Copyright
©2014 Baishideng Publishing Group Inc.
World J Diabetes. Jun 15, 2014; 5(3): 385-392
Published online Jun 15, 2014. doi: 10.4239/wjd.v5.i3.385
Published online Jun 15, 2014. doi: 10.4239/wjd.v5.i3.385
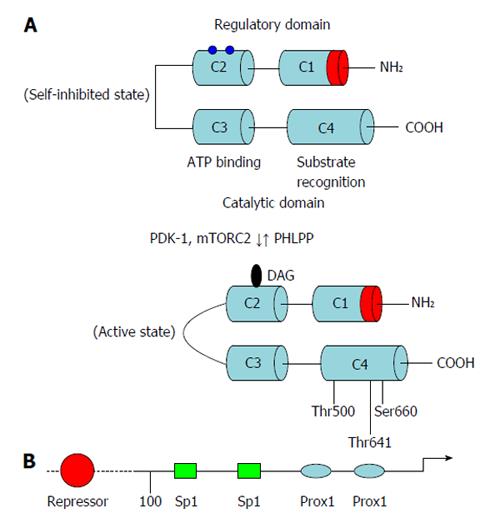
Figure 1 Domain composition of protein kinase C-β and its regulation at the transcriptional and posttranscriptional levels.
A: Membrane-targeting modules (C1 and C2), pleckstrin homology domain, the pseudosubstrate region, the kinase core and the C-terminal tail; B: Schematic representation of promoter structure of protein kinase C-β gene. Approximate locations of known regulatory regions are indicated. ATP: Adenosine-5’-triphosphate; PHLPP: PH domain and leucine rich repeat protein phosphatases; PDK-1: 3-phosphoinositide-dependent protein kinase 1.
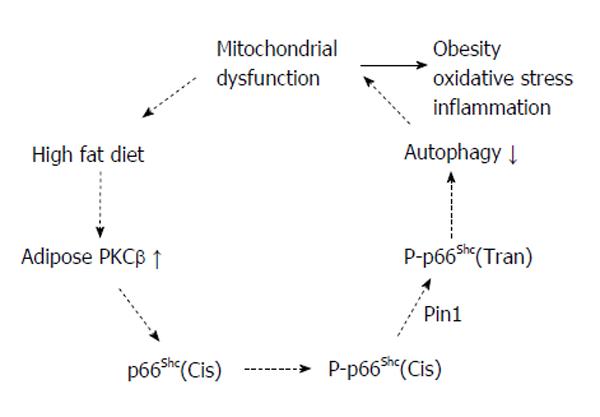
Figure 2 Proapoptotic signals, including reactive oxygen species, activate protein kinase C-β, which in turn phosphorylates p66Shc at serine 36.
Phosphorylated p66Shc translocates to the inner mitochondrial membrane and acts as a redox enzyme to amplify oxidative stress by generating H2O2. Increased H2O2, in turn, causes opening of the mitochondrial permeability transition pore and apoptosis. Protein kinase C-β (PKCβ) activated by reactive oxygen species further induces p66Shc phosphorylation. This event in turn perturbs mitochondria structure and function.
- Citation: Mehta KD. Emerging role of protein kinase C in energy homeostasis: A brief overview. World J Diabetes 2014; 5(3): 385-392
- URL: https://www.wjgnet.com/1948-9358/full/v5/i3/385.htm
- DOI: https://dx.doi.org/10.4239/wjd.v5.i3.385