Copyright
©The Author(s) 2016.
World J Diabetes. Jul 10, 2016; 7(13): 260-270
Published online Jul 10, 2016. doi: 10.4239/wjd.v7.i13.260
Published online Jul 10, 2016. doi: 10.4239/wjd.v7.i13.260
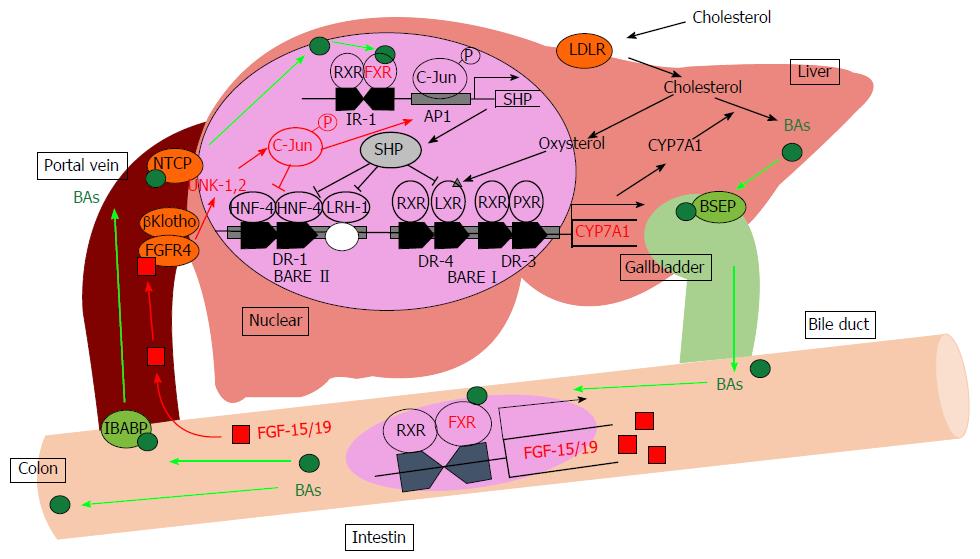
Figure 1 Bile acid metabolism in the liver.
BAs induce the FXR-SHP-mediated pathway and repress BA synthesis enzyme gene expression such as CYP7A1 and CYP8B1. Synthesized BAs increase the expression of FGF-15/19 in the small intestine. FGF-15/19 signaling induces JNK pathway activation resulting in the repression of CYP7A1 transcription. AP1: Activator protein 1; BAs: Bile acids; BARE: Bile acid response element; BSEP: Bile salt export pump; CYP7A1: Cholesterol-7α-hydroxylase; DR: Direct repeat element; FGF-15/19: Fibroblast growth factor-15/19; FGFR4: Fibroblast growth factor receptor 4; FXR: Farnesoid X receptor; HNF-4: Hepatocyte nuclear factor; IBABP: Intestinal bile acid-binding protein; IR-1: Inverted repeat element-1; JNK: Jun-N-terminal kinase; LDLR: Low-density lipoprotein receptor; LRH-1: Liver receptor homolog-1; LXR: Liver X receptor; NTCP: Sodium-taurocholate cotransporting polypeptide; PXR: Pregnane X receptor; RXR: Retinoid X receptor; SHP: Small heterodimer partner.
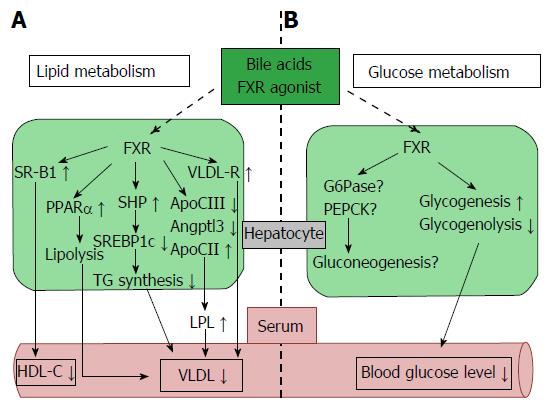
Figure 2 Farnesoid X receptor-dependent metabolic regulation in the liver.
Hepatic FXR signaling regulates lipid and glucose metabolism. A: FXR signaling reduces lipogenesis (SREBP1c) and induces fatty acid β oxidation (PPARα) and plasma TG clearance (LPL and VLDL-R), resulting in decreased plasma VLDL levels. Plasma HDL-C uptake is also increased by FXR and SRB1 activity; B: FXR signaling up-regulates glycogenesis, down-regulates glycogenolysis, and reduces blood glucose levels. Hepatic FXR signaling is also associated with gluconeogenesis, but the controlling mechanism is still unclear. Angptl3: Angiopoietin-like protein 3; ApoCII/CIII: Apolipoprotein-CII/CIII; FXR: Farnesoid X receptor; G6Pase: Glucose-6-phosphatase; HDL-C: High density lipoprotein-cholesterol; LPL: Lipoprotein lipase; PEPCK: Phosphoenolpyruvate carboxykinase; PPARα: Peroxisome proliferator-activated receptor α; SR-B1: Scavenger receptor-B1; SREBP1c: Sterol regulatory element-binding protein 1c; TG: Triglyceride; VLDL-R: Very low density lipoprotein-receptor.
