Copyright
©The Author(s) 2023.
World J Diabetes. May 15, 2023; 14(5): 494-511
Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.494
Published online May 15, 2023. doi: 10.4239/wjd.v14.i5.494
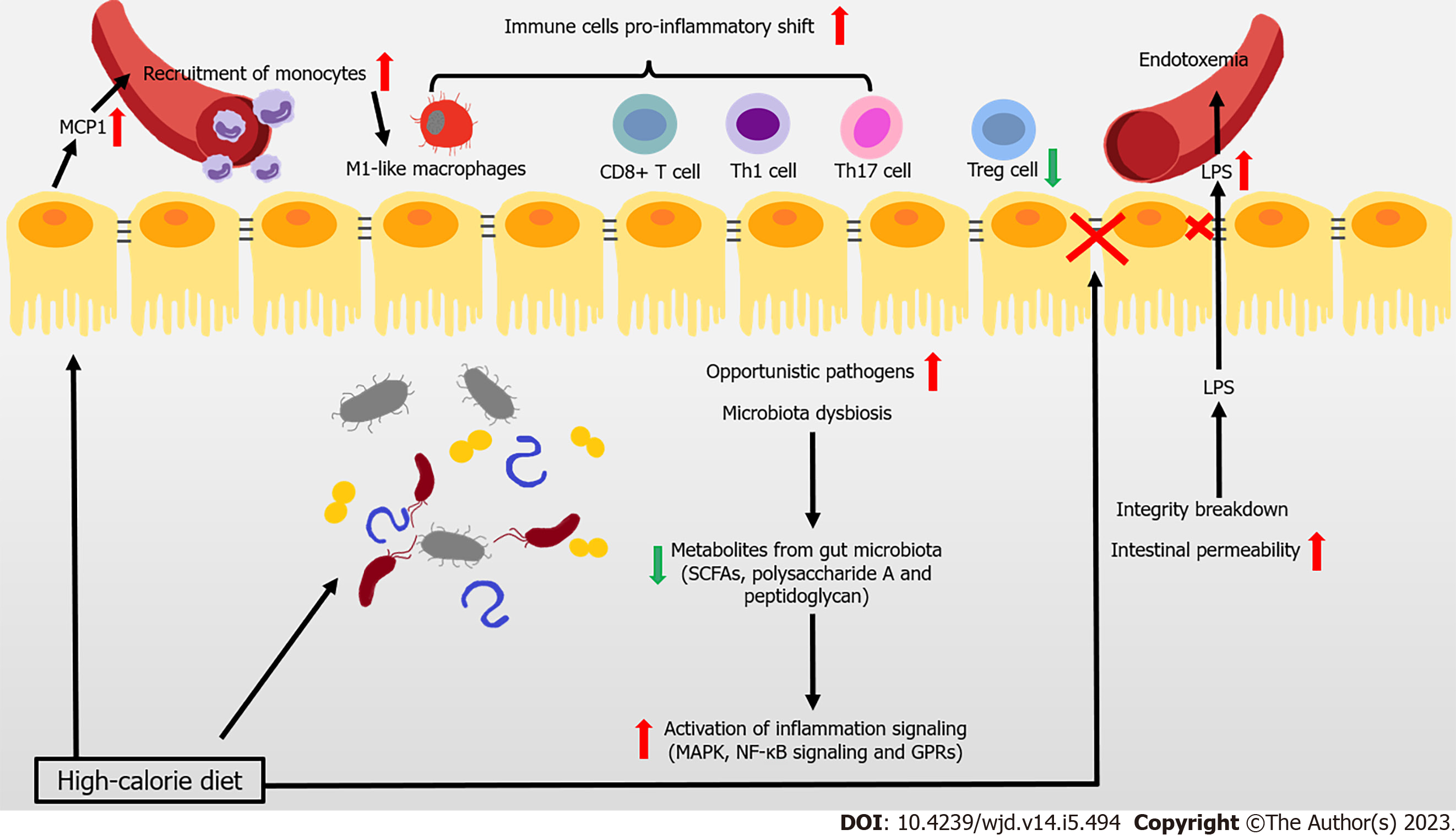
Figure 1 Immune attack and inflammation in the gut during obesity-related type 2 diabetes.
