Copyright
©The Author(s) 2023.
World J Diabetes. Mar 15, 2023; 14(3): 130-146
Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.130
Published online Mar 15, 2023. doi: 10.4239/wjd.v14.i3.130
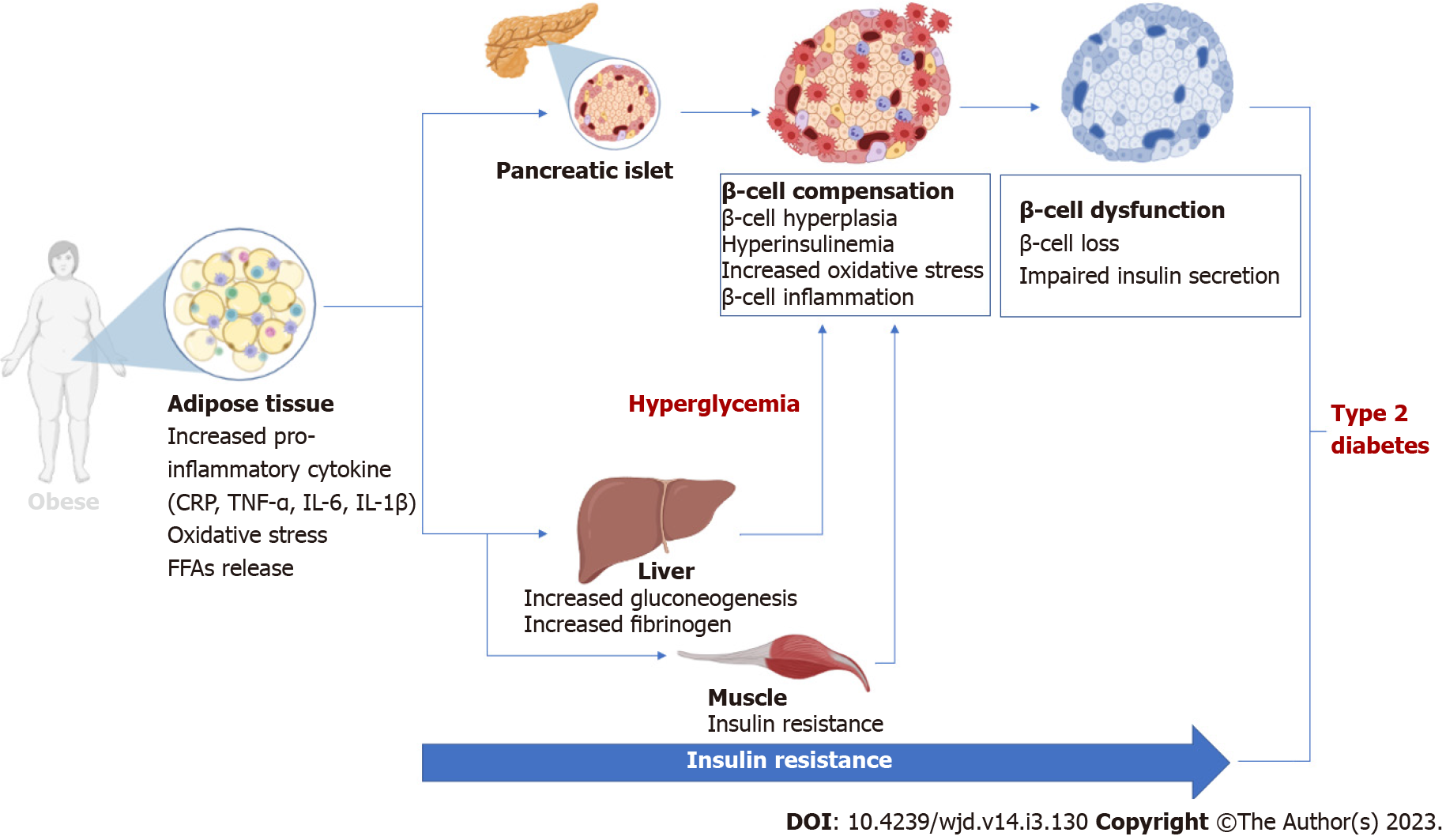
Figure 1 An overview of the pathological implications of adipose tissue hypertrophy and insulin resistance during the development and progressive loss of β-cell function in conditions of obesity to type 2 diabetes.
Briefly, adipose tissue expansion (usually seen in obesity or type 2 diabetes) is associated with enhanced secretion of pro-inflammatory markers and generation of oxidative stress that directly or indirectly cause pancreatic β-cell loss, leading to impaired insulin secretion. CRP: C-reactive protein; TNF-α: Tumor necrosis factor-alpha; IL: Interleukin; FFAs: Free fatty acids.
