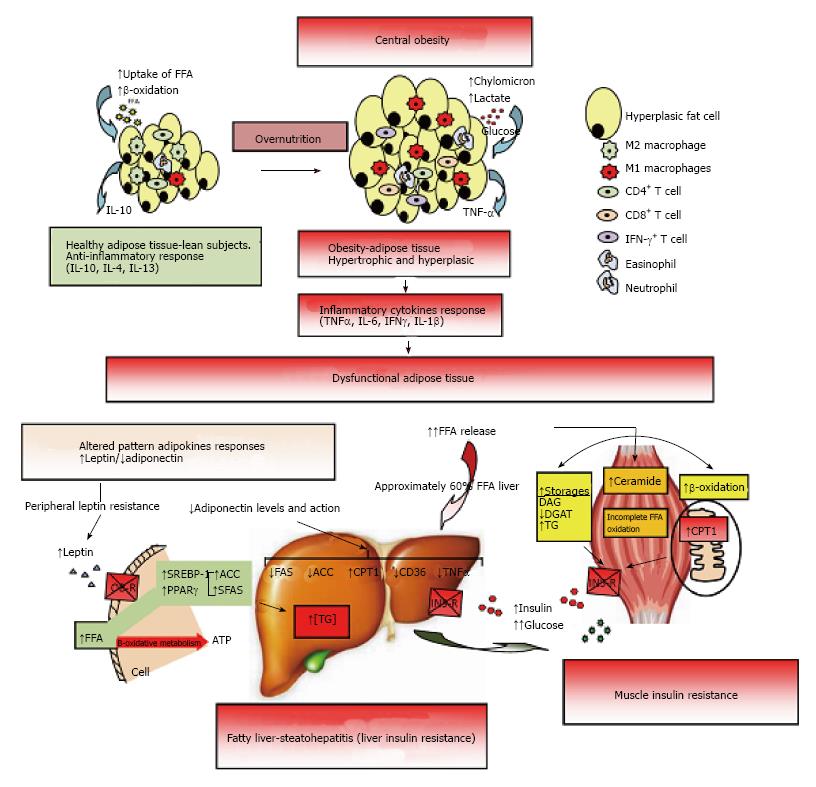
Figure 2 Dysfunctional adipose tissue.
Early central obesity is associated with a low-grade chronic inflammatory state characterized by slow infiltration of macrophages which are an important source of inflammation of this adipose tissue[275,276]. Several macrophage subtypes can be found, and simply put, are divided in pro-inflammatory M1 or alternatively activated M2, although in vivo studies reveal a spectrum of macrophage phenotypes[277]. Adipocytes and immune cells such as T cells and macrophages participate in the activation and production of inflammatory cytokines[170,275,278,279]. The M1 macrophages mainly found in obesity, are induced from precursor M0 macrophages by stimulation of components of bacteria (lipopolysaccharide) and type 1 T-helper (Th1) inflammatory cytokines like IFN-γ and TNF-α. The M2 macrophages are activated by type 2 (Th2) cytokines such as IL-4 and IL-13. The M2 macrophages are abundant in adipose tissue of lean subjects and appear to be involved in remodeling, tissue repair, and maintenance of insulin sensitivity through the production and expression of IL-10, IL-1 receptor antagonist, and arginase-1. Whereas M1 macrophages use glucose for energy, M2 macrophages activate the β-oxidation of fatty acids[277,280]. Finally, M1 macrophages are the major source of inflammatory cytokines including TNF-α which inhibits adipose cell differentiation by activating Wnt signaling and suppressing expression of PPAR-γ transcription factor essential for the development and function of adipocyte, and reducing the effect on stored triglycerides[281,282]. The subcutaneous adipose tissue will continue to expand to an equilibrium point. When this capacity is exceeded, glucose and lipid uptake begins to decline and insulin levels are raised to maintain serum glucose in the normal range[215]. In addition, when WAT is unable to expand (inflexibility), associated with insulin resistance state, a continuous release of FFA to interstice begins, generating a systemic lipotoxic effect in muscle, liver, etc., (lipotoxicity). The adipose