Copyright
©The Author(s) 2025.
World J Diabetes. Mar 15, 2025; 16(3): 92003
Published online Mar 15, 2025. doi: 10.4239/wjd.v16.i3.92003
Published online Mar 15, 2025. doi: 10.4239/wjd.v16.i3.92003
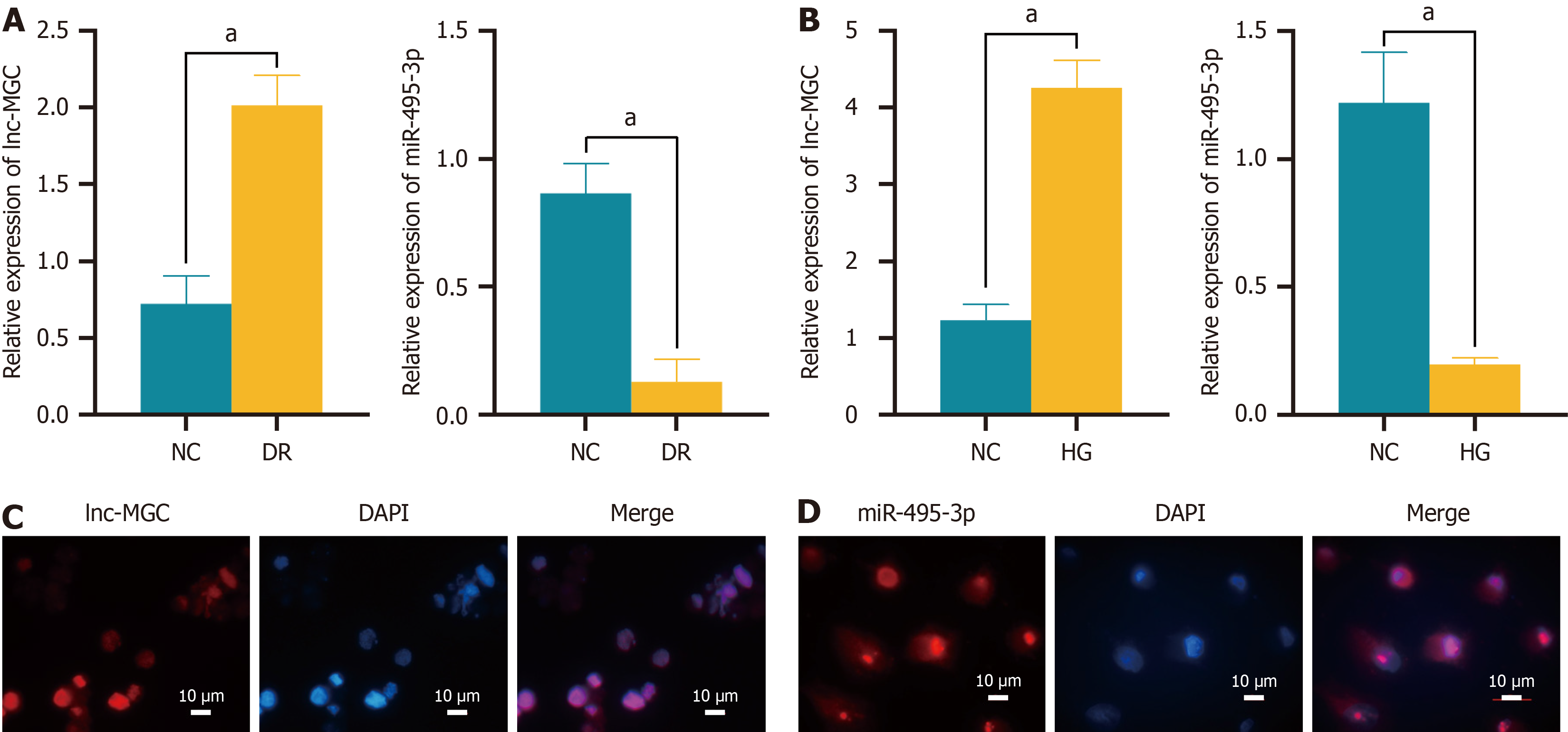
Figure 1 Differential expression of lnc-MGC and miR-495-3p in the diabetic retinopathy rat retina and cell model.
