Published online Sep 16, 2017. doi: 10.4253/wjge.v9.i9.464
Peer-review started: March 2, 2017
First decision: May 26, 2017
Revised: June 15, 2017
Accepted: July 14, 2017
Article in press: July 17, 2017
Published online: September 16, 2017
Processing time: 194 Days and 1.2 Hours
To compared individuals with serrated polyposis syndrome (SPS) to those with sessile serrated adenoma (SSA) and adenomas in the setting of endoscopists with high adenoma detection rates at a secondary and tertiary academic centre.
Retrospectively we collated the clinical, endoscopic and histological features of all patients with SPS at St Vincent’s public and private hospital in the last 3 years. Patients were identified by searching through 2 pathology databases. Variables explored included smoking status, symptoms, and family history of concurrent colorectal cancer, number and location of polyps. Patients with SPS were matched to two cohorts (1) patients with SSA not meeting World Health Organization (WHO) criteria for SPS over 3 years; and (2) patients with exclusively adenomas. The control cases were also matched according to gender and endoscopist. Adenoma detection rates ranged from 25% to 40%.
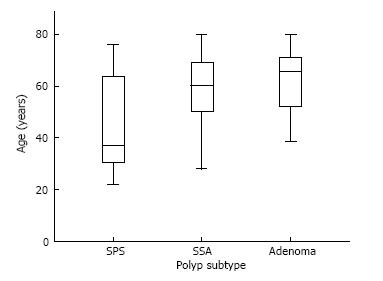
Forty patients with SPS were identified and matched with 40 patients in each control group. In total 15452 colonoscopies were performed over the study period which amounts to a prevalence of 1: 384 patients (0.26%) with SPS. Fourteen patients (35%) required more than 1 year to accumulate enough polyps to reach WHO criteria for SPS. The diagnosis of SPS was largely incidental and 5% SPS patients were diagnosed with colorectal cancer over 3 years. The chance of detecting a meta-synchronous adenoma was similar in those with SPS (42%) and those with SSA (55%), P = 0.49. The majority of patients (75%) meeting criteria for SPS were women. The mean age of those with SPS (45 years) was significantly lower than both cohorts with SSA (57 years) and adenomas (63 years), P = 0.01. On univariate analysis cigarette exposure, first-degree family history of colorectal cancer and a high BMI weren’t significantly more associated with SPS compared to patients with SSA or patients with adenomas. However, patients with SPS (97%) and patients with SSAs not meeting SPS criteria (98%) were significantly more likely to be Caucasian compared to patients with adenomas (79%), P = 0.01.
The prevalence of SPS in our study was 0.26%. The vast majority of patients diagnosed with SPS were women. As a group, they were significantly younger compared to patients with SSA not meeting WHO criteria and patients with adenomatous polyps by more than a decade. Patients with SPS were no more likely to have a first degree relative with colorectal cancer or smoking history than the other two groups. Patients with serrated polyps were more likely to be Caucasian than patients with adenomas.
Core tip: At the time of diagnosis, sessile serrated polyposis syndrome (SPS) is associated with a high risk of concurrent colorectal cancer. Early diagnosis of SPS is crucial and this case-control study aim to delineate differences in risk factors for SPS and other types of polyps. The vast majority of patients diagnosed with SPS in our study were women. They were younger and more likely to be Caucasian compared to patients with adenomatous and patients with serrated adenomas not meeting World Health Organization criteria. SPS patients were no more likely to have a family history of colorectal cancer or cigarette exposure than other polyp groups.
- Citation: Wu Y, Mullin A, Stoita A. Clinical predictors for sessile serrated polyposis syndrome: A case control study. World J Gastrointest Endosc 2017; 9(9): 464-470
- URL: https://www.wjgnet.com/1948-5190/full/v9/i9/464.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i9.464
In the past decade, serrated polyps have garnered rapid interest as they cause colorectal cancer via a different pathway compared to adenomas. This pathway features hyper-methylation, CpG island methylator phenotype (CIMP) and mutations in proto-oncogene BRAF. Sessile Serrated adenoma (SSA), hyperplastic polyps and traditional serrated adenoma all fall under the classification of serrated polyps.
