Copyright
©The Author(s) 2024.
World J Diabetes. Feb 15, 2024; 15(2): 287-304
Published online Feb 15, 2024. doi: 10.4239/wjd.v15.i2.287
Published online Feb 15, 2024. doi: 10.4239/wjd.v15.i2.287
Figure 1 The expression of differential proteins in the hypothalamus after duodenal jejunal bypass intervention.
A: Heat map of the expression of the 120 DEPs between the T [type 2 diabetes mellitus (T2DM)-Sham] rats and D [T2DM-duodenal jejunal bypass (DJB)] rats. The colored column represents the sample number, the row name indicates the DEGs, each rectangle in the graph corresponds to the expression value of a sample, red indicates high expression and blue indicates low expression; B: Statistics of significantly enriched Kyoto encyclopedia of genes and genomes pathways (T2DM-Sham vs T2DM-DJB); C: GO term statistics for significantly enriched genes (T2DM-Sham vs T2DM-DJB).
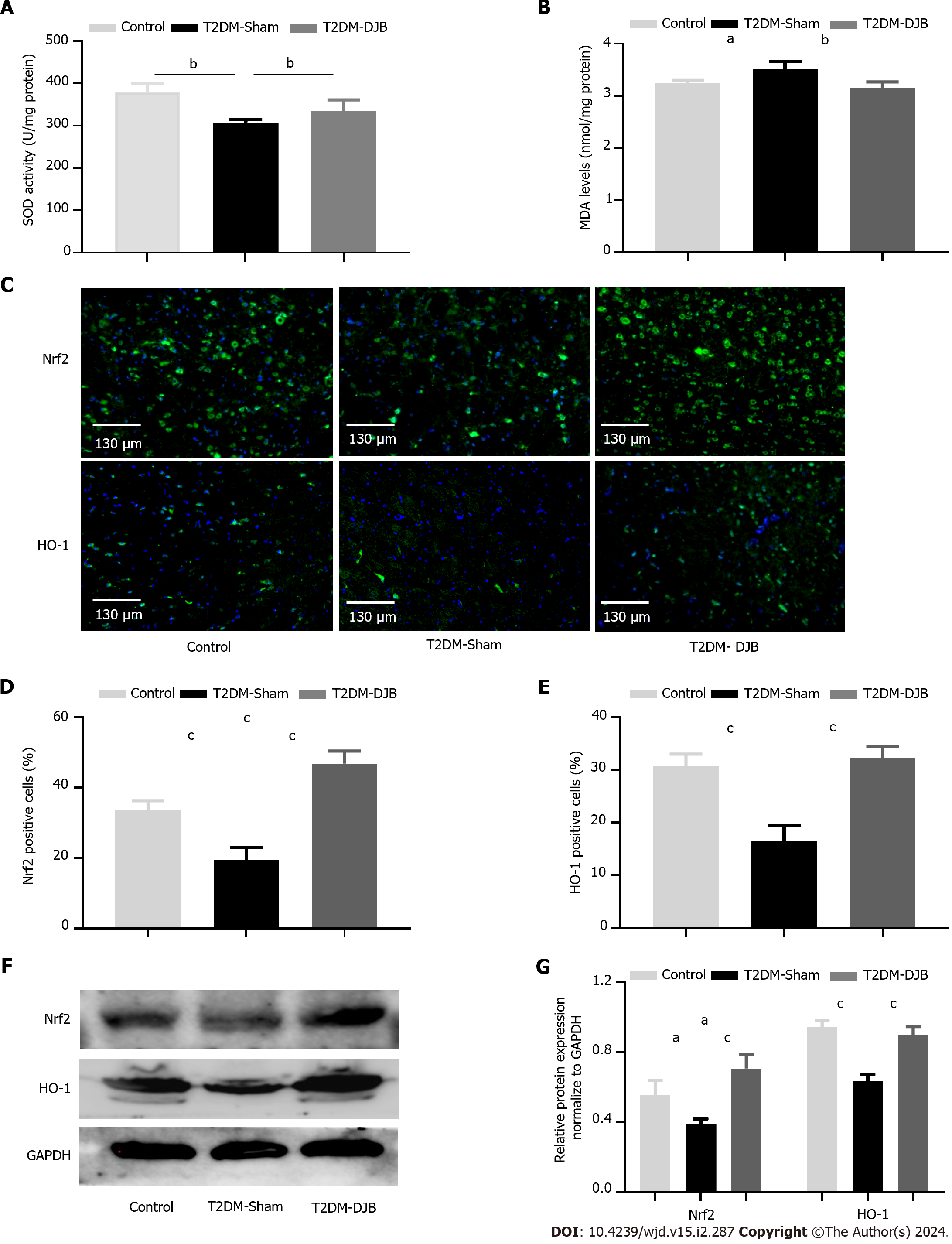
Figure 2 Duodenal jejunal bypass surgery inhibits hypothalamic oxidative stress in rats with type 2 diabetes mellitus by activating the Nrf2/HO-1 signaling pathway.