A: Detection of lnc-MGC and miR-495-3p expression in diabetic retinopathy rat retina tissue by RT-qPCR; B: Expression levels of lnc-MGC and miR-495-3p in the cell model were detected by RT-qPCR; C: Localization of lnc-MGC in ARPE-19 cells was detected by fluorescence in situ hybridization (FISH). The scale bar represents 10 μm; D: Localization of miR-495-3p in ARPE-19 cells was detected by FISH. The scale bar represents 10 μm. aP < 0.001. NC: Normal control; DR: Diabetic retinopathy; HG: High glucose.
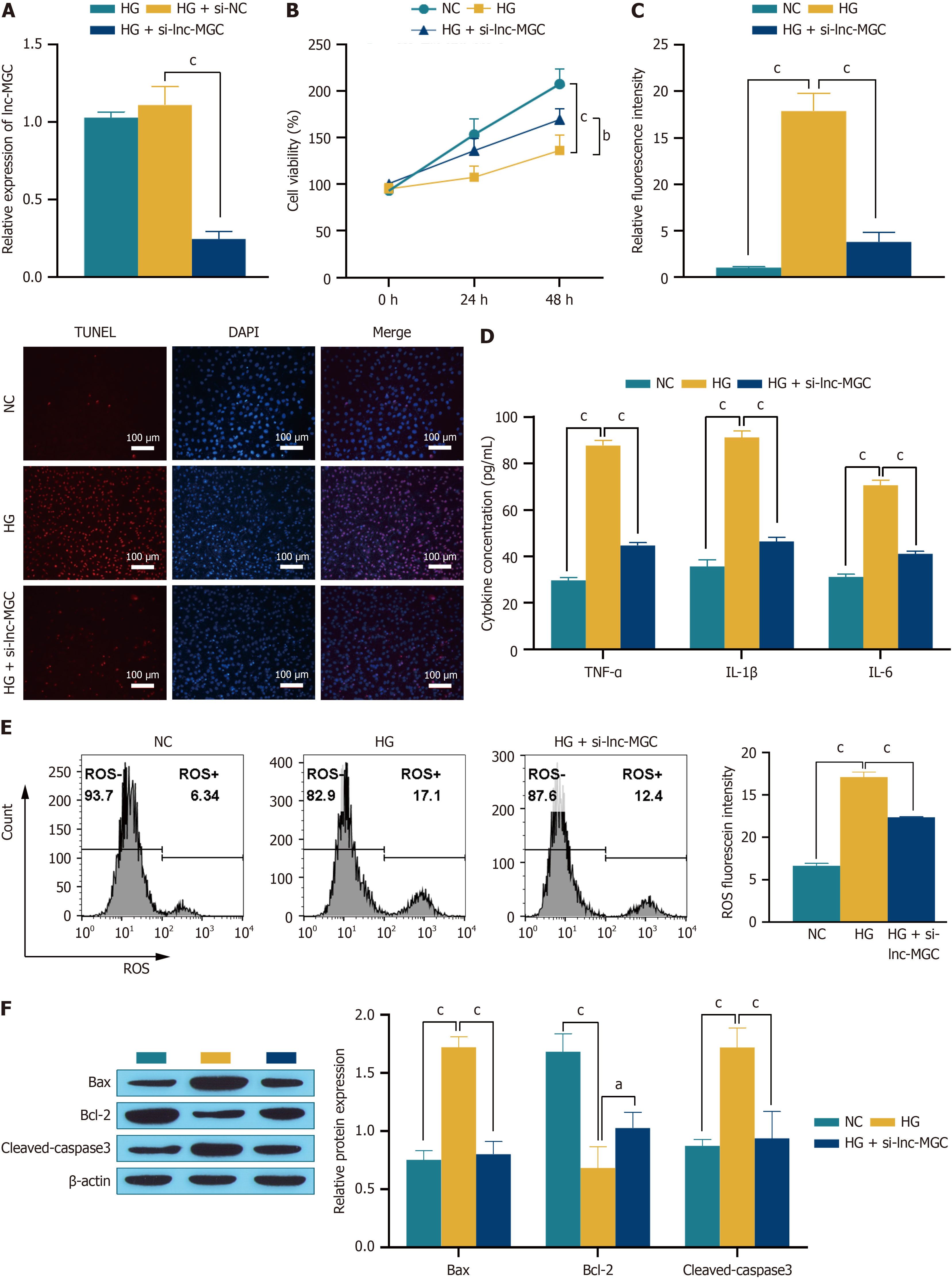
Figure 2 Inhibition of lnc-MGC protects retinal pigment epithelial cells.