Serrated polyposis syndrome (SPS), distinguished by numerous serrated polyps distributed throughout the colon is associated with increased risk of colorectal cancer. Its prevalence is unclear. In a systematic review, it ranges from as low as 1:1800 in average risk screening population and as high as 1:150 in patients in a Faecal immunochemical testing programme[1,2]. In comparison, Familial Adenomatous Polyposis Syndrome (FAP), has lower prevalence rate of 1:13000 in average risk screening population and has a known genetic mutation[3]. However a genetic basis for SPS has not been discovered despite large multi-centre studies looking into a variety of germline mutations[4]. The definition of SPS is essentially a clinical one, based on endoscopic findings and histopathology of polyps removed. In 2010, the condition was arbitrarily defined by the World Health Organization (WHO) as follows: (1) at least 5 histologically diagnosed serrated lesions proximal to the sigmoid colon, of which 2 should be > 10 mm in diameter; (2) any number of serrated polyps proximal to the sigmoid colon in an individual who has a first-degree relative (FDR) with SPS; or (3) > 20 serrated polyps distributed throughout the colon. The definition is a cumulative one, whereas many years and multiple colonoscopies can pass before patient reach the criteria for the diagnosis of SPS[5].
Concurrent colorectal cancer (CRC) is often encountered at the time of SPS diagnosis, at rates as high as 16%-29%[5]. The propensity for a delay in diagnosis in SPS and the inclusion of patients with symptoms suspicious for cancer accounts for the high prevalence rates in these previous studies. Once patients enter a surveillance program and undergo the recommended yearly colonoscopies, the absolute 5-year CRC risk falls to 1.5%[2]. Hence, early diagnosis of SPS is crucial as it radically change the natural history of patients with the disorder. Serrated and adenomatous polyps share many clinical risk factors, however it is unclear if certain risk factors have a stronger association with one polyp subtype compared to another[6]. Despite there being a small number of studies investigating the differences in risk factors for distinct polyp subtypes, no study to date has included SPS as one of the comparators.
A case-control retrospective study was conducted in 2 centres; St Vincent’s public hospital, Sydney, Australia a tertiary referral teaching hospital and St Vincent’s clinic, a secondary referral private centre in the same campus. The colonoscopies were performed by 7 experienced endoscopists, each performing more than 450 colonoscopies per year and having high adenoma detection rate between 25%-40%. Patients undergoing a colonoscopy with polypectomy with a diagnosis of SPS between December 2013 and December 2016 were identified by searching through two pathology databases. The colonoscopies were performed with high definition white light endoscope with NBI capability (190 series, Evis Extera III CV190 processor Olympus, Tokyo, Japan). Bowel preparation was used using either Moviprep split dose (a polyethylene glycol preparation) or Prep Kit C (Sodium picosulfate and Glycoprep). Bowel preparation was considered adequate if the Boston scale score was more than 6 points. Those with poor bowel preparation were excluded. Procedures were performed with the patients under deep sedation with Propofol administered by an anaesthetist. We collated the clinical, endoscopic and histological features of all SPS patients and matched them to two cohorts (1) patients with SSA not meeting WHO criteria for SPS over 3 years; and (2) patients with exclusively adenomas. The control cases were matched according to gender and endoscopist in order to minimise bias secondary to variations in endoscopic recognition and technique.
Variables collected for each patient included age, ethnicity, smoking status, weight, height, symptoms, family history of colorectal cancer, presence/absence of concurrent colorectal cancer, number, size and location of polyps. Since the diagnosis of SPS is dependent on a cumulative polyp count, the number of colonoscopies and years required to meet criteria were also analysed. Whether the diagnosis of SPS was made on the first “index” colonoscopy or subsequent followup colonoscopy during followup was also distinguished. Adenoma detection rates for each endoscopist involved in the study was extracted from Quality Assurance data from each centre. The study was approved by the Institutional Review Board.