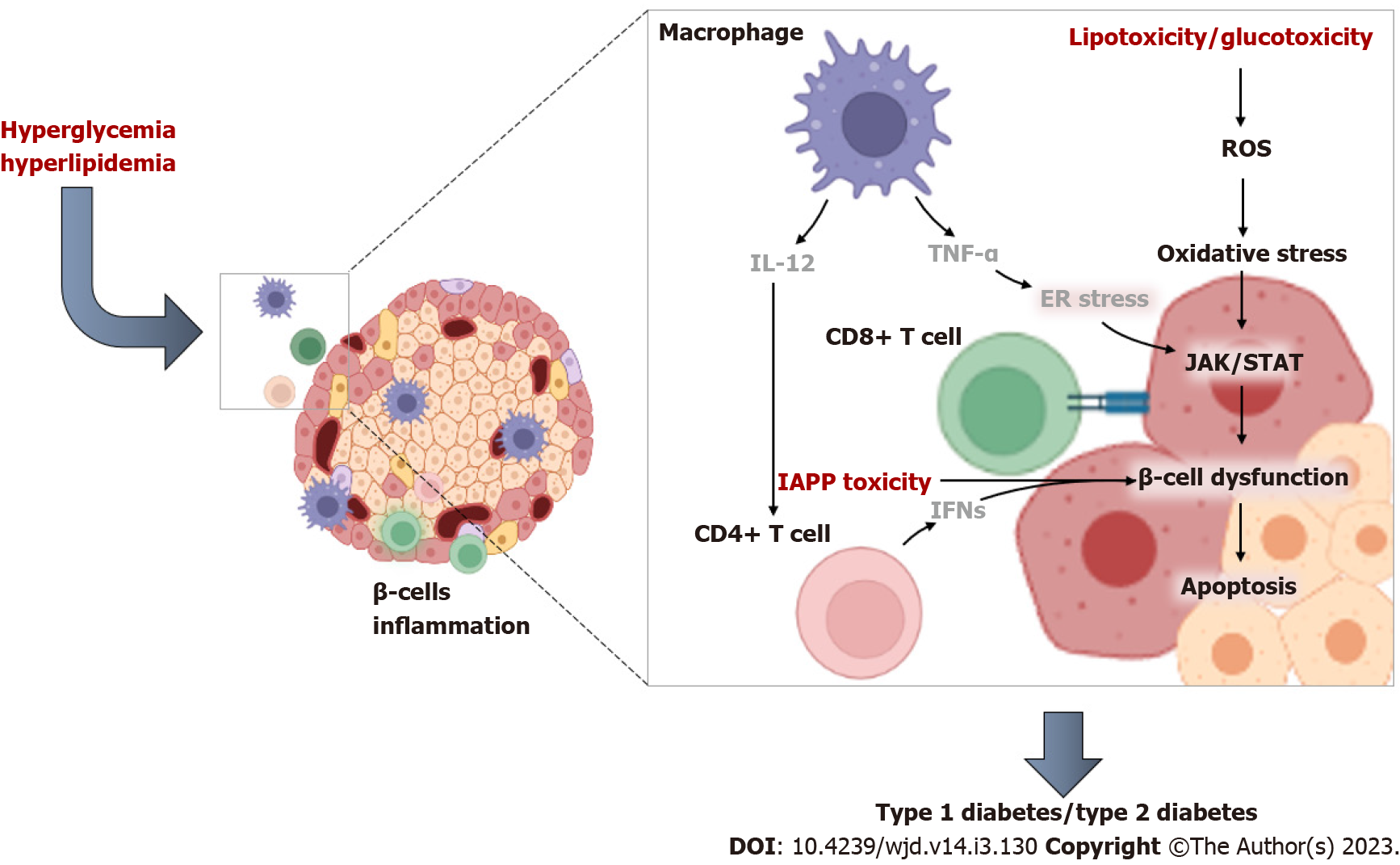
Figure 2 An overview of the pathological mechanisms linking impaired immune function and inflammation during β-cell dysfunction in conditions of type 2 diabetes (characterized by hyperglycemia and hyperlipidemia).
Briefly, CD4+ T cells can be activated by interleukin-12 produced from macrophages and dendritic cells, and this consequence occurs as part of a vicious process involving cytotoxic T cells and recruitment by the pancreatic islets. Notably, elevated levels of tumor necrosis factor-alpha are linked with activation of pro-inflammatory signals such as Janus kinase/signal transducer and activator of transcription that promote β-cell failure. IL-12: Interleukin-12; TNF-α: Tumor necrosis factor-alpha; INFs: Interferons; IAPP: Islet amyloid polypeptide; ROS: Reactive oxygen species; ER stress: Endoplasmic reticulum stress; JAK/STAT: Janus kinase/signal transducer and activator of transcription.