A: RT-qPCR was used to detect the transfection efficiency; B: CCK-8 was used to detect cell proliferation activity; C: TUNEL was used to detect cell apoptosis. The scale bar represents 100 μm; D: ELISA was used to detect the expression of the inflammatory factors TNF-α, IL-6 and IL-1β; E: Flow cytometry was used to detect reactive oxygen species; F: Western blotting was used to detect the expression of the apoptosis-related proteins Bax, Bcl-2 and cleaved-caspase 3. aP < 0.05, bP < 0.01, and cP < 0.001. NC: Normal control; HG: High glucose; ROS: Reactive oxygen species.
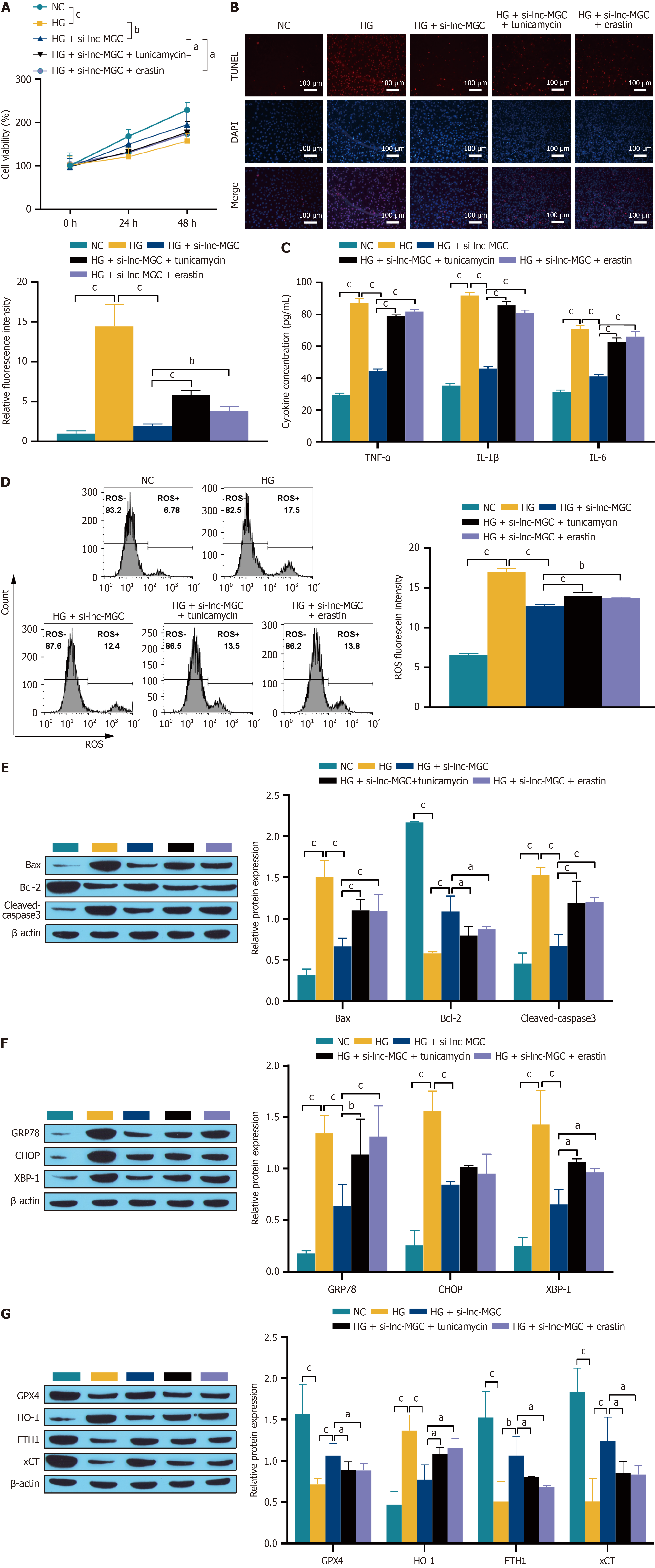
Figure 3 Downregulation of lnc-MGC can mediate the protective effect of retinal pigment epithelial cells through ER stress and ferroptosis.