Univariate analysis of co-variates was analysed using Fisher’s exact test or χ2 test as appropriate. Age and BMI was assessed using the Independent student T-test. Quantitative variables were summarized using mean standard variation and mean values. Frequencies were used to summarise categorical values. SPSS statistics software version X (SPSS, Inc Chicagi, Illionois, United States) was used to analyze the data. The statistical methods of this study were reviewed by Dr. Yang Wu from Department of Gastroenterology, St Vincent’s Hospital, NSW, Australia. Nancy Briggs from Stats Central, statistical consulting unit established at University of NSW, also reviewed the statistical methods of this study.
During the 3 years period, 40 patients were diagnosed with SPS and equal number of patients matched in the control groups: Patients with SSA not meeting the WHO criteria for SPS and patients with only adenomatous polyps. Bowel prep was reported as adequate in all the cases. The majority, 26 patients had enough polyps to meet criteria at their first “index” colonoscopy. However 14 patients (35%) required more than 1 year to accumulate enough polyps to reach WHO criteria for SPS, with 7 patients requiring 2 years and 7 patients requiring 3 years to reach criteria. In total 15452 colonoscopies were performed over the study period across the two sites which amounts to a prevalence of 1: 384 patients (0.26%) with SPS.
The mean age of the patients with SPS was 45 years old and women made up 75% of the cohort. Characteristics of the 40 patients with SPS are included in Table 1. There was substantial heterogeneity in the indications for colonoscopy in patients with SPS, ranging from abdominal pain to per rectal bleeding. 7/40 patients underwent colonoscopy due to a history of colonic polyps in the past, sometimes discovered at other endoscopy centres not involved in the study. For the remaining patients, SPS diagnosis was largely unrelated to the pretest suspicion of the referring doctor. Only 1/40 patient had their procedure initiated by a positive Faecal Occult Blood Test (FOBT). The indication for colonoscopy is not a reliable predictor factor for finding SPS.
| Study population (n = 40) | |
| Women | 30 (75) |
| Age in years, mean ± SD | 45 ± 18.54 |
| Ethnicity | |
| Caucasian | 39 (98) |
| Other | 1 (2) |
| First Degree Relative with CRC | |
| Present | 12 (35) |
| Absent | 22 (65) |
| Smoking status | |
| Current Smoker/Ex-smoker | 318 (48) |
| BMI (kg/m2), mean ± SD | 25 ± 4.7 |
| WHO Criteria | |
| 1 | 30 (75) |
| 2 | 0 |
| 3 | 10 (25) |
| Mean number of total polyps | 13 |
| Mean number of serrated polyps during first colonoscopy | 7 |
| Mean size of largest polyp (mm) | 17 |
| Location of largest polyp | |
| Caecum | 13 (32) |
| Ascending colon | 16 (40) |
| Transverse colon | 5 (13) |
| Descending colon | 2 (5) |
| Sigmoid colon | 4 (10) |
The mean number of serrated polyps removed during the first colonoscopy was 7 (range 1 to 30) and the mean total number of polyps detected over 3 years was 13 (range 6 to 30). The mean size of the largest serrated polyp removed was 17 mm, ranging from 10 mm to 40 mm. Interestingly, 17/40 patients with SPS also had concurrent adenomas ranging from 1-6 adenomas detected over 3 years. The age of this subpopulation ranged from 32 to 72 years old.
A small proportion 5% (2/40), were diagnosed with colorectal cancer during the 3 years and neither of the patient displayed symptoms classic of malignancy. Interestingly, both patients met WHO criteria for SPS not during their index colonoscopy but during the followup colonoscopy when their colorectal cancer was diagnosed. Neither patients had a family history of colorectal cancer. One patient was a 76 years old woman with history of one SSA removed from ascending colon in 2014 and subsequently developed a colorectal cancer in hepatic flexure plus five right-sided SSA in 2016. Molecular markers for the colorectal cancer showed positivity for BRAF, Microsatellite instability (MSI) and MLH1. The second patient was a 64 years old woman with one SSA removed in 2013 at different endoscopy centre and subsequently developed a colorectal cancer in the ascending colon along with three adenomas and thirteen SSAs in 2016. This cancer was BRAF and MSI negative. Both patients had localised disease and had surgery with curative intent in 2016.