A: Analysis of SOD content in the hypothalamus of rats; B: Analysis of MDA content in the hypothalamus of rats; C: Nrf2 and HO-1 expression in the hypothalamus detected by immunofluorescence (scale bar, 130 µm); D: The percentage of cells expressing Nrf2; E: The percentage of cells expressing HO-1; F: Expression levels of Nrf2 and HO-1 in hypothalamus detected by Western blotting; G: The quantitative densitometric analysis of Nrf2 and HO-1. aP < 0.05, bP < 0.01, cP < 0.001. T2DM: Type 2 diabetes mellitus; DJB: Duodenal jejunal bypass.
Figure 3 Duodenal jejunal bypass surgery increases type 2 diabetes mellitus glucagon-like peptide 1 signals and enhances the expression of Nrf2/HO-1.
A: Serum glucagon-like peptide 1 (GLP-1) secretion of rats in the three groups; B: The quantitative real-time PCR results of GLP-1 receptor (GLP-1R) expression; C: Expression levels of GLP-1R in the hypothalamus detected by Western blotting; D: The quantitative densitometric analysis of GLP-1R. aP < 0.05, bP < 0.01, cP < 0.001. T2DM: Type 2 diabetes mellitus; DJB: Duodenal jejunal bypass; GLP-1: Glucagon-like peptide 1; GLP-1R: Glucagon-like peptide 1 receptor.
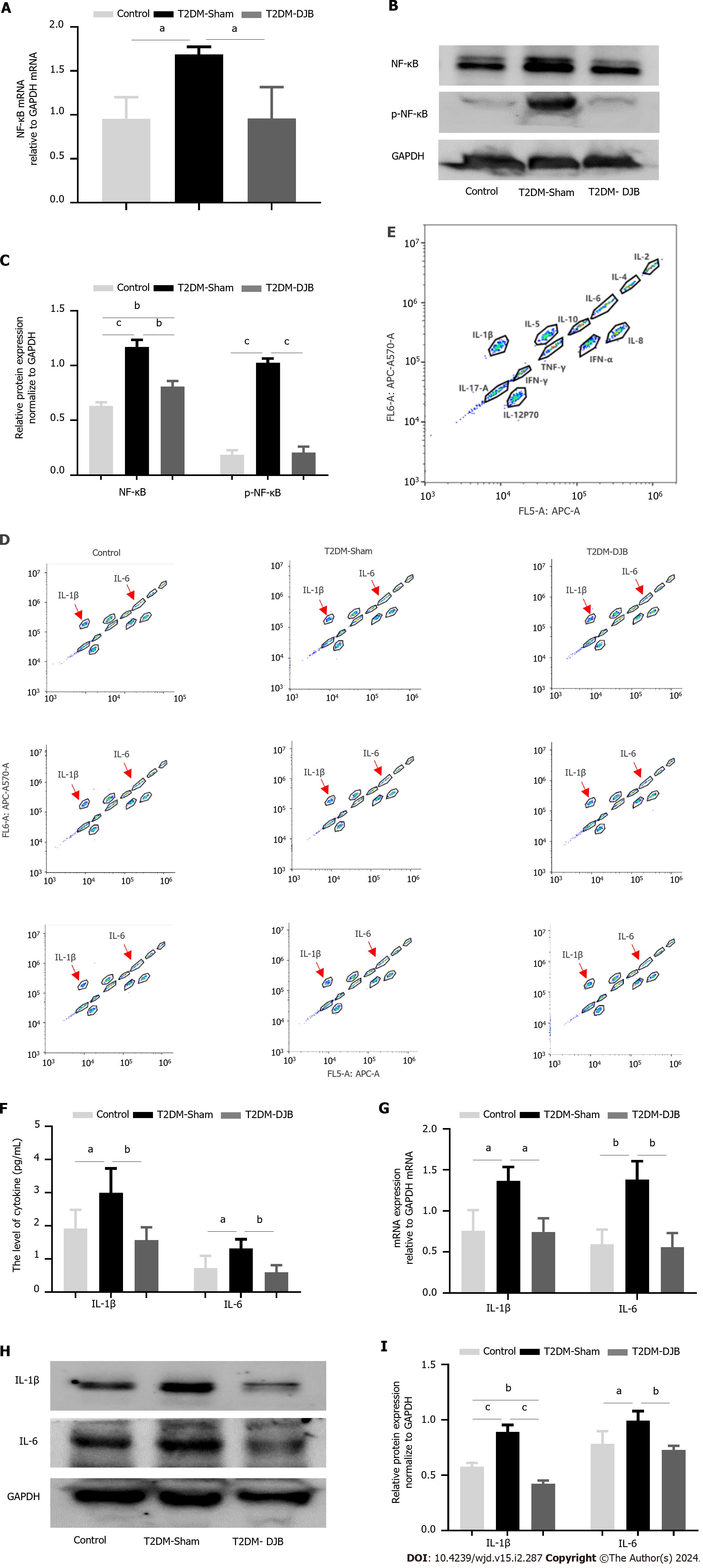
Figure 4 Duodenal jejunal bypass inhibits the production of proinflammatory cytokines by blocking the activation of NF-κB signaling in the hypothalamus of type 2 diabetes mellitus rats.
A: The quantitative real-time (qRT)-PCR results of NF-κB and p-NF-κB expression; B: The expression levels of NF-κB and p-NF-κB detected by Western blotting; C: The quantitative densitometric analysis of NF-κB and p-NF-κB; D: Flow cytometry results for standards; E: Flow cytometry results for samples; F: Flow cytometry results for the levels of proinflammatory cytokines interleukin (IL)-1β and IL-6; G: The qRT-PCR results of IL-1β and IL-6 expression; H: Expression levels of IL-1β and IL-6 detected by Western blotting; I: The quantitative densitometric analysis of IL-1β and IL-6. aP < 0.05, bP < 0.01, cP < 0.001. T2DM: Type 2 diabetes mellitus; DJB: Duodenal jejunal bypass; IL: Interleukin.