A: CCK-8 detection of cell proliferation activity; B: TUNEL detection of cell apoptosis. The scale bar represents 100 μm; C: ELISA detection of the expression of the inflammatory cytokines TNF-α, IL-1β and IL-6; D: Flow cytometry detection of reactive oxygen species; E: Western blotting was used to detect the expression of the apoptosis-related proteins Bax, Bcl-2 and cleaved-caspase 3; F: Western blotting was used to detect the expression of the ER-related proteins GRP78, CHOP and XBP-1; G: Western blotting was used to detect the expression of the ferroptosis-associated proteins GPX4, HO-1, FTH1, and xCT. aP < 0.05, bP < 0.01, and cP < 0.001. NC: Normal control; HG: High glucose; ROS: Reactive oxygen species.
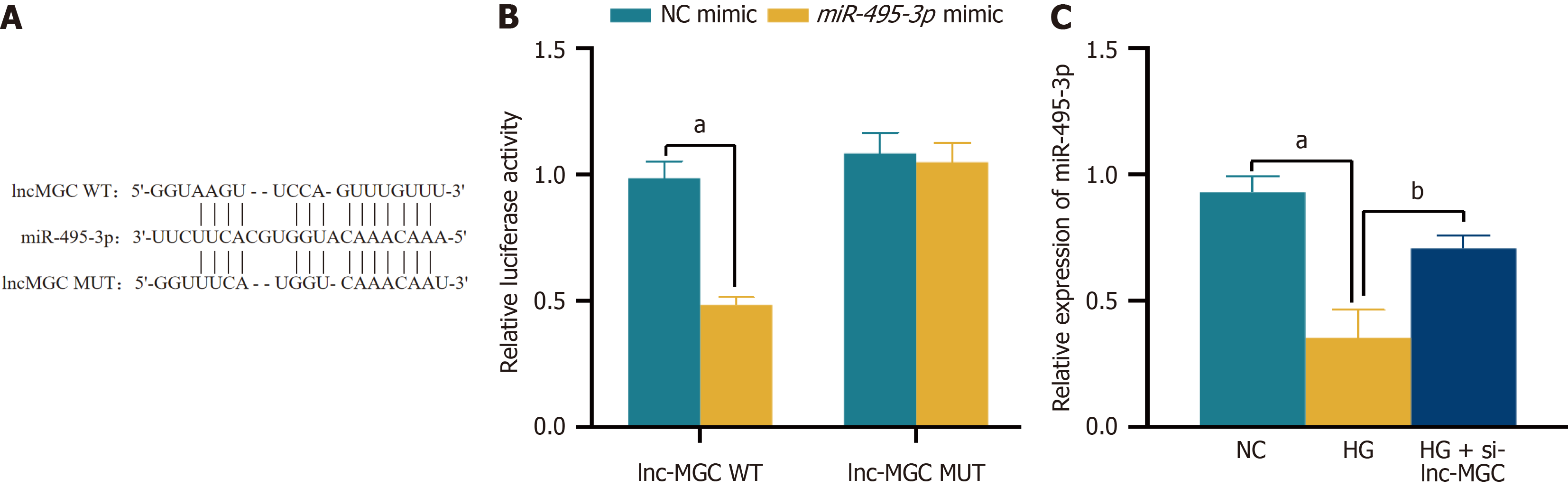
Figure 4 Correlation between lnc-MGC and miR-495-3p.
A: The StarBase was used to predict the binding sites of lnc-MGC and miR-495-3p; B: Dual-luciferase gene analysis was used to verify the targeting binding relationship between lnc-MGC and miR-495-3p; C: The expression of miR-495-3p was detected by RT-qPCR. aP < 0.001, bP < 0.01. WT: Wild type; MUT: Mutation; NC: Normal control; HG: High glucose.