The mean age of patients with SPS was 45 years (SD: 18.54), significantly lower than 57 years (SD: 15.56) in patients SSAs not meeting SPS criteria and 63 years (SD: 10.58) in patients with adenomas, P = 0.01. Figures 1 and 2 shows the difference in age distribution between the three polyp groups. The SPS polyp group had a higher rate of family history of colorectal cancer in a first degree relative (31%) compared to the SSA group (26%) and adenoma group (30%), but this didn’t reach statistically significance. Patients with SPS (97%) and patients with SSAs not meeting SPS criteria (98%) were significantly more likely to be Caucasian compared to patients with adenomas (79%), P = 0.01. Past or present cigarette exposure was reported at a higher proportion in patients with SPS (47%) compared to SSA group (32%) and Adenoma group (33%) but this wasn’t statistically significant, P = 0.39. BMI was not significantly different across the three groups with the SPS group associated with mean BMI of 26 kg/m2, SSA group associated with mean BMI of 23 kg/m2 and Adenoma group with mean BMI of 25 kg/m2, P = 0.61.
Evaluating clinical risk factors between the 26 patients who met WHO criteria for SPS at their first “index” vs 14 patients who required more than 1 year to accumulated enough polyps to be diagnosed with SPS, there was no significant difference in rates of family history of colorectal cancer, smoking or age.
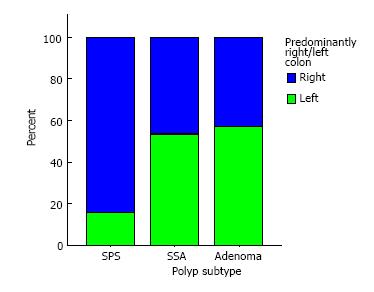
Endoscopically, each polyp group has distinct characteristics. Polyps were predominantly discovered in the right colon in 84% of SPS patients vs 46% of patients with SSAs and 43% of patients with exclusively adenomas, P = 0.003.
In 4/40 patients with SPS and in 4/40 patients with SSA not meeting WHO criteria, an adenoma was the largest polyp discovered, not a serrated polyp. In the rest of the group, 36 patients, the largest polyp was a serrated polyp. Concentrating only on patients whose largest polyp was serrated, the distribution of largest or dominant polyp was significantly different between the SPS patients and SSA patients.
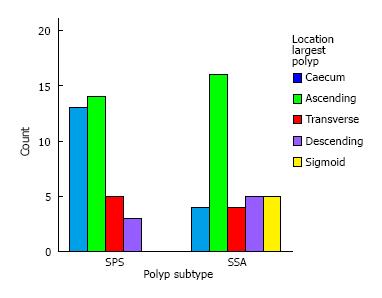
Figure 3 illustrates the differences in the distribution of the largest Serrated polyp in the SPS group compared with the SSA group. In both groups, the largest serrated polyp was most frequently found in ascending colon. However in SSA group, there was a more even spread of large serrated polyps throughout the colonic segments with 5 patients having their largest polyp in his/her sigmoid colon.
The proportion concurrent adenoma 17 (42%) in patients who were diagnosed with SPS was comparable to those with SSAs not meeting SPS criteria 21 (52%). Table 2 describes that patients with SPS have a mean total polyp count significantly higher than the other two groups, but this is expected given the intrinsic requirement of multiple polyps to meet the criteria for SPS. Similarly a greater proportion of patients with SPS have a polyp > 15 mm compared to the other groups. The mean size of largest polyp for SPS (17 mm) was significantly greater than the mean size of largest polyp found in the SSA group (14.3 mm) and the adenoma group (7.8 mm), P = 0.014.
