Copyright
©The Author(s) 2016.
World J Diabetes. May 25, 2016; 7(10): 198-208
Published online May 25, 2016. doi: 10.4239/wjd.v7.i10.198
Published online May 25, 2016. doi: 10.4239/wjd.v7.i10.198
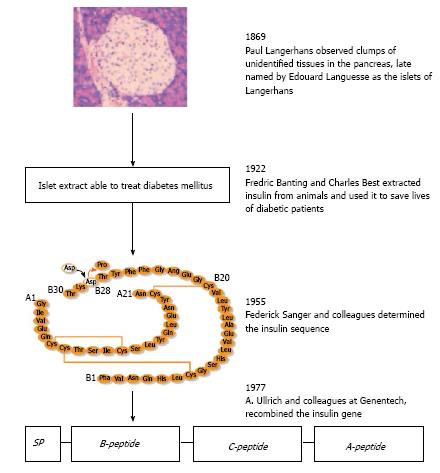
Figure 1 Historical account of the discovery of insulin.
Presented four landmarked discoveries of the islets of Langerhans, insulin, the sequencing of insulin and insulin gene recombinant technology.
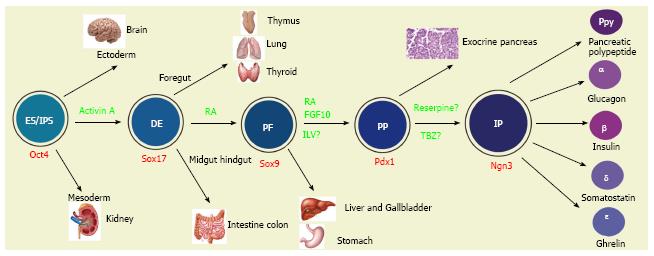
Figure 2 Multiple fate commitments of pluripotent stem cells lead to the development of insulin-secreting β cells.
Whereas inner cell mass (ICM) gives rise to three germ layers (the ectoderm, mesoderm and endoderm) during gastrulation, embryonic stem cells (ESC) or induced pluripotent stem cells (iPSC) preferentially differentiate into definitive endodermal cells [DE, marked by the expression of Sox17 (the Sry-related HMG box transcription factor 17) and Foxa2 (foxhead homeobox 2a)] in the presence of activin A. Along the anterior-posterior axis the DE is divided into foregut (giving rise to the lung, thyroid and oesophagus), posterior foregut [PF, marked by the expression of the transcription factor Sox9 and hindgut (committing the intestine and colon)]. In vitro, retinoid acid would direct the DE cells to PF cells. Rather than to the stomach, liver and gallbladder, the PF cells preferentially give rise to pancreatic progenitors (PP, marked by the expression of the transcription factor Pdx1) in the presence of retinoid acid (RA) and fibroblast growth factor 10 (FGF10) or indolactam (ILV). Principally towards the exocrine and ductal tissues, the PP also commits to progenitors of the endocrine islet lineages [IP, marked by the expression of a high level of Ngn3, as well as NeuroD1 (neural differentiation 1), IA1 (insulinoma associated 1), Isl1 (Islet 1), Pax6 (paired box factor 6) and Rfx6]. The ES/iPSC-derived Pdx1+ cells gave rise to Ngn3+ cells in the presence of tetrabenzine (TBZ). The IP then differentiates into five types of islet cells [α, β, δ (somatostatin), PP (pancreatic polypeptide) and ε (ghrelin)]. The “?” indicates that the differentiation factors have not yet been completely validated.
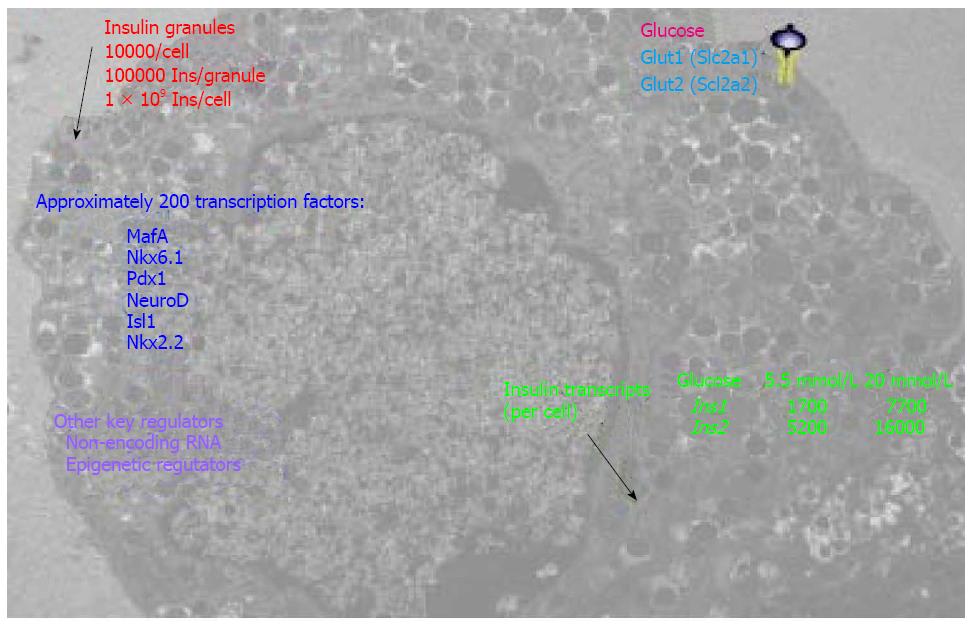
Figure 3 An integral view of insulin-secreting β cells.
The highly specialized cells have a powerful function that is regulated by multiple layers of signaling.
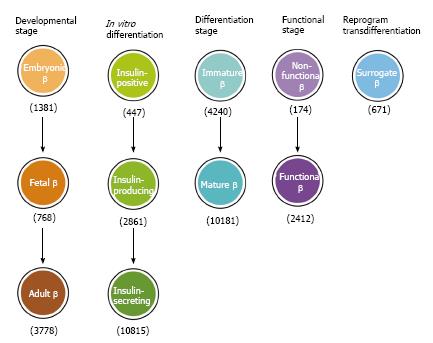
Figure 4 Conceptual confusions in insulin-secreting β cells.
Insulin-secreting cells have been given a variety of nomenclature depending on the developmental stages, in vitro differentiation, functional states or reprogram/transdifferentiation. The number of used nomenclature in parenthesis was from a Pubmed search in May 2015.
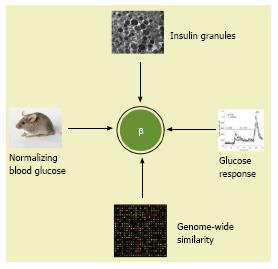
Figure 5 A post-genomic concept of insulin-secreting β cells.
Whereas four criteria are proposed, a highly similar transcriptomic profile to adult β cells is essential to establish pluripotent stem cells-derived β cells.
- Citation: Jiang FX, Morahan G. Insulin-secreting β cells require a post-genomic concept. World J Diabetes 2016; 7(10): 198-208
- URL: https://www.wjgnet.com/1948-9358/full/v7/i10/198.htm
- DOI: https://dx.doi.org/10.4239/wjd.v7.i10.198