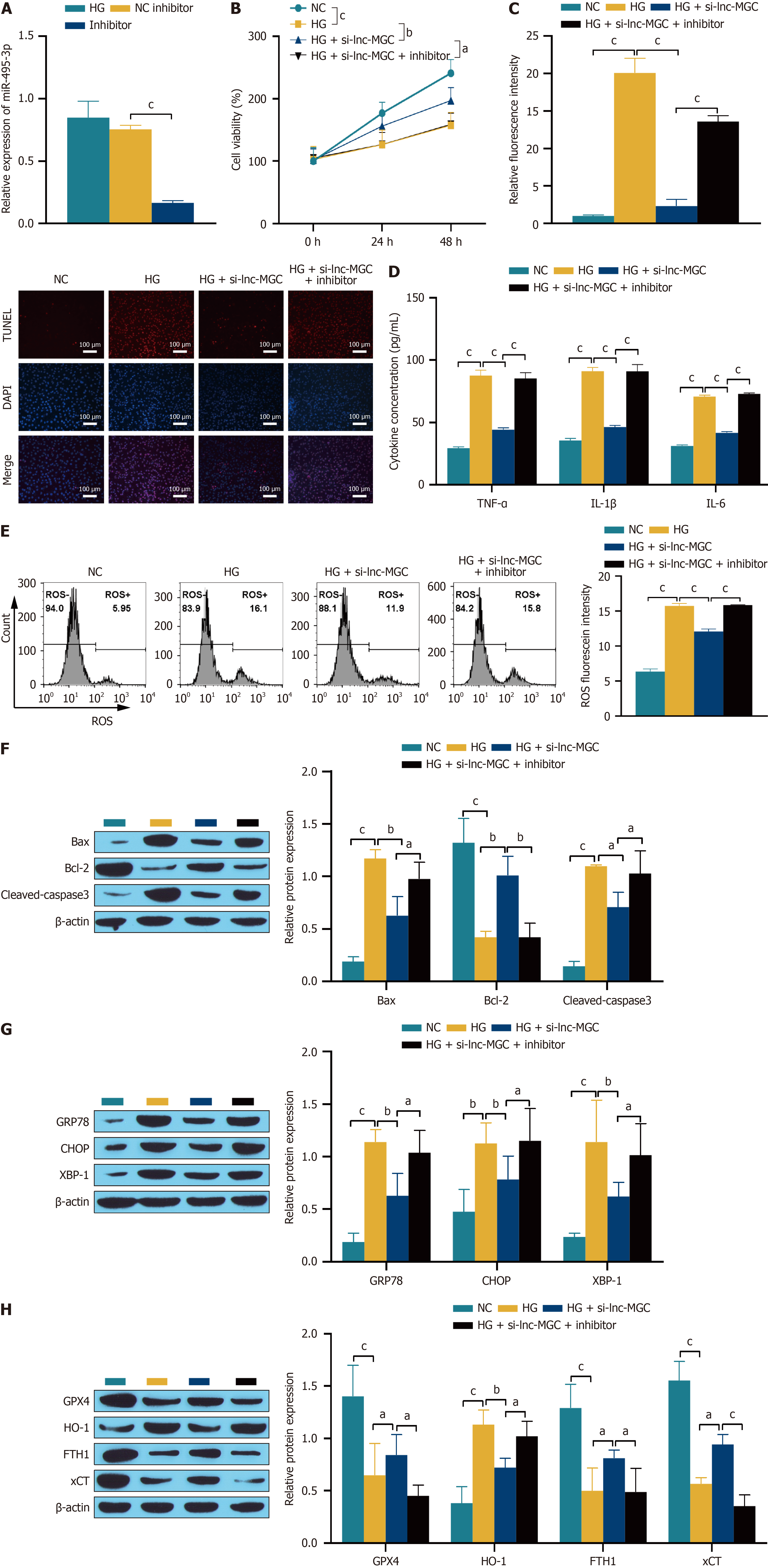
Figure 5 lnc-MGC affects the progression of diabetic retinopathy through miR-495-3p.
