Copyright
©2011 Baishideng Publishing Group Co.
World J Diabetes. Nov 15, 2011; 2(11): 176-188
Published online Nov 15, 2011. doi: 10.4239/wjd.v2.i11.176
Published online Nov 15, 2011. doi: 10.4239/wjd.v2.i11.176
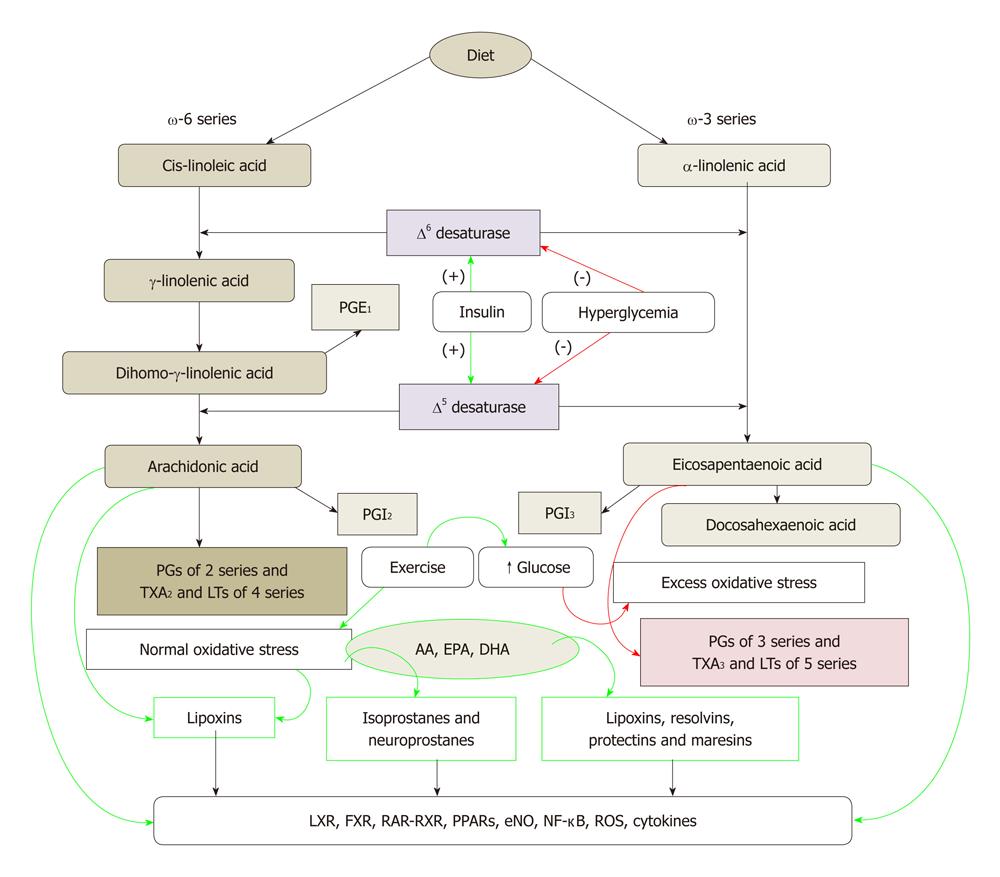
Figure 1 Metabolism of essential fatty acids and their modulation by insulin, glucose, exercise and oxidative stress.
Calorie restriction increases the activity of Δ6 and Δ5 desaturases. High-fat diet, trans-fats and cholesterol block the activities of Δ6 and Δ5 desaturases. (-): Inhibition of synthesis or action; (+): Enhancement of synthesis or action. Green arrows indicate beneficial action and/or anti-inflammation whereas red arrows indicate harmful action and/or pro-inflammation. PG: Pro-inflammatory prostaglandin; AA: Arachidonic acid; EPA: Eicosapentaenoic acid; DHA: Docosahexaenoic acid; LTs: Leukotrienes.