In the context of obesity and type 2 diabetes mellitus, overnutrition leads to the reduced gut microbiota, and even the increase of opportunistic pathogens. At the same time, the occurrence of decreased metabolites levels with anti-inflammatory effects, is accompanied by the activation of inflammation signaling. During obesity, imbalance of pro- and anti-inflammatory immune cells occurs in the gut. The intestinal epithelial cell-produced monocyte chemoattractant protein-1 (MCP1) recruits the circulating monocytes to the gut and they shift to the pro-inflammatory phenotype. High fat diet also induces a pro-inflammatory shift in T cells, accompanied with decreased regulatory T cells. Immunoglobulin A (IgA)-secreting immune cells and IgA secretion are both decreased. High-calorie diet and several recruited immune cells also impair intestinal barrier and increase intestinal epithelial and gut vascular permeability, leading to the leakage of microbiota-derived molecules (such as lipopolysaccharide [LPS]) into blood. High levels of LPS and other bacterial products cause endotoxemia and inflammation in multiple organs that further aggravate the metabolic diseases. GPR: G protein-coupled receptor; SCFAs: Short-chain fatty acids.
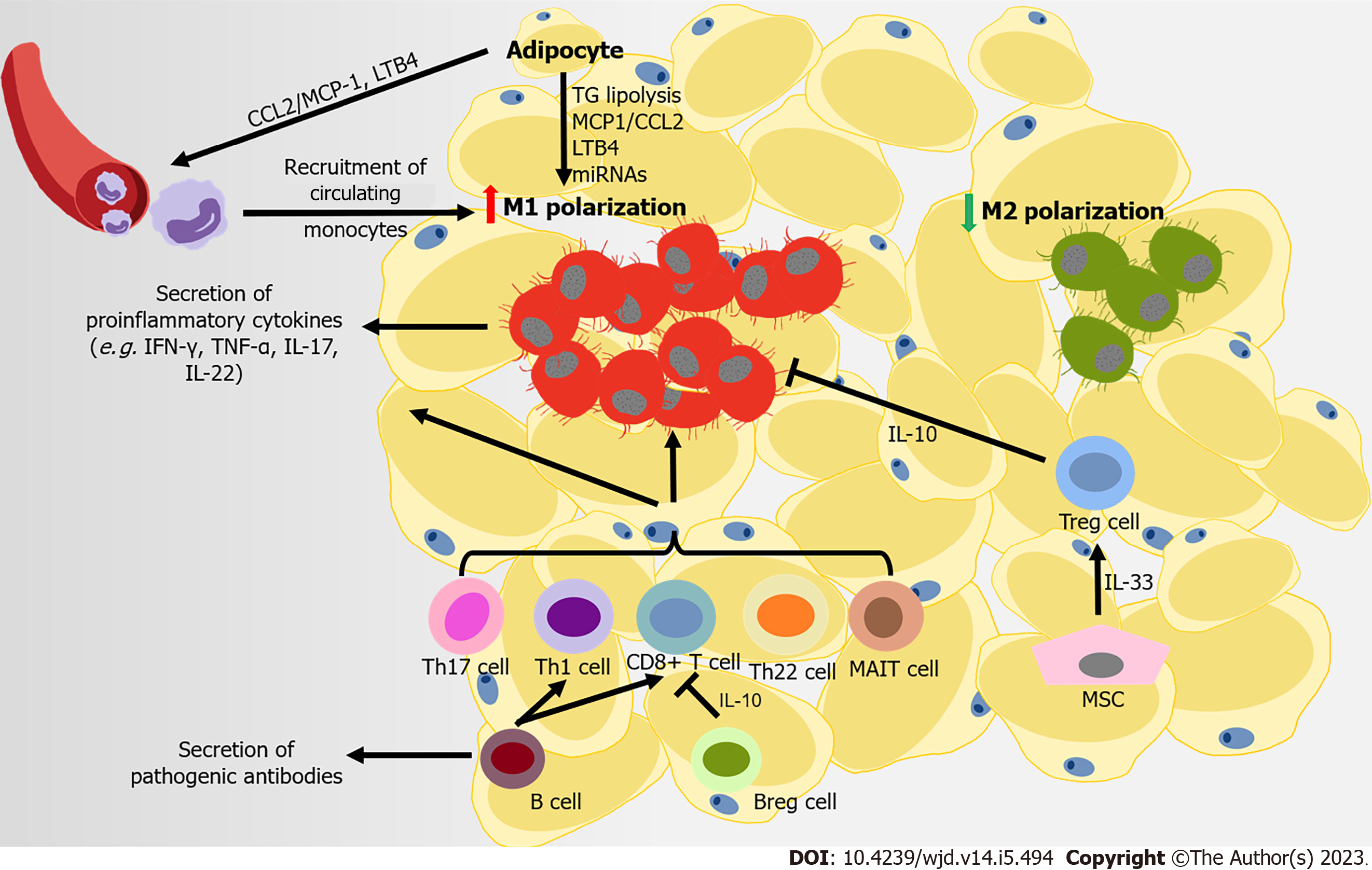
Figure 2 Immune attack and inflammation in the white adipose tissue during obesity-related type 2 diabetes.