A: Transfection efficiency was detected by RT-qPCR; B: Cell proliferation was detected by CCK-8 assay; C: Cell apoptosis was detected by TUNEL assay. The scale bar represents 100 μm; D: The expression of the inflammatory cytokines TNF-α, IL-1β, IL-6 was detected by ELISA; E: reactive oxygen species were detected by flow cytometry; F-H: Western blotting was used to detect the expression of related proteins. aP < 0.05, bP < 0.01, and cP < 0.001. Inhibitor: MiR-495-3p inhibitor; NC: Normal control; HG: High glucose; ROS: Reactive oxygen species.
Figure 6 miR-495-3p targets GRP78.
A: The TargetScan was used to predict binding sites for miR-495-3p and GRP78; B: Dual-luciferase verification that miR-495-3p targets GRP78. aP < 0.01. WT: Wild type; MUT: Mutation; NC mimic: Negative control mimic.
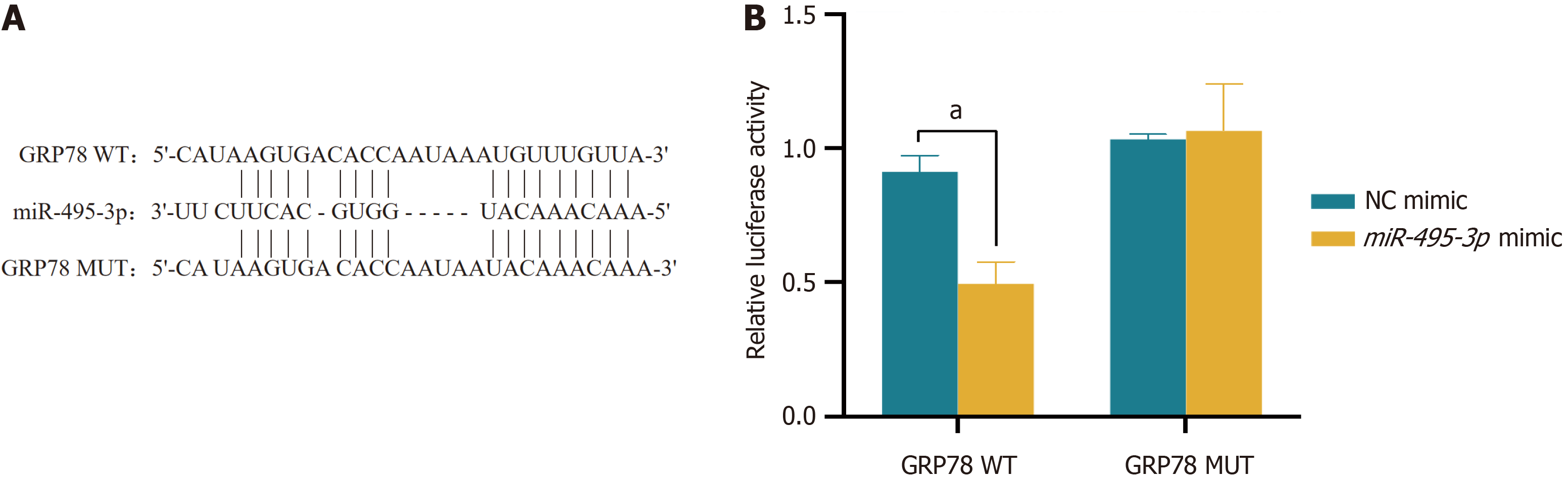
Figure 7 miR-495-3p regulates endoplasmic reticulum stress and ferroptosis through GRP78.
A: RT-qPCR was used to detect transfection efficiency; B: Western blot was used to detect transfection efficiency; C: CCK-8 was used to detect cell proliferation activity; D: A TUNEL assay was used to detect apoptosis. The scale bar represents 100 μm; E: The expression of TNF-α, IL-1β and IL-6 was detected by ELISA; F: Reactive oxygen species were detected by flow cytometry; G-I: Western blotting was used to detect the expression of related proteins. aP < 0.05, bP < 0.01, and cP < 0.001. NC: Normal control; HG: High glucose; ROS: Reactive oxygen species; mimic: miR-495-3p mimic.