tissue itself begins a slow process of low-level chronic inflammation (macrophages, lymphocytes, etc.) which increases local release of TNF-α and IL-6[166]. TNF-α and IL-6 levels are inversely related with peripheral and hepatic glucose-uptake which is insulin-mediated[283]. The liver keeps excess uptake of FFA in serum to capacity by joining with glycerol (TAG) and slowly fatty liver is developed (NAFLD). It has been shown that peripheral fatty acids contribute approximately 60% of total TAG stored in the liver, whereas the novo lipogenesis in the liver is approximately 26% and approximately 15% is from the diet[284]. On the other hand, leptin levels respond directly to adipose expansion, while adiponectin levels tend to decrease when metabolic syndrome is developed. The elevated leptin levels should increase lipolysis in non-adipose tissues, decreasing excess fatty acids in these cells. However, this action of leptin may be partially blocked by the anabolic effect established by hyperinsulinemia, settling down leptin system dysfunction (peripheral leptin resistance)[115]. In addition, the decreased adiponectin levels are inversely related to peripheral glucose uptake and directly related with progressive development of chronic liver disease by fat infiltration. Adiponectin exerts a protective action on liver fat accumulation, favoring lipolysis by promoting the action of CPT-1, while interfering with the action of FAS, ACO and TNF-α, and decreasing the expression and action of CD-36 protein that promotes the transport of fatty acids[129]. Finally, both leptin and adiponectin seem to regulate the deposition of fat in insulin-sensitive tissues by increasing fat oxidation. IFN-γ: Interferon-γ; TNF-α: Tumor necrosis factor-α; IL: Interleukin; PPAR-γ: Peroxisome proliferator-activated receptor-γ; WAT: White adipose tissue; FFA: Free fatty acids; NAFLD: Non alcoholic fatty liver disease; CPT-1: Carnitine palmitoyltransferase-1; FAS: Fatty acid synthase; ACO: Acyl CoA carboxylase.
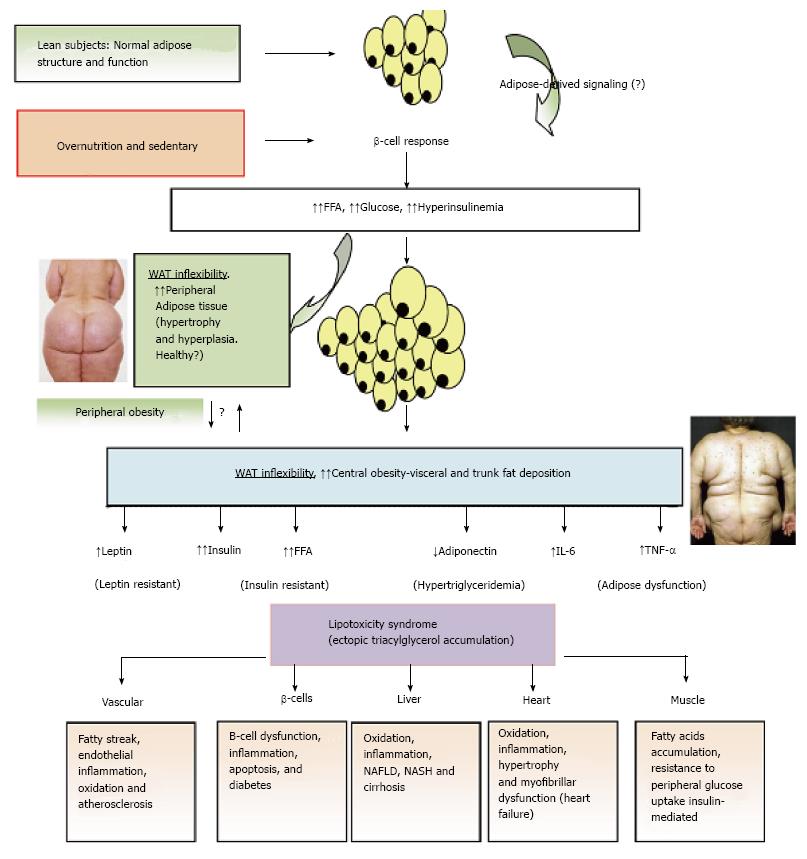
Figure 3 Adipose tissue expandability and metabolic syndrome.