At the later stage of obesity, recruited monocytes mainly contribute to the accumulation of macrophages in adipose tissue, following the secretion of monocyte chemoattractant protein-1 (MCP1) and leukotriene B4 (LTB4) by adipocytes to the microenvironment. Free fatty acids from the diet and in triglyceride (TG) lipolysis in adipocytes promote M1-like polarization. Several adipocyte-derived microRNAs also regulate the immune balance between M1- and M2-macrophage polarization. CD8+ T cells, pro-inflammatory CD4+ T cells (T helper type 1 [Th1], Th17, and Th22) and mucosal-associated invariant T cells are also recruited into adipose tissue, promoting M1-like polarization. Regulatory B cells (Bregs) and regulatory T cells (Tregs) can negatively control the local inflammation by secreting interleukin-10 (IL-10), but B cells contribute to systemic inflammation by activating CD8+ and Th1 cells, and releasing pathogenic antibodies. Some mesenchymal stromal cells in visceral adipose tissue can improves insulin resistance and inflammation in adipose tissues through expanding and sustaining the resident Treg population via IL-33 secretion.
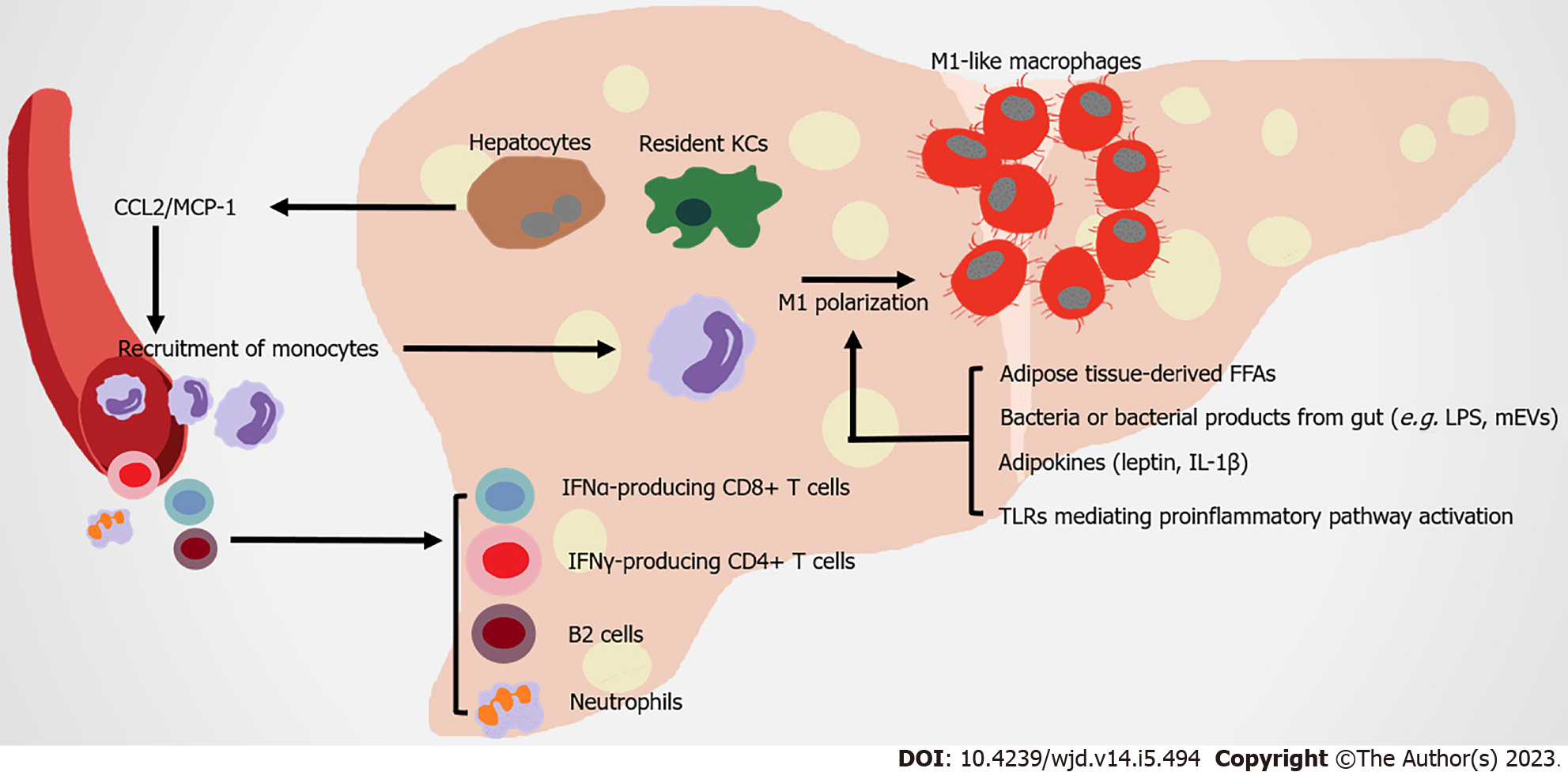
Figure 3 Immune attack and inflammation in the liver in obesity-related type 2 diabetes.
Under metabolic stress, recruited hepatic macrophages, which are derived from circulating monocytes, are recruited by steatosis hepatocytes and Kupffer cells secreting monocyte chemoattractant protein-1 (MCP1). Expanded adipose tissue-derived free fatty acids, leptin, interleukin-1 beta (IL-1β) and bacteria with their products from gut, contribute to the M1 polarization of hepatic macrophages. Nonalcoholic steatohepatitis (NASH) is a severe form of nonalcoholic fatty liver disease, which is associated with more severe hepatic insulin resistance and inflammation. The infiltration of neutrophils, B2 cells, interferon gamma (IFN-γ)-producing CD4+ T cells and IFN-α-producing CD8+ T cells occur in NASH liver, promoting insulin resistance under diet-induced metabolic stress. FFAs: Free fatty acids; KCs: Kupffer cells; LPS: Lipopolysaccharide; mEVs: Extracellular vesicles.