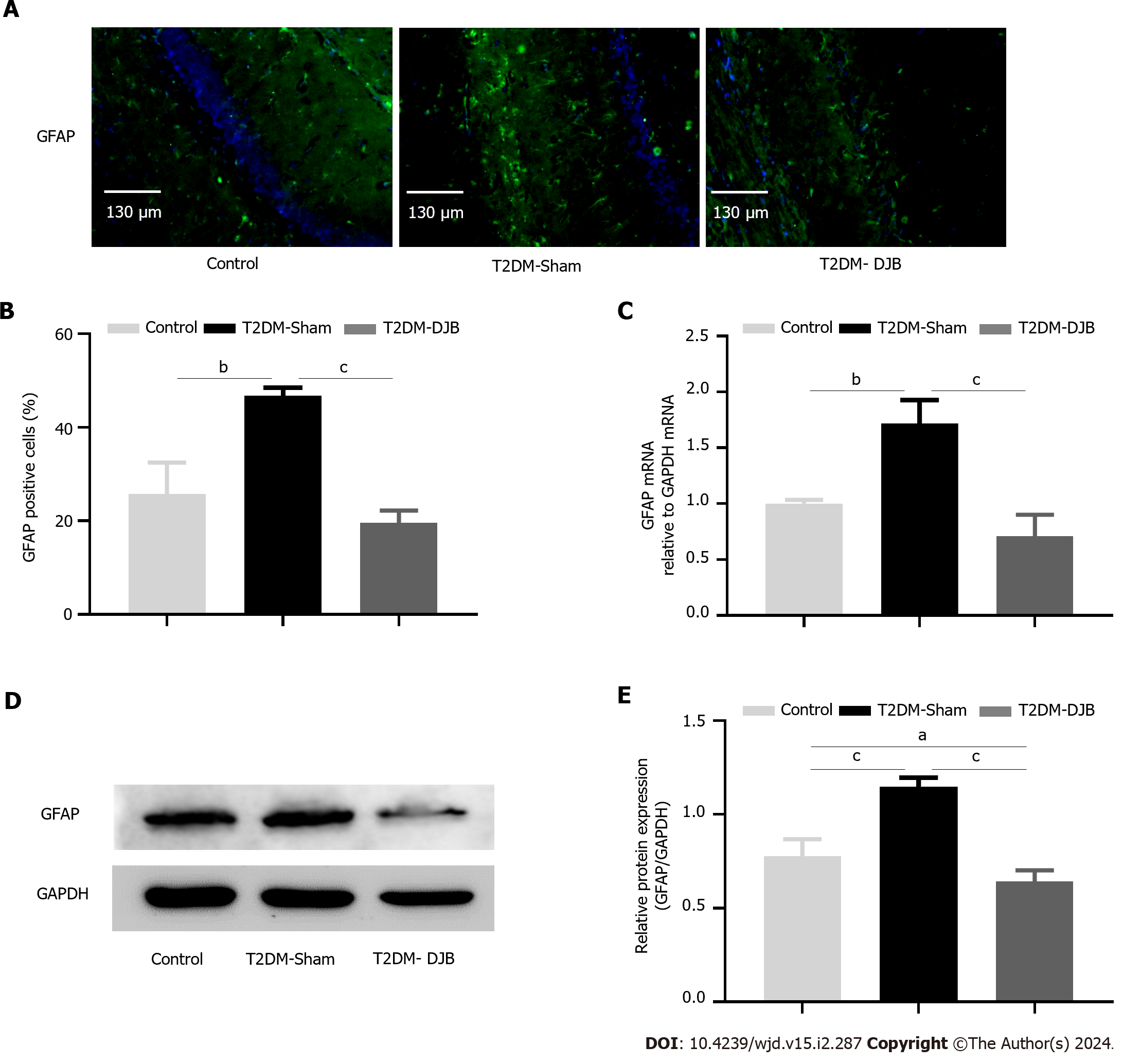
Figure 5 Duodenal jejunal bypass surgery improves hypothalamic cell injury induced by oxidative stress.
A: The expression of glial fibrillary acidic protein (GFAP) in the hypothalamus determined by immunohistochemistry (scale bar, 130 µm); B: The percentage of cells expressing GFAP; C: The quantitative real-time PCR results of GFAP expression in the hypothalamus; D: Expression levels of GFAP detected by Western blotting; E: The quantitative densitometric analysis of GFAP. aP < 0.05, bP < 0.01, cP < 0.001. T2DM: Type 2 diabetes mellitus; DJB: Duodenal jejunal bypass; GFAP: Glial fibrillary acidic protein.
Figure 6 Duodenal jejunal bypass surgery improves diabetes-induced hypothalamic cell apoptosis in type 2 diabetes mellitus rats.