| SPS n = 40Number/mean | Adenoma n = 40Number/mean | SSA n = 40Number/mean | P value for comparison between groups | |
| Patients with concurrent adenoma | 0.49 | |||
| Yes | 17 (42) | 21 (52) | ||
| No | 23 (58) | 19 (48) | ||
| Total Number of Polyps | 13 | 1.4 | 3.4 | 0.001 |
| Patients with a polyp > 15 mm | ||||
| Yes | 30 (75) | 3 (7.5) | 21 (52) | 0.04 |
| No | 10 (25) | 37 (92.5) | 19 (48) | |
| Size of largest polyp (mm) | 17 | 7.8 | 13.5 | 0.014 |
Missing data was most frequently encountered in collating BMI (10%) and documentation of family history of colorectal cancer (15%). Information on smoking status and indication for colonoscopy were almost universally available. Alcohol intake of the patients were sparsely and inconsistently documented in the clinical records. We were unable to precisely quantify alcohol intake for the majority of the patients, hence this variable, an important risk factor for adenoma polyp development, wasn’t analysed in our study.
It is estimated that 20%-30% of all CRCs develop through the serrated neoplasia pathway[7]. It has been hypothesised that the imperceptible nature of serrated polyps and inconsistent bowel preparation have been responsible for the relative failure of colonoscopy to protect from proximal colon carcinoma.
In this study, we describe the phenotypic features in a group of patients with SPS from a secondary and tertiary gastroenterology centre over 3 years. The prevalence of SPS in our study, 1:384, is higher than the measurements reported in previous studies performed in screening populations 1:1800[1]. The contrast between these figures may reflect the expertise and vigilance of the local gastroenterologist in detecting serrated lesions. All adenoma detection rates of the endoscopists involved in this study were above the national benchmark on quality in colonoscopy. A shift towards switching split preparation in recent years may have also influenced the ability to detect serrated lesions. Another strength in our data is that there is little ascertainment bias as our data isn’t collected from genetic clinics or bowel cancer screening programs. Limitations in our study include the retrospective assessment of the data, the smaller number of patients with SPS relative to recent multi-center studies[8], and the inability to analyse alcohol intake as a variable due to missing data points.
Two of our 40 patient with SPS (5%) were diagnosed with colorectal cancer during the 3 year evaluation period, neither of whom reported symptoms suggestive of malignancy. Quantification of the magnitude in which SPS increases the risk of CRC is difficult. Early case series have reported rates of 16%-29% in synchronous cancer at the time of SPS diagnosis, however theses risk estimates were likely inflated due to inclusion of many patients symptomatic of cancer not patients undergoing screening. In 2015 Carballal et al[8] described a cumulative CRC incidence of 1.9% over 5 years in a group of 296 patients with SPS in Spain. Forty-seven patients (15.8%) developed cancer during follow-up, with 4/47 (8.5%) developing tumours during surveillance. One of our two patients who developed colorectal cancer had a previous colonoscopy at a different endoscopy centre, where the optical training of the endoscopist and bowel preparation quality is unclear and the diagnosis of SPS could have been missed.
Consistent with previous reports, our cohort with SPS demonstrated some phenotypic heterogeneity[9]. Patients diagnosed with the syndrome were aged from 23 years to 73 years and had an overall polyp count ranging from 7 to 40 polyps, with the largest polyp resected ranging from 10 mm to 40 mm. However, there were some clinical features that were distinct for patients with SPS compared to those with SSAs not meeting WHO criteria and patients with adenomas. SPS patients were significantly younger and more likely to be caucasian. The polyp groups were matched according to gender hence the gender distribution across the three groups were identical, but the over-representation of women in 75% of patients with SPS suggests that being a women is likely a positive predictor for SPS. Having a first degree relative with CRC was no more strongly associated with SPS than any other polyp groups, somewhat supportive of the lack of genetic causality found in SPS patients so far. Cigarette exposure was found in greater number of patients with SPS than other groups but this didn’t reach statistical significance. Our data adds to the controversy in current literature on whether or not smoking is a risk factor in the serrated pathway[10,11]. Past population-based studies have shown that cancers arising from serrated pathways are specifically associated with cigarette smoking but Liang et al[12] published a meta-analysis in 2009 showing a stronger association with smoking and distal/rectal cancer.