After a long period of overeating with positive energy balance, associated with increased hormones such as insulin, adipose tissue responds by increasing its storage capacity, which is determined by a number of factors. Individuals with a higher capacity for storing fat, mainly when peripheral WAT is expanded (WAT flexibility), most subjects will remain metabolically normal for a longer period, despite obesity developing. These subjects are observed to be metabolically healthy (MHO). Chronic inflammatory response leads to dysfunctional adipose tissue with increased local and endocrine secretion of acute phase reactants and inflammatory signaling pathways[285]. Abnormal cytokine and adipokines production is related to insulin resistance, hyperglycemia, altered lipid profile and cardiovascular diseases[115,286,287]. Insulin resistance slowly results from increased accumulation of lipids in other nonadipose tissues such as muscle (lipotoxicity) due to enhanced release of fatty acids from hypertrophic and hyperplasic adipocyte cells. In addition, when adipocytes achieve their maximal storage capacity, they begin to alter their adipokynes secretion profile. Therefore, a proinflammatory milieu with elevation in IL-6 and TNF-α and altered adipokines profile, with decreased adiponectin and increased leptin levels, with peripheral leptin resistance, in a dysfunctional adipose system is observed. This suggests that the limitation in storage capacity could be necessary and even precedes the development of metabolic factors. Ectopic lipid accumulation in non-adipocyte cells causes lipotoxicity in these organs and tissues, including inflammation and finally apoptosis. Thus, lipotoxicity in β-cell could decrease beta cell mass (dysfunction of β-cell secretion) and would cause diabetes. Increased fat in liver leads to hepatic steatosis (NAFLD) and steatohepatitis (NASH) and would cause hepatic dysfunction, in the heart would cause myocardiac dysfunction, in the endothelial fatty streak would be precursor of generalized arteriosclerosis, etc. At what point the adipose tissue begins to fail is likely to be determined by genetic and epigenetic factors. However, the question is: Can storage capacity in WAT be enhanced to meet an increased demand[288]? So far, in human trials, the PPAR-γ agonists (TZDs), that remove fat from central deposits toward more favorable peripheral deposits, have been shown to improve lipid profile, insulin-sensitivity, and reduce diabetes and NAFLD[269]. WAT: White adipose tissue; MHO: Metabolically healthy obese; IL: Interleukin; TNF-α: Tumor necrosis factor-α; NAFLD: Non alcoholic fatty liver disease; NASH: Nonalcoholic steatohepatitis; PPAR-γ: Peroxisome proliferator-activated receptor-γ; TZD: Thiazolidinedione.
Figure 4 Insulin resistant syndrome and lipid metabolism.
When obesity is developing, early abnormalities are observed at this time including hyperinsulinemia and low grade of proinflammatory state (↑ cytokines and PCR-hs), increase liberation of free fatty acids from adipose tissue (↑ lipolysis) and altered release of adipokines (↓ adiponectin, ↑leptin with leptin resistance). In some subjects, fatty liver develops later and consequently affects some functions of the liver. These include an early altered postprandial state (increasing glucose and triglyceride-rich VLDL particles), but finally these finding are observed in fasting state[289]. The VLDL particles undergo reduction by LPL and triglycerides are taken up by adipose tissue. The final result is the increase of cholesterol-rich small and dense LDL particles in serum. These LDL particles are highly susceptible to modifications like oxidation and glycation and the result is the increasing levels of ox-LDL, gly-LDL and the generation of antibodies to ox-LDL[190]. Finally, modified LDL are phagocytosed by macrophages in endothelial blood vessels and an inflammatory pattern that alters endothelial function initiating arteriosclerosis begins[177]. On the other hand, through ABC1 ligand the lipid efflux from peripheral cells to start the reverse transport of cholesterol is mediated. Mature HDL3 are generated from lipid-free apo A1 or lipid-poor pre-β1-HDL as the precursors, and LCAT-mediated sterification of cholesterol generates mature HDL3 and HDL2[189]. In T2D insulin-resistant patients, after adequate metabolic control the HDL3 cholesterol and APO A1 levels were increased. These findings were associated with a higher specific binding activity of HDL3 in those patients that showed improved insulin resistance[190]. Cholesterol efflux capacity has a strong inverse association with carotid intima-media thickness and was inversely associated with the incidence of cardiovascular events in a population-based cohort[188,290]. LCAT-mediated cholesterol esterification generates large spherical HDL2 particles, but large HDL2 can be converted in turn to small HDL3 upon CETP-mediated transfer of CE from HDL to apoB-containing lipoproteins, interfering with reverse cholesterol transport. Finally, SR-BI mediates the selective uptake of cholesteryl esters from HDL particles into mainly liver and steroidogenic organs[291]. VLDL: Very light density lipoprotein; LPL: Lipoprotein lipase; ox-LDL: Oxidized-LDL; gly-LDL: Glycated-LDL; ABC1: ATP-binding cassette transporter 1; LCAT: Lecithin cholesterol acyltransferase; CETP: Cholesteryl ester transfer protein; SR-BI: Scavenger receptor class-B, type I.