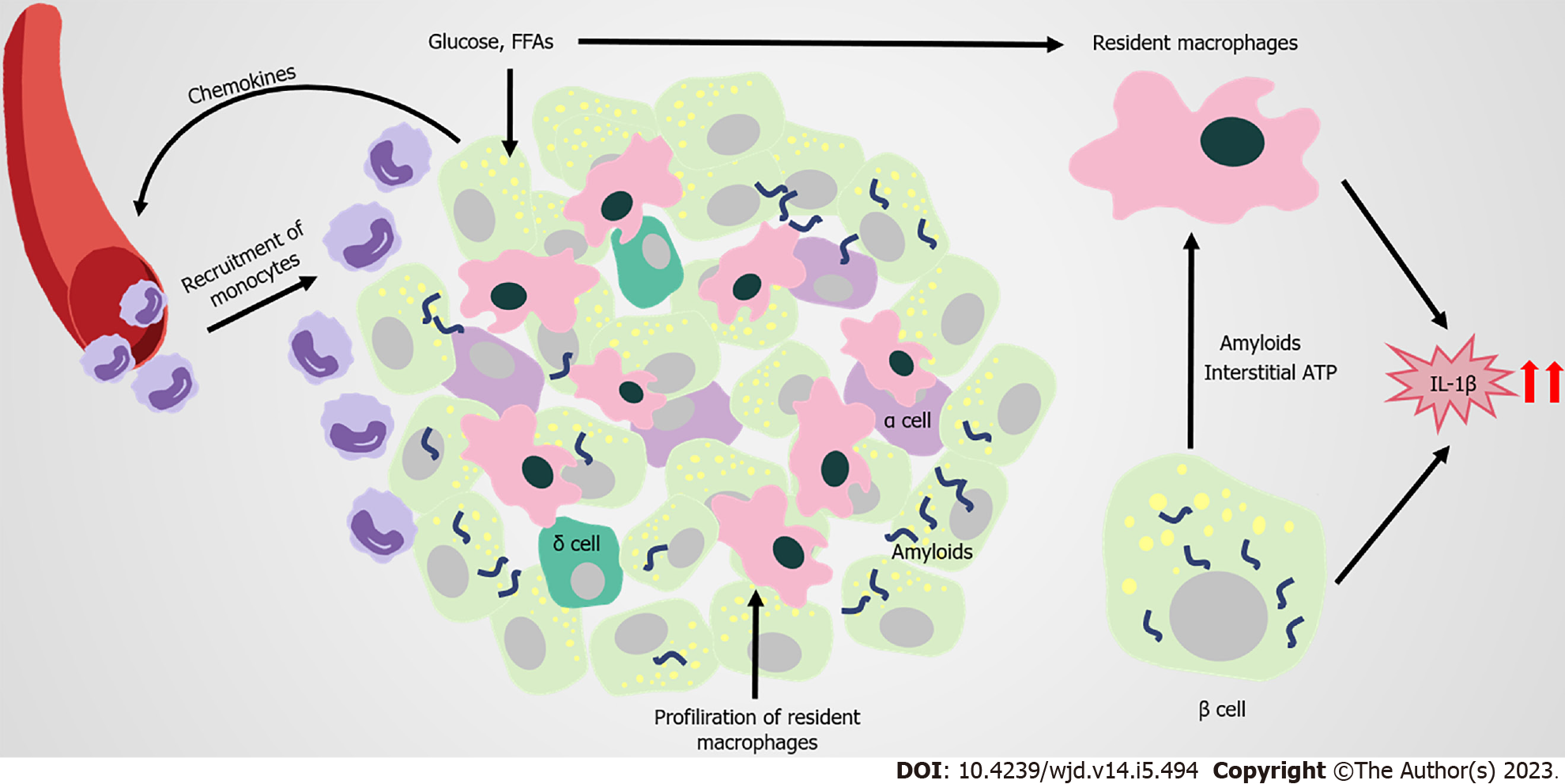
Figure 4 Immune attack and inflammation in the islet in obesity-related type 2 diabetes.
In obesity and type 2 diabetes mellitus (T2DM), the proliferation of islet resident macrophages causes accumulation of macrophages in islets with elevated inflammatory cytokines and chemokines (such as interleukin-1 beta [IL-1β], tumor necrosis factor-alpha [TNF-a]). Β cells respond to saturated fatty acids recruit Ly6C+ monocytes to the islets; however, these recruited monocytes remain at the boundary of the exocrine and endocrine pancreas. High concentrations of glucose or free fatty acids and amyloids deposition, promote islet macrophages to produce more IL-1β. Glucose-activated insulin and ATP secretion of β cells also trigger the production of cytokines from macrophages. Elevated IL-1β levels can promote inflammation in islets, and are closely related to the development of prediabetes and T2DM. FFAs: Free fatty acids.