A: The quantitative real-time (qRT)-PCR results of Caspase-3 expression; B: The qRT-PCR results of Bcl-2 expression; C: Expression levels of apoptosis-related proteins in the hypothalamus by Western blotting; D: The quantitative densitometric analysis of apoptosis-related proteins. aP < 0.05, bP < 0.01, cP < 0.001. T2DM: Type 2 diabetes mellitus; DJB: Duodenal jejunal bypass.
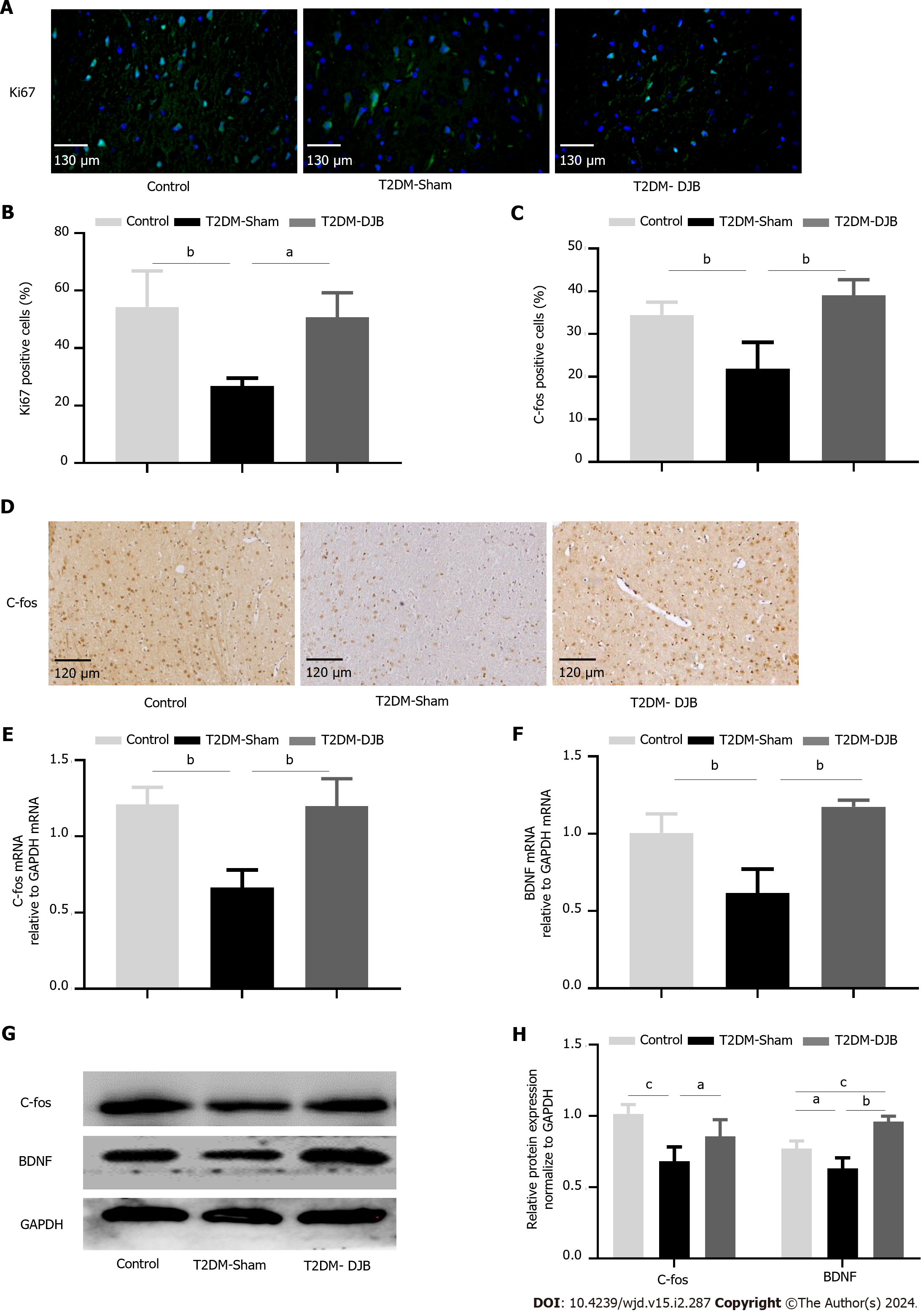
Figure 7 Duodenal jejunal bypass surgery promotes hypothalamic neurogenesis in type 2 diabetes mellitus rats.
A: Immunofluorescence analysis the expression of Ki67 (scale bar, 130 µm); B: The percentage of cells expressing Ki67; C: The percentage of cells expressing C-fos; D: Immunofluorescence analysis the expression of C-fos (scale bar, 120 µm); E: The quantitative real-time (qRT)-PCR results of C-fos expression; F: The qRT-PCR results of BDNF expression; G: Expression levels of C-fos and BDNF detected by Western blotting. The quantitative densitometric analysis of C-fos and BDNF. aP < 0.05, bP < 0.01, cP < 0.001. T2DM: Type 2 diabetes mellitus; DJB: Duodenal jejunal bypass.