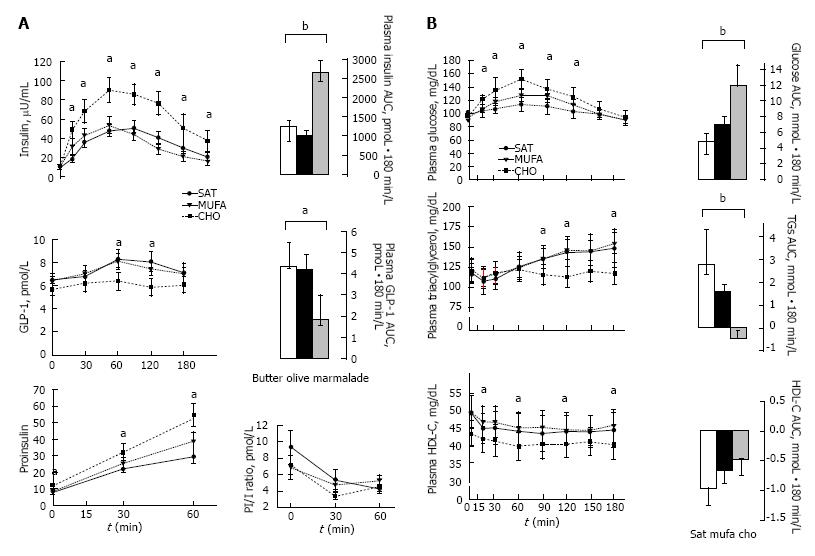
Figure 5 Mean (± SE) postprandial responses of insulin, proinsulin and glucagon-like peptide-1 levels (A), and glucose, triacylglycerol and high-density lipoprotein cholesterol levels (B), in 11 insulin-resistant subjects to three isocaloric (443 kcal) standard breakfasts.
A breakfast rich in carbohydrates, a Mediterranean breakfast enriched with extra-virgin olive oil and standard breakfast high in saturated fat. The incremental AUC was calculated by the formula based on the trapezoid rule with adjustment for baseline concentrations. Repeated measures ANOVA and Tukey’s test. aP < 0.05; bP < 0.01[237]. CHO: High-carbohydrate; MUFA: Monounsaturated fatty acids; AUC: Area under the curve; ANOVA: Analysis of variance.
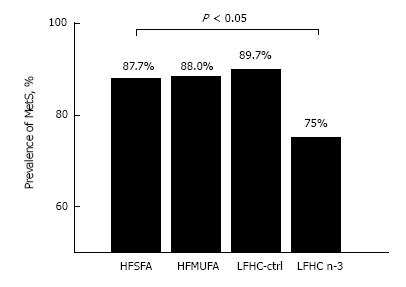
Figure 6 Prevalence of metabolic syndrome after 12-wk of diet assignment.
HFSFA is a high fat diet rich in saturated fat and HFMUFA is a high fat diet rich in monounsaturated fat. LFHC-control is a low-fat, high complex carbohydrate diet and LFHC n-3 is a low-fat, high complex carbohydrate diet supplemented with 1.24 g/d of very long chain n-3 polyunsaturated fatty acid (VLC n-3 PUFA). χ2 test, P < 0.05)[261]. MetS: Metabolic syndrome; HFSFA: High fat diets rich in saturated fat; HFMUFA: High fat diets rich in monounsaturated fat; PUFA: Polyunsaturated fatty acids.
- Citation: Paniagua JA. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J Diabetes 2016; 7(19): 483-514
- URL: https://www.wjgnet.com/1948-9358/full/v7/i19/483.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i19.483