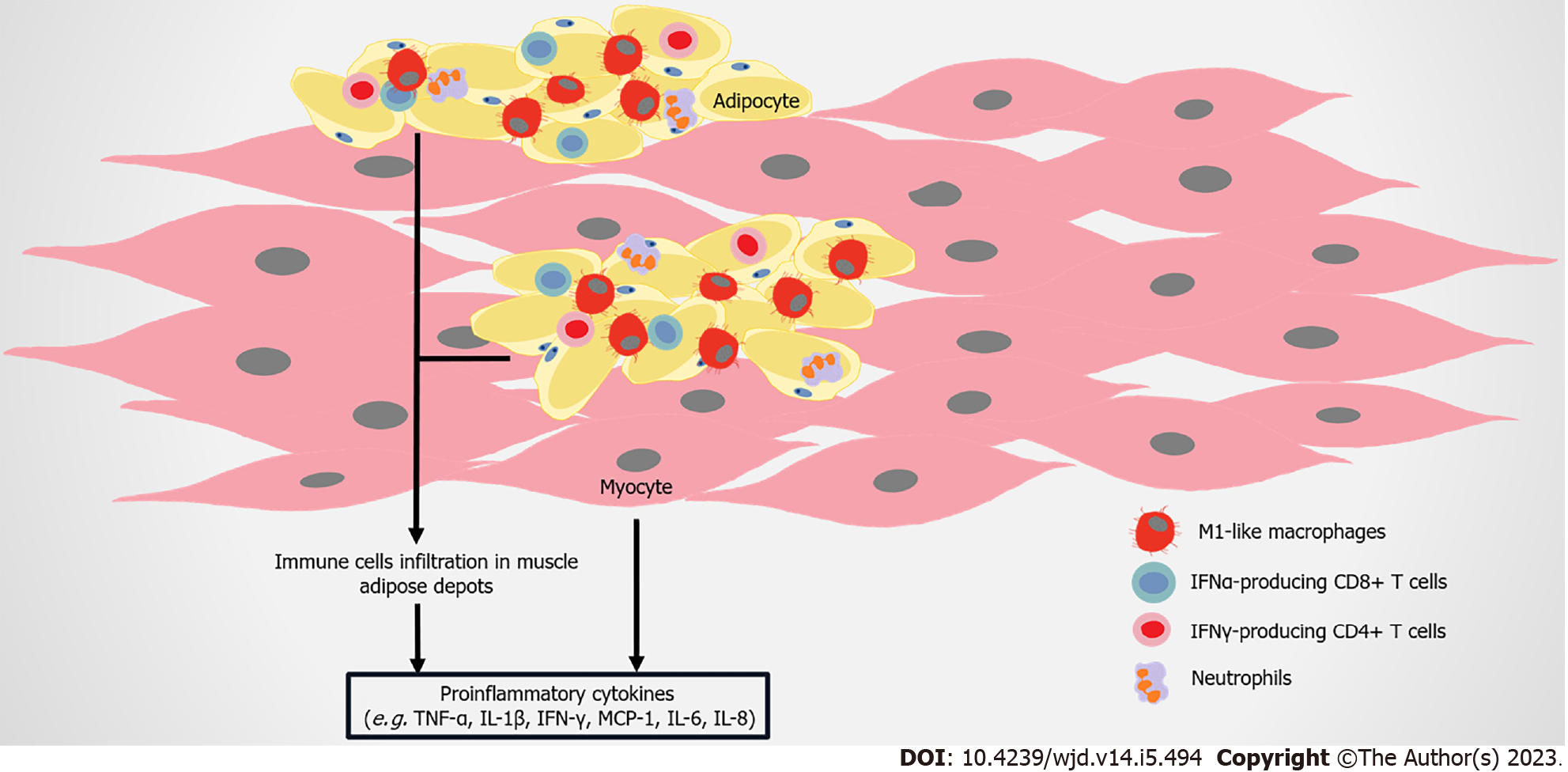
Figure 5 Immune attack and inflammation in the skeletal muscle in obesity-related type 2 diabetes.
As obesity develops, adipose depots between skeletal muscles or surrounding muscles continuously further expand. Immune cells including M1-like macrophages, CD8+ T cells and interferon-gamma (IFN-γ)–producing CD4+ cells, infiltrate into adipose depots in skeletal muscles. Skeletal muscle cells can also produce monocyte chemoattractant protein-1 (MCP1), interleukin-6 (IL-6), IL-8, tumor necrosis factor-alpha (TNF-α), and other molecules, and lead to the infiltration of macrophages, finally inducing insulin resistance.
- Citation: Wang HW, Tang J, Sun L, Li Z, Deng M, Dai Z. Mechanism of immune attack in the progression of obesity-related type 2 diabetes. World J Diabetes 2023; 14(5): 494-511
- URL: https://www.wjgnet.com/1948-9358/full/v14/i5/494.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i5.494