The endoscopic features of our SPS cohort were intrinsic to definition of the disease. Compared to other polyp groups, a greater total number of polyps were discovered in SPS and the size of the dominant polyp was also bigger. SPS was also significantly associated with polyps found with predominance for the right colon. Somewhat surprisingly, the spread of the largest polyp in patients with SSA not meeting WHO criteria was evenly across both right and left colon. In Carballal’s study on CRC risk factors, 53% of the cancers found in a cohort of 293 patients with SPS were discovered in the distal colon[8]. Coupled with the fact that 42% of our SPS patients and 52% of our SSA patients had concurrent adenomas, it seems the pathway to colorectal cancer is indeed a complex interplay between various carcinogenic pathways we are yet to fully untangle.
The cumulative definition of SPS isn’t widely known as it not consistently included in published versions of the WHO criteria. In our study, 14/40 patients could have potentially been mis-diagnosed if the endoscopist didn’t recognise that their cumulative polyp load over successive colonoscopy during the study period were enough to qualify for SPS diagnosis. Although no pre-endoscopic risk factors was found be distinguish the subgroup of SPS diagnosed at index colonoscopy from the subgroup with cumulated polyp count, the fact that both patients with colorectal cancer were diagnosed at a follow-up colonoscopy suggest that there may be important differences in the two groups. Discussion about how to manage patients who may soon develop SPS seems to be sparse. Currently ASGE and ESGE guidelines for patients with SSAs that don’t meet WHO criteria is very similar to adenomas, stratified according to size and number. The earliest recommended followup colonoscopy for serrated polyps without dysplasia is 3 years[13]. Contrasting this with patients with established SPS, the recommended followup colonoscopy is 1 year. Recently, Rivera-Sanchez et al[14] assessed the incremental rate of SPS diagnosis in 191 patients with proximal serrated lesions larger than 5 mm after a positive FOBT. One year after the index colonoscopy’s where they found 11 patients with SPS, they performed a reassessment colonoscopy in 71 patients and found 20 new patients with SPS. It is postulated that the polyps found on reassessment colonoscopy were either missed during initial colonoscopy or newly grown and early reassessment colonoscopy at 1 year substantially improved SPS detection rate in patients with proximal serrated lesions. Results of our study indicate that there is a substantial number of patients with a polyp load that is borderline for fulfilling WHO criteria and Rivera-Sanchez showed there is good yield in performing reassessment colonoscopy as early as 1 year. Hence, we suggest that there is a population of patients with SSAs that are at risk of developing SPS in near future or have missed serrated polyps that would benefit from earlier endoscopic follow up than currently recommended. Larger prospective studies are required to stratify exactly which threshold qualify a patient for earlier screening, but a revision of guidelines to encourage more accurate and earlier detection SPS would be prudent.
At the time of diagnosis, sessile serrated polyposis syndrome (SPS) is associated with high risk of concurrent colorectal cancer. However once patients enter a surveillance program and undergo the recommended yearly colonoscopies, the absolute 5-year colorectal cancer (CRC) risk falls dramatically. Hence, early diagnosis of SPS is crucial as it radically change the natural history of patients with the disorder. Serrated and adenomatous polyps share many clinical risk factors; however it is unclear if certain risk factors have a stronger association with one polyp subtype compared to another.
Despite there being a small number of studies investigating the differences in risk factors for distinct polyp subtypes, no study to date has included SPS as one of the comparators.
Patients diagnosed with SPS were younger and more likely to be Caucasian compared to patients with adenomatous polyps and patients with serrated adenomas not meeting World Health Organization (WHO) criteria. The vast majority of SPS patients diagnosed were women. Patients with SPS were no more likely to have a family history of colorectal cancer or cigarette exposure than other polyp groups. In addition, 14 out of 40 patients with SPS could have potentially been mis-diagnosed if the endoscopist didn’t recognise that their cumulative polyp load over successive colonoscopy over 3 years were enough to qualify for the diagnosis. This suggest that patients with a polyp load that is borderline for fulfilling WHO criteria have a good chance of qualifying for a SPS diagnosis if reassessment colonoscopy is performed as early as 1 year.