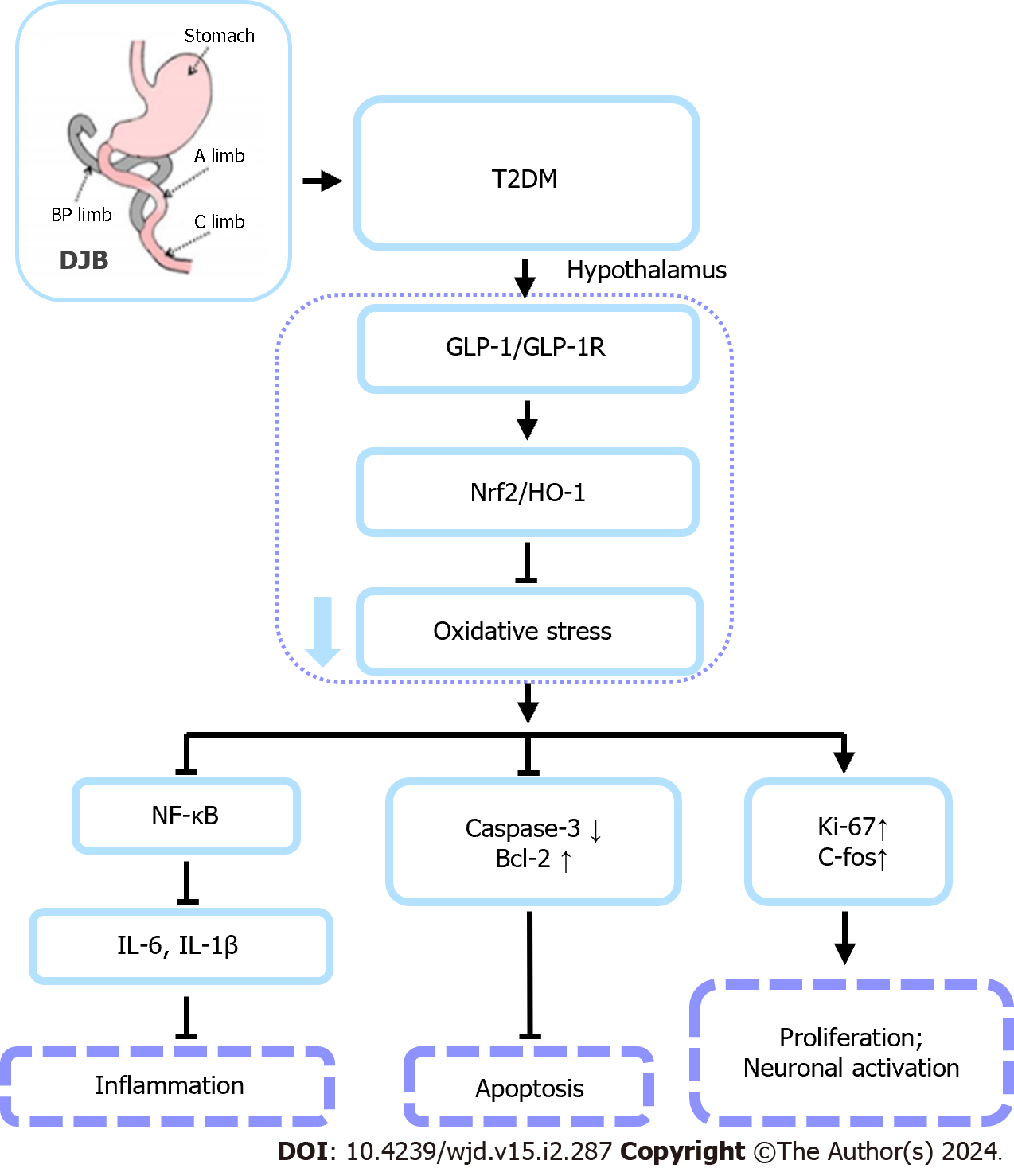
Figure 8 A schema summarizing the protective effects of duodenal jejunal bypass surgery on hypothalamic cell injury induced by diabetes.
Duodenal jejunal bypass (DJB) surgery significantly reduces hypothalamic oxidative stress injury and inflammation in type 2 diabetes mellitus rats by a mechanism depending on glucagon-like peptide 1 (GLP-1)-mediated activation of Nrf2/HO-1 signaling pathway. DJB therapy effectively inhibits hypothalamic oxidative stress and inflammatory damage caused by diabetes by GlP-1-mediated activation of Nrf2/HO-1 to inhibit oxidative stress damage in the hypothalamus. In addition, DJB inhibits inflammation by upregulating Nrf2 expression, activating the Nrf2/HO-1 axis, and inhibiting the NF-κB pathway. T2DM: Type 2 diabetes mellitus; DJB: Duodenal jejunal bypass; GLP-1: Glucagon-like peptide 1; GLP-1R: Glucagon-like peptide 1 receptor; IL: Interleukin.
- Citation: Wang HJ, Zhang LB, Sun SP, Yan QT, Gao ZQ, Fu FM, Qu MH. Duodenal-jejunal bypass improves hypothalamic oxidative stress and inflammation in diabetic rats via glucagon-like peptide 1-mediated Nrf2/HO-1 signaling. World J Diabetes 2024; 15(2): 287-304
- URL: https://www.wjgnet.com/1948-9358/full/v15/i2/287.htm
- DOI: https://dx.doi.org/10.4239/wjd.v15.i2.287