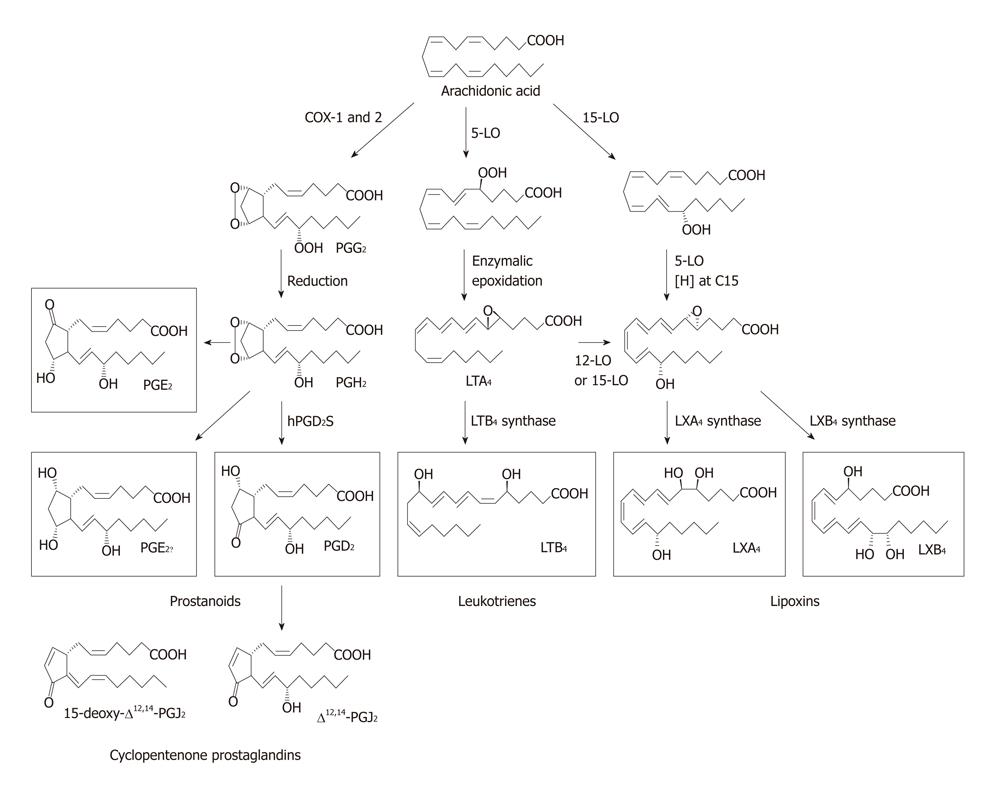
Figure 2 Metabolism of arachidonic acid showing different metabolites formed from it.
LO: Lipoxygenase; LXs: Lipoxins; LTs: Leukotrienes; PG: Pro-inflammatory prostaglandin.
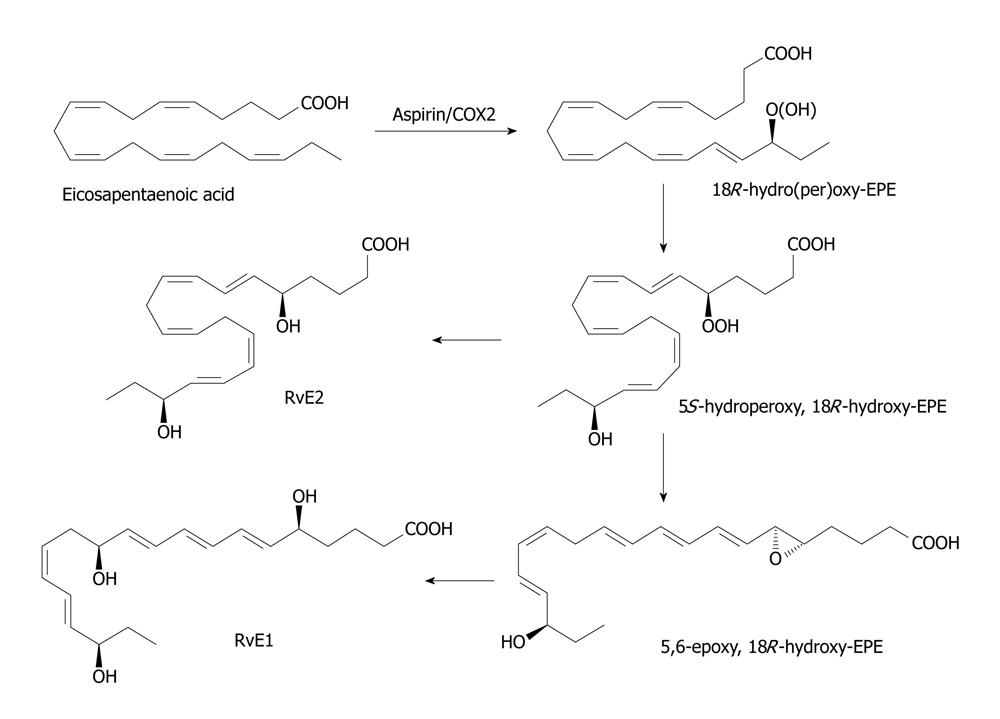
Figure 3 Scheme showing the formation of resolvin E derived from eicosapentaenoic acid.
In the endothelial cells, the cyclo-oxygenase (COX)-2 enzyme that has been acetylated introduces an 18R hydroperoxy-group into the eicosapentaenoic acid molecule (c.f. the role of aspirin in the biosynthesis of the epi-lipoxins). This is reduced to the corresponding hydroxy compound before a 5S-hydroperoxy group is introduced into the molecule by the action of 5-lipoxygenase as in the biosynthesis of leukotrienes. A further reduction step produces 15S,18R-dihydroxy-EPE or resolvin E2. Alternatively, the 5S-hydrpperoxy, 18R-hydroxy-EPE intermediates is converted to a 5,6-epoxy fatty acids in polymorphonuclear leukocytes I humans and eventually to 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eiocsapentaenoic acid or resolvin E1 by process similar to the formation of leukotrienes in leukocytes.
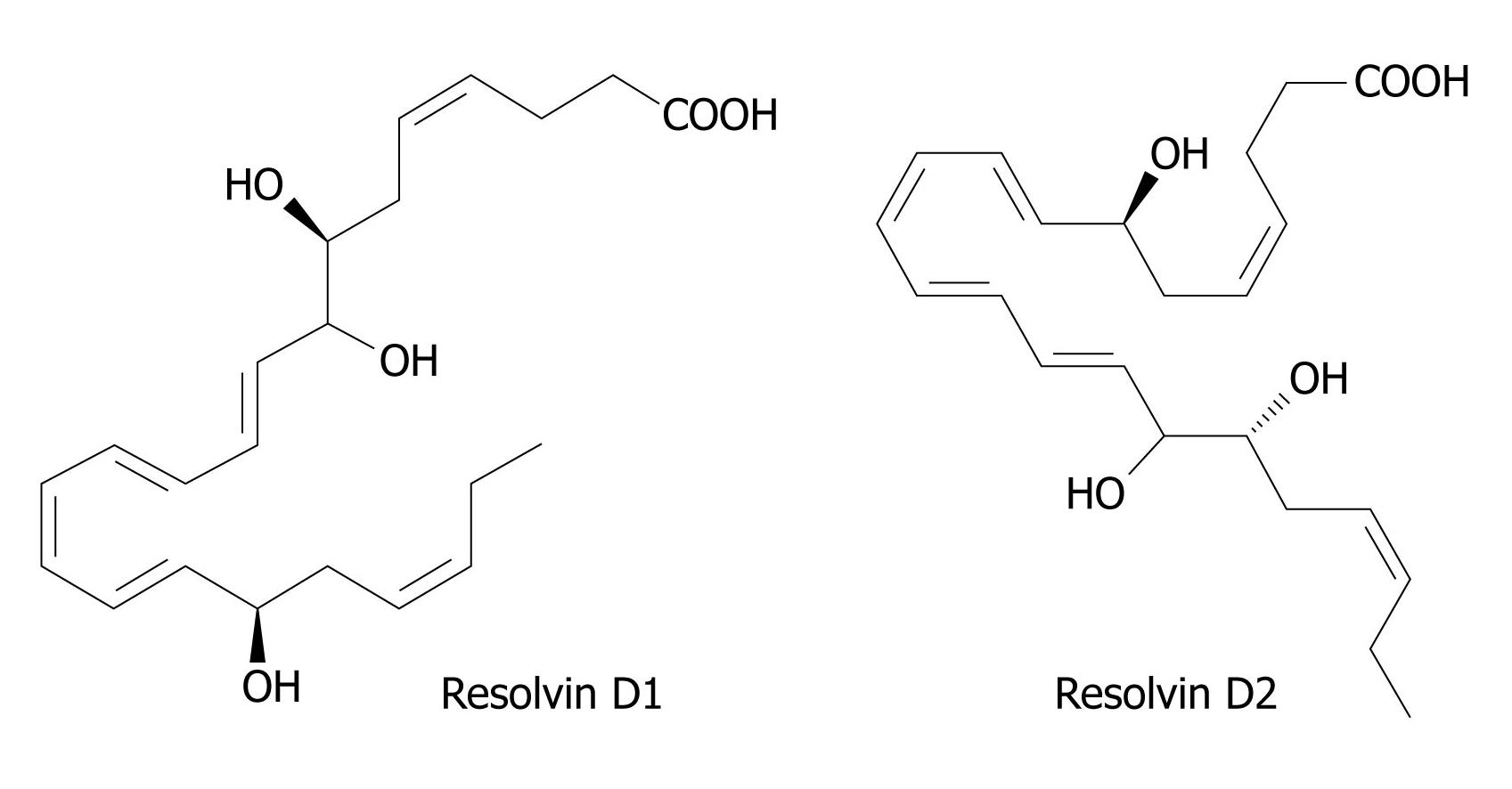
Figure 4 Structures of Resolvin D1 and D2.
DHA is converted to 17R-resolvins by a similar aspirin-triggered mechanism similar to the scheme shown in Figure 2. In the absence of aspirin, COX-2 of endothelial cells converts DHA to 13S-hydroxy-DHA. In the presence of aspirin, the initial product is 17R-hydroxy-DHA, which is converted to 7S-hydroperoxy, 17R-hydroxy-DHA by the action of a lipoxygenase, and thence via an epoxy intermediate to epimeric resolvins D1 and D2. An alternative lipoxygenase-generated intermediate, 4S-hydroperoxy, 17R-hydroxy-DHA, is transformed via an epoxide to epimeric resolvins D3 and D4. 17S Resolvins of the D series are produced in cells in the absence of aspirin by a reaction catalyzed in the first step by a lipoxygenase. COX: Cyclo-oxygenase; DHA: Docosahexaenoic acid.
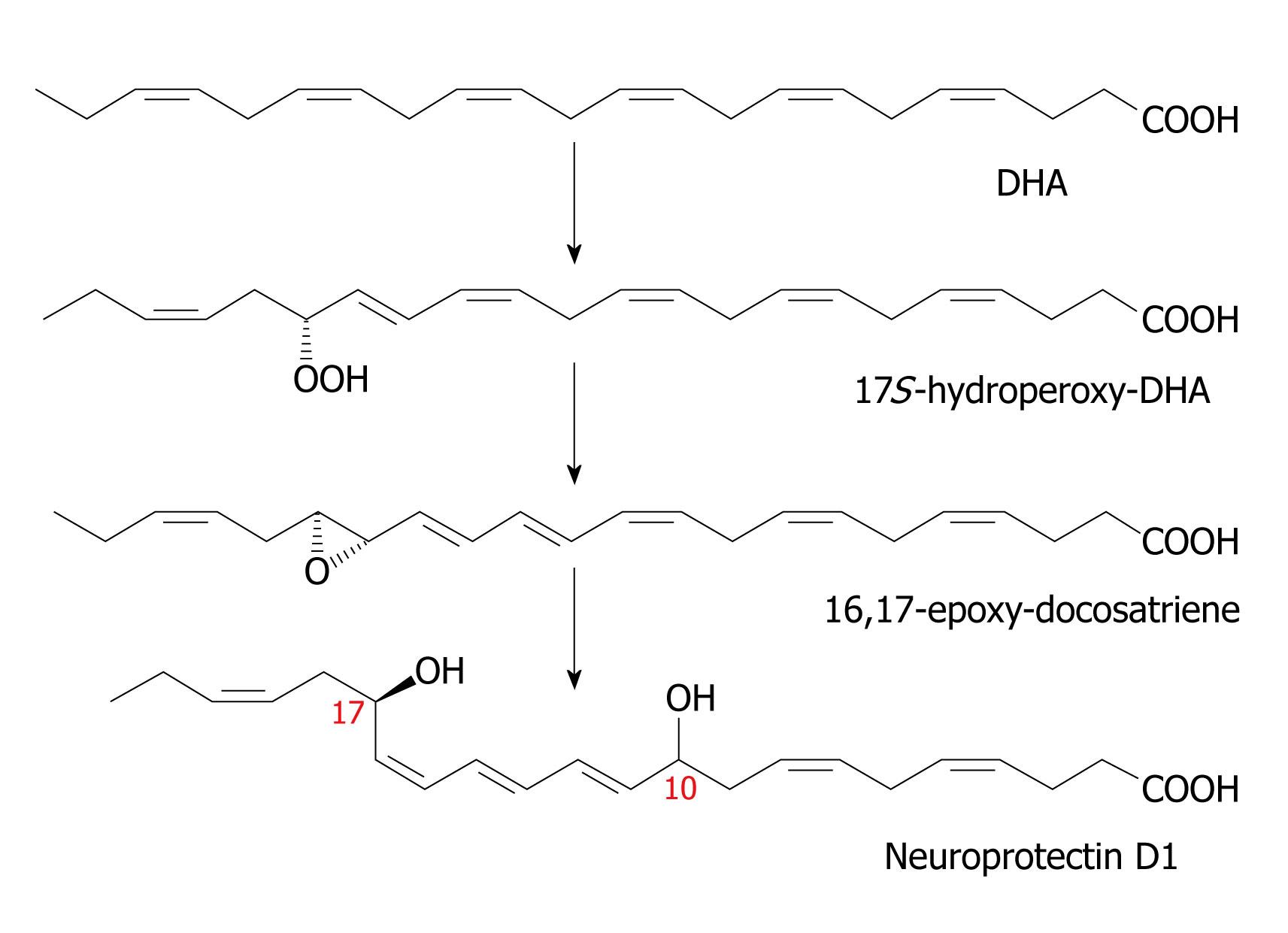
Figure 5 Scheme showing the synthesis of neuroprotectin D1.
Resolvins are generated in brain tissue in response to aspirin treatment and in addition, docosatrienes termed neuroprotectins are also produced. The lipoxygenase product 17S-hydroperoxy-DHA is converted first to a 16(17)-epoxide and then to the 10, 17-dihydroxy docosatriene denoted as 10, 17 S-DT or NPD1. As with the leukotrienes, there are three double bonds in conjugation, hence the term “triene”, although there are six double bonds in total. Figures 2-5 are from[27,31,32,64,119]. DHA: Docosahexaenoic acid.
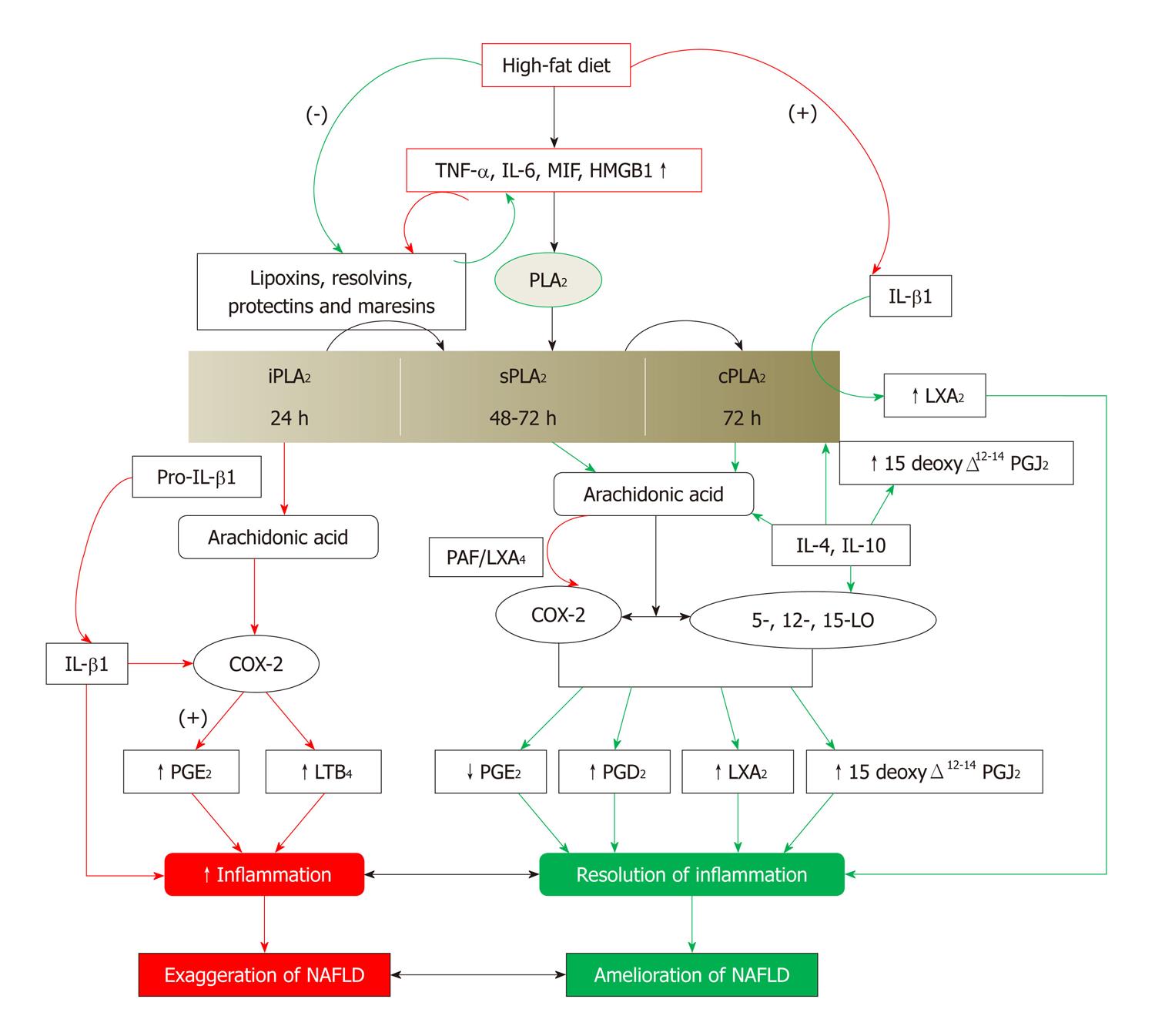
Figure 6 Scheme showing the role of eicosanoids, lipoxins, resolvins, protectins and maresins in resolution of non-alcoholic fatty liver disease.
Green arrows indicate events that will lead to resolution of non-alcoholic fatty liver disease (NAFLD). Red arrows indicate events that will lead to initiation and/or progression of NAFLD. (-): Inhibition or suppression of action; (+): Activation or enhancement of action; TNF-α: Tumor necrosis factor-α; LXs: Lipoxins; COX: Cyclo-oxygenase; LO: Lipoxygenase; PG: Pro-inflammatory prostaglandin. For further details see[82-90,119].
- Citation: Das UN. A defect in the activities of Δ6 and Δ5 desaturases and pro-resolution bioactive lipids in the pathobiology of non-alcoholic fatty liver disease. World J Diabetes 2011; 2(11): 176-188
- URL: https://www.wjgnet.com/1948-9358/full/v2/i11/176.htm
- DOI: https://dx.doi.org/10.4239/wjd.v2.i11.176