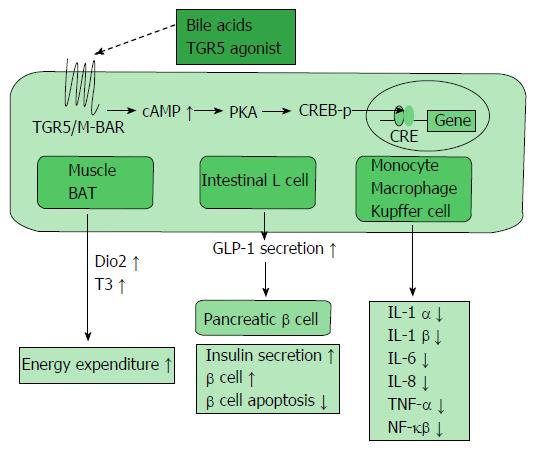
Figure 3 TGR5/M-BAR-dependent metabolic regulation.
TGR5/M-BAR activation leads to increased intracellular cAMP levels, the activation of PKA and induction of CREB phosphorylation. This series of signaling activity induces the expression of genes bearing CRE and exists in various tissues. TGR5/M-BAR signaling induces energy expenditure in the muscle and BAT, increases GLP-1 secretion in the intestinal L cell, and reduces inflammatory cytokine release in immune cells. CREB-p: cAMP response element-binding protein phosphorylation; Dio2: Deiodinase iodothyronine type II; T3: Tri-iodothyronine; BAT: Brown adipose tissue; GLP-1: Glucagon-like peptide-1.
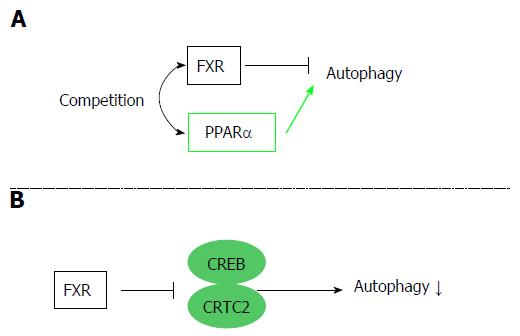
Figure 4 Autophagy regulation by the farnesoid X receptor.
FXR is associated with regulation of autophagy. Two different mechanisms are reported. A: FXR and PPARα competitively bind to the promoter regions of autophagic genes, and FXR activation suppresses autophagy; B: FXR stimulation disrupts the functional CREB–CRTC2 complex and suppresses autophagy. FXR: Farnesoid X receptor; PPARα: Peroxisome proliferator-activated receptor α; CREB: cAMP response element-binding protein; CRTC2: CREB regulated transcription coactivator 2.
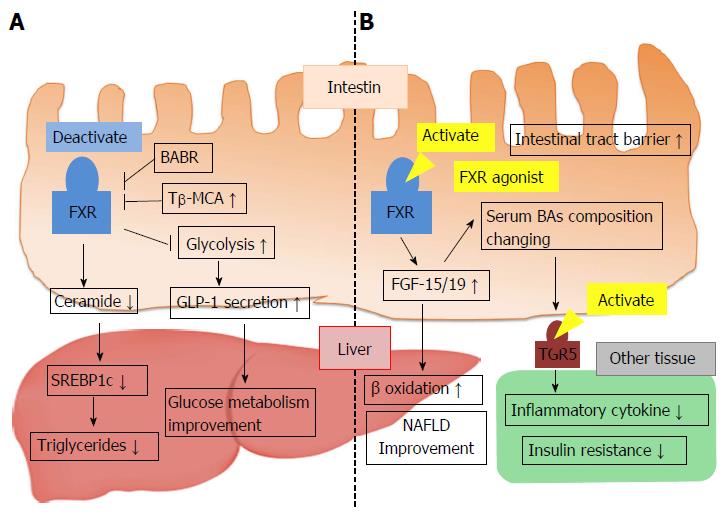
Figure 5 Conflicting mechanisms of metabolic regulation via intestinal farnesoid X receptor activity.
A: FXR activation decreases hepatic TG levels and improves glucose metabolism; B: Intestinal FXR activation of FXR agonist leads to FGF-15/19 production and improves nonalcoholic fatty liver disease. Synthesized FGF-15/19 changes BA metabolism and serum BA composition, which causes TGR5/M-BAR activation, reduced inflammatory cytokine release, and improved insulin resistance. BABR: Bile acid binding resin; FGF-15/19: Fibroblast growth factor-15/19; FXR: Farnesoid X receptor; NAFLD: Nonalcoholic fatty liver disease; SREBP1c: Sterol regulatory element-binding protein 1c; Tβ-MCA: Tauro-β-muricholic acid; GLP-1: Glucagon-like peptide-1; TG: Triglyceride.
- Citation: Taoka H, Yokoyama Y, Morimoto K, Kitamura N, Tanigaki T, Takashina Y, Tsubota K, Watanabe M. Role of bile acids in the regulation of the metabolic pathways. World J Diabetes 2016; 7(13): 260-270
- URL: https://www.wjgnet.com/1948-9358/full/v7/i13/260.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i13.260