There is a population of patients with sessile Serrated adenoma that are at risk of developing SPS in near future or have missed serrated polyps that would benefit from earlier endoscopic follow up than currently recommended. Larger prospective studies are required to stratify exactly which threshold qualifies a patient for earlier screening, but a revision of guidelines to encourage more accurate and earlier detection SPS would be prudent.
SPS refers to sessile serrated polyposis syndrome. It is defined by a set of clinical and histo-pathological features by World Health Organisation.
Well-documented study highlighting the main possible risk factors which could interfere in SPS development. Even though the number of participants in the study is small, the statistical analysis seems to reveal interesting information as concerns the SPS background and the potential of shortening the surveillance interval of the syndrome.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Galanopoulos M S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | van Herwaarden YJ, Verstegen MH, Dura P, Kievit W, Drenth JP, Dekker E, IJspeert JE, Hoogerbrugge N, Nagengast FM, Nagtegaal ID. Low prevalence of serrated polyposis syndrome in screening populations: a systematic review. Endoscopy. 2015;47:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Biswas S, Ellis AJ, Guy R, Savage H, Madronal K, East JE. High prevalence of hyperplastic polyposis syndrome (serrated polyposis) in the NHS bowel cancer screening programme. Gut. 2013;62:475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Bisgaard ML, Fenger K, Bülow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat. 1994;3:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 295] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 4. | Clendenning M, Young JP, Walsh MD, Woodall S, Arnold J, Jenkins M, Win AK, Hopper JL, Sweet K, Gallinger S. Germline Mutations in the Polyposis-Associated Genes BMPR1A, SMAD4, PTEN, MUTYH and GREM1 Are Not Common in Individuals with Serrated Polyposis Syndrome. PLoS One. 2013;8:e66705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Buchanan DD, Sweet K, Drini M, Jenkins MA, Win AK, Gattas M, Walsh MD, Clendenning M, McKeone D, Walters R. Phenotypic diversity in patients with multiple serrated polyps: a genetics clinic study. Int J Colorectal Dis. 2010;25:703-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Burnett-Hartman AN, Passarelli MN, Adams SV, Upton MP, Zhu LC, Potter JD, Newcomb PA. Differences in epidemiologic risk factors for colorectal adenomas and serrated polyps by lesion severity and anatomical site. Am J Epidemiol. 2013;177:625-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 362] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 8. | Carballal S, Rodríguez-Alcalde D, Moreira L, Hernández L, Rodríguez L, Rodríguez-Moranta F, Gonzalo V, Bujanda L, Bessa X, Poves C. Colorectal cancer risk factors in patients with serrated polyposis syndrome: a large multicentre study. Gut. 2016;65:1829-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Hazewinkel Y, Koornstra JJ, Boparai KS, van Os TA, Tytgat KM, Van Eeden S, Fockens P, Dekker E. Yield of screening colonoscopy in first-degree relatives of patients with serrated polyposis syndrome. J Clin Gastroenterol. 2015;49:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Samowitz WS, Albertsen H, Sweeney C, Herrick J, Caan BJ, Anderson KE, Wolff RK, Slattery ML. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006;98:1731-1738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 222] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Barault L, Charon-Barra C, Jooste V, de la Vega MF, Martin L, Roignot P, Rat P, Bouvier AM, Laurent-Puig P, Faivre J. Hypermethylator phenotype in sporadic colon cancer: study on a population-based series of 582 cases. Cancer Res. 2008;68:8541-8546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 233] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 12. | Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009;124:2406-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 369] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 13. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR; United States Multi-Society Task Force on Colorectal Cancer. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1446] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 14. | Rivero-Sanchez L, Lopez-Ceron M, Carballal S, Moreira L, Bessa X, Serradesanferm A, Pozo A, Augé JM, Ocaña T, Sánchez A. Reassessment colonoscopy to diagnose serrated polyposis syndrome in a colorectal cancer screening population. Endoscopy. 2017;49:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |