Copyright
©The Author(s) 2025.
World J Diabetes. Jun 15, 2025; 16(6): 105709
Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.105709
Published online Jun 15, 2025. doi: 10.4239/wjd.v16.i6.105709
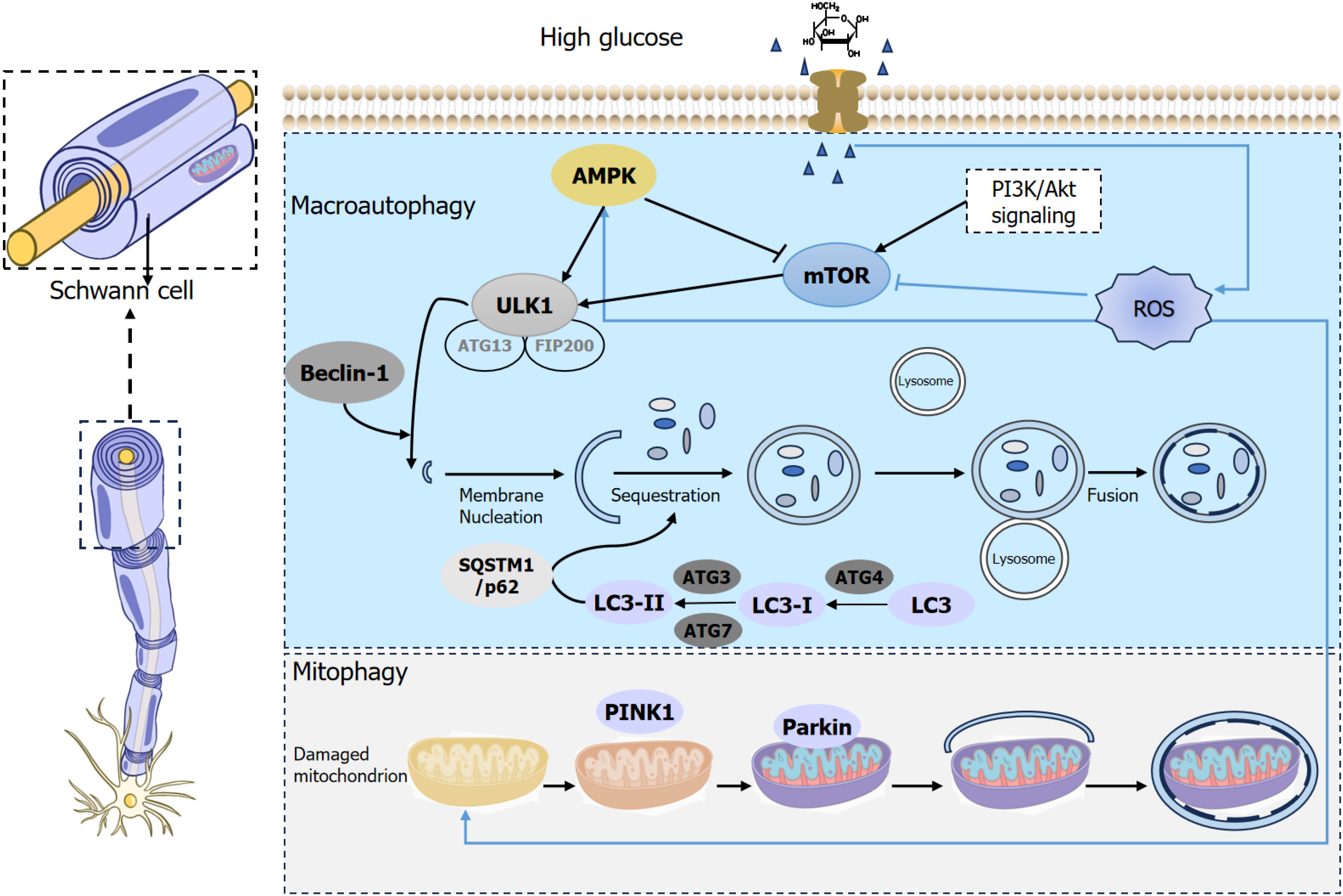
Figure 1 Autophagic imbalance leads to Schwann cell dysfunction.
High glucose-induced oxidative stress triggers reactive oxygen species release, activates protein kinase B via phosphatidylinositol 3-kinase, inhibits tuberous sclerosis complex, and promotes mammalian target of rapamycin phosphorylation. Subsequently mRNA translation is enhanced and autophagosome formation is inhibited. Lysosomal damage induces transforming growth factor-β-activated protein kinase 1-galectin-9 binding, which activates adenosine monophosphate-activated protein kinase and unc-51-like kinase 1 to promote autophagy. Mitochondrial damage under high glucose leads to phosphatase and tensin homolog induced putative kinase 1 accumulation and parkin phosphorylation. AMPK: Adenosine monophosphate-activated protein kinase; mTOR: Mammalian target of rapamycin; PI3K: Phosphatidylinositol 3-kinase; ULK1: Unc-51-like kinase 1; ATG13: Autophagy related 13; FIP200: Focal adhesion kinase family kinase-interacting protein 200kD; ROS: Reactive oxygen species; SQSTM1: Sequestosome 1; LC3: Light chain 3; PINK1: Phosphatase and tensin homolog-induced putative kinase 1.
- Citation: Xing QC, Chen J, Liu Z, Li WC, Liu X, Li W. Autophagy in Schwann cells: A potential pharmacotherapeutic target in diabetic peripheral neuropathy. World J Diabetes 2025; 16(6): 105709
- URL: https://www.wjgnet.com/1948-9358/full/v16/i6/105709.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i6.105709