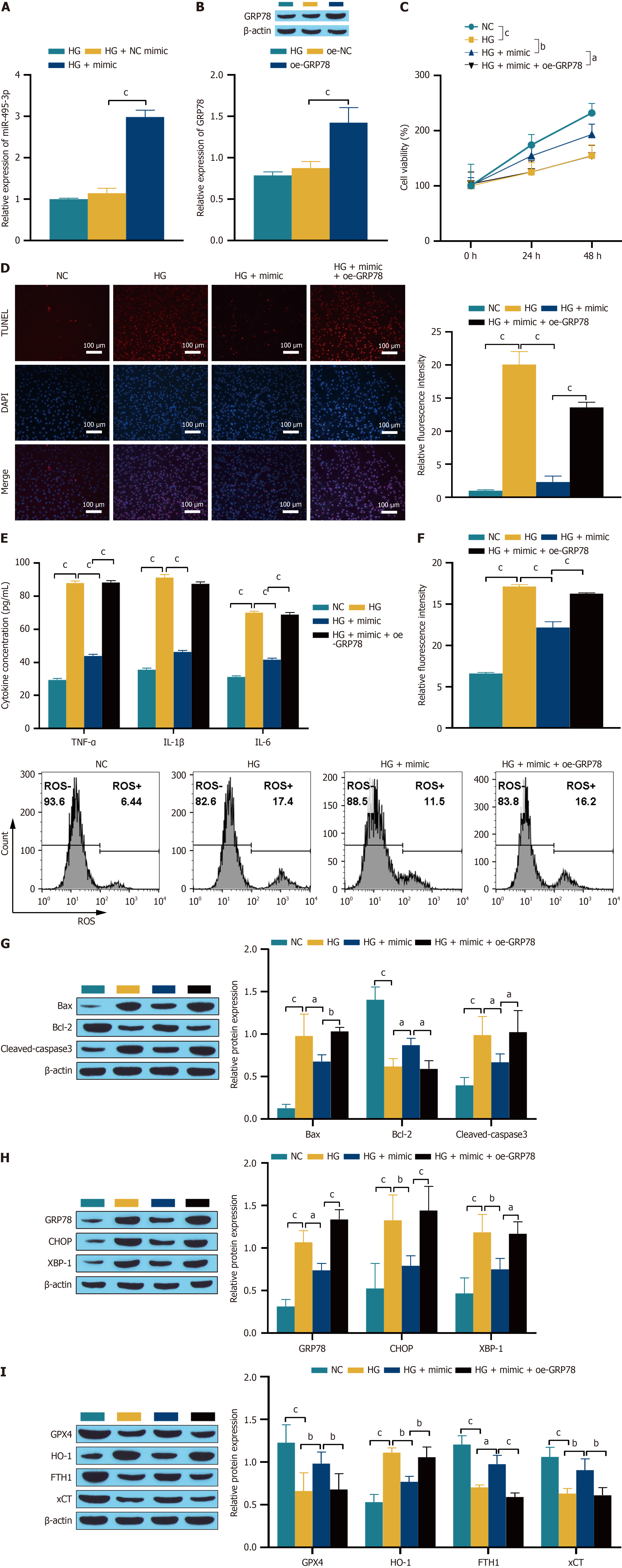
Figure 8 LEF1, as the binding protein of lnc-MGC, regulates the progression of diabetic retinopathy.
A and B: LEF1 can bind to the lnc-MGC promoter; C: Western blotting was used to detect the inhibition efficiency of LEF1; D: RT-qPCR was used to detect the transfection efficiency; E: CCK-8 was used to measure the cell proliferation activity; F: TUNEL was used to detect cell apoptosis. The scale bar represents 100 μm; G: ELISA was used to determine the expression of the inflammatory cytokines TNF-α, IL-1β and IL-6; H: Flow cytometry was used to detect reactive oxygen species; I-K: Western blotting was used to detect the expression of related proteins. aP < 0.05, bP < 0.01, and cP < 0.001. NC: Normal control; HG: High glucose; ROS: Reactive oxygen species.
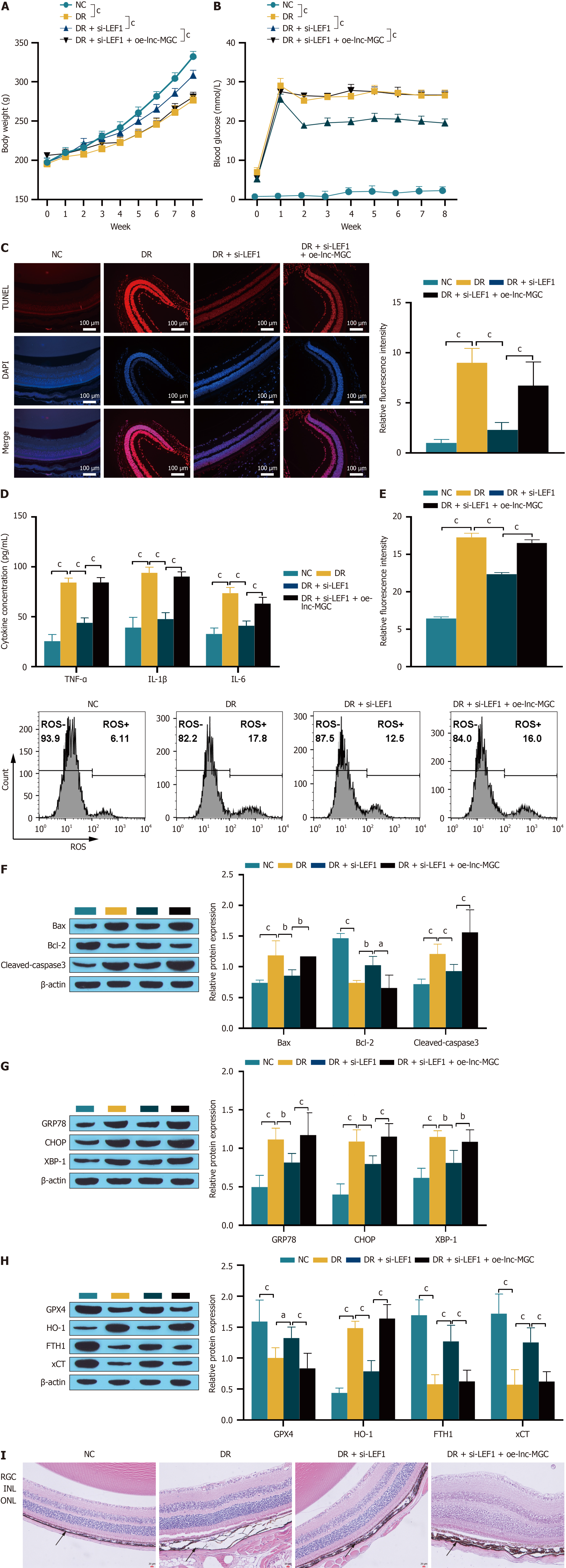
Figure 9 Animal experiments to verify that LEF1 combined with lnc-MGC regulates diabetic retinopathy progression.
A and B: Diabetic retinopathy rat body weight and blood glucose detection; C: A TUNEL assay was used to detect apoptosis. The scale bar represents 100 μm; D: ELISA was used to detect the expression of the inflammatory factors TNF-α, IL-1β and IL-6; E: Reactive oxygen species were detected by flow cytometry; F-H: Western blotting was used to detect the expression of related proteins; I: HE staining was used to observe the retinal structure. The scale bar represents 20 μm. aP < 0.05, bP < 0.01, and cP < 0.001. ONL: Outer nuclear layer; INL: Inner core layer; RGC: retinal ganglion cells; NC: Normal control; DR: Diabetic retinopathy; ROS: Reactive oxygen species.
- Citation: Luo YY, Ba XY, Wang L, Zhang YP, Xu H, Chen PQ, Zhang LB, Han J, Luo H. LEF1 influences diabetic retinopathy and retinal pigment epithelial cell ferroptosis via the miR-495-3p/GRP78 axis through lnc-MGC. World J Diabetes 2025; 16(3): 92003
- URL: https://www.wjgnet.com/1948-9358/full/v16/i3/92003.htm
- DOI: https://dx.doi.org/10.4239/wjd.v16.i3.92003