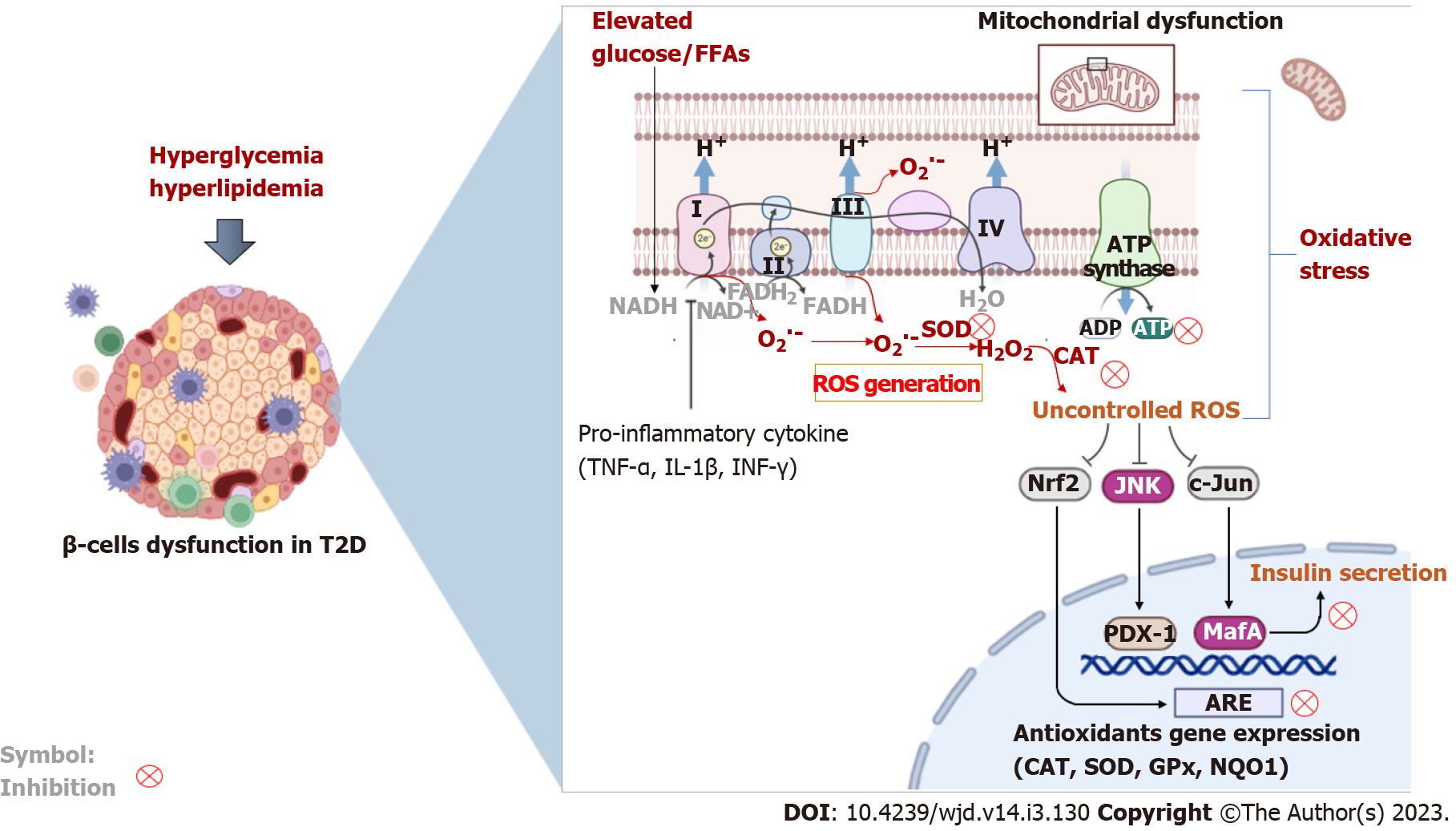
Figure 3 An overview of some pathophysiological mechanisms that highlight the interrelated link between inflammation and oxidative stress during β-cell dysfunction in type 2 diabetes.
Briefly, elevated levels of glucose (hyperglycemia) and lipids (hyperlipidemia) are consistent with an abnormal inflammatory response (due to increased levels of tumor necrosis factor-alpha, interferon-gamma, and interleukin 1 beta) and impaired mitochondrial electron transport chain that causes enhanced generation of reactive oxygen species implicated in β-cell dysfunction. Both inflammation and oxidative stress are responsible for activating cellular destructive mechanisms such as the c-Jun N-terminal kinase pathway and suppressing intracellular antioxidant responses (e.g., nuclear factor erythroid 2-related factor 2, catalase, superoxide dismutase and others), leading to accelerated β-cell injury. ROS: Reactive oxygen species; SOD: Superoxide dismutase; CAT: Catalase; Gpx: Glutathione peroxidase; NQO1: NAD(P)H quinone dehydrogenase 1; NAD(H): Nicotinamide adenine dinucleotide; FADH: Flavin adenine dinucleotide; Nrf2: Nuclear factor erythroid 2-related factor 2; JNK: c-Jun N-terminal kinase; PDX-1: Pancreatic duodenal homeobox 1; ARE: Antioxidant response element; TNF-α: Tumor necrosis factor-alpha; IL-1β: Interleukin 1 beta; INF-γ: Interferon-gamma; ADP: Adenosine diphosphate; ATP: Adenosine triphosphate; ROS: Reactive oxygen species; T2D: Type 2 diabetes; FFAs: Free fatty acids.
- Citation: Dludla PV, Mabhida SE, Ziqubu K, Nkambule BB, Mazibuko-Mbeje SE, Hanser S, Basson AK, Pheiffer C, Kengne AP. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J Diabetes 2023; 14(3): 130-146
- URL: https://www.wjgnet.com/1948-9358/full/v14/i3/130.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i3.130