Published online Aug 16, 2017. doi: 10.4253/wjge.v9.i8.405
Peer-review started: January 22, 2017
First decision: March 28, 2017
Revised: April 23, 2017
Accepted: May 22, 2017
Article in press: May 24, 2017
Published online: August 16, 2017
Processing time: 201 Days and 5 Hours
To describe all abnormal histological findings and their associated endoscopic presentation in patients using mycophenolate mofetil (MMF).
A retrospective review of all individuals prescribed MMF within 6 mo of a colonoscopy or flexible sigmoidoscopy between 07/2009 and 09/2015 was performed within Northwell Health system. Records were analyzed for age, gender, procedure indication, MMF indication, and both gross and microscopic findings. Only reports with abnormal histology were included.
One hundred and eighty-four procedures from 170 patients were found, of which 39 met inclusion criteria. Fifty-one point three percent were female. MMF was used for solid organ transplant in 71.8%. Diarrhea was the indication for 71.8% of colonoscopies. Fifty-nine percent of reports revealed gross and microscopic abnormalities while 41.0% had only microscopic findings. Only 11 patients’ reports (28.2%) indicated a specific histopathology of MMF colitis. Among the entire group, only 23.1% of abnormal histology was isolated proximal to the splenic flexure.
Our results demonstrate a high rate of left sided disease and microscopic findings without gross mucosal abnormalities among patients using MMF. Also, a broader definition of MMF-colonopathy may be appropriate, with a majority of our abnormal histology falling outside of the more narrowly defined MMF-colitis category. Given the high frequency of isolated microscopic abnormalities and distal disease, sigmoidoscopy with random biopsies may be an appropriate, less invasive initial endoscopic examination in selected MMF patients.
Core tip: Gastrointestinal complaints are common among patients using mycophenolate mofetil (MMF). Little information exits to guide an effective endoscopic workup in this population. A retrospective review of all patients prescribed mycophenolate within 6 mo of an endoscopic procedure was performed. Our results demonstrate a high rate of left sided disease and microscopic findings without gross mucosal abnormalities among patients using mycophenolate. A broader definition of MMF-colonopathy may be appropriate, with a majority of our abnormal histology falling outside of the more narrowly defined MMF-colitis category. Our findings suggest sigmoidoscopy with random biopsies may be an appropriate initial evaluation.
- Citation: Izower MA, Rahman M, Molmenti EP, Bhaskaran MC, Amin VG, Khan S, Sultan K. Correlation of abnormal histology with endoscopic findings among mycophenolate mofetil treated patients. World J Gastrointest Endosc 2017; 9(8): 405-410
- URL: https://www.wjgnet.com/1948-5190/full/v9/i8/405.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i8.405
Mycophenolate mofetil (MMF) is an immunosuppressive agent that is used mainly for the prevention of organ transplant rejection, but is increasingly used for autoimmune and hematologic disorders[1,2]. Mycophenolic acid (MPA) is the active metabolite of MMF. MPA prevents the proliferation of lymphocytes by inhibiting inosine monophosphate dehydrogenase, an enzyme in the de novo pathway of purine synthesis[3]. Other mechanisms of MPA immunosuppression have been reported, including apoptosis of activated T-lymphocytes, decreased recruitment of lymphocytes to sites of inflammation, and decreased nitric oxide-mediated tissue damage[4]. Enterocytes are also dependent on the de novo pathway of purine synthesis and become potential targets for MPA[1,4]. This can lead to gastrointestinal toxicity, which typically manifests as diarrhea, and can occur in up to 36% of patients[1].
For many patients using MMF who develop diarrhea, the severity of complaints may prompt a formal workup. In cases with negative stool studies for infectious causes, endoscopic examination either with flexible sigmoidoscopy or colonoscopy may be performed[5]. In those individuals with abnormal histology, it is then critical to differentiate MMF-related colitis from colitis of other etiologies such as new onset inflammatory bowel disease (IBD) or atypical infection. Accurate diagnosis is critical to proper use of MMF, as dose modification and/or discontinuation of MMF risks organ rejection or reactivation of autoimmune disease[2,6].
Prototypical histopathology of MMF colitis has been described as “prominent crypt cell apoptosis and reactive/reparative changes including enterocyte cytologic atypia, increased neuroendocrine cells, and glandular architectural distortion”[7]. While a pathologist informed of MMF usage may identify a typical pattern of MMF related injury[1,2,4], and specify a finding as “MMF colitis”, a broader spectrum of abnormal histology associated with MMF appears to exist[2]. In addition, the endoscopic findings related to MMF colitis, and other MMF associated abnormal histology, are not well described - including the gross nature of lesions and typical distribution within the colon. Most prior studies have been limited to case reports and retrospective studies and have focused on abnormal histopathology associated with MMF usage without addressing the endoscopic appearance or patterns of distribution within the GI tract[4].
As with other disorders such as Crohn’s disease and microscopic colitis, knowledge of anatomic disease distribution and associated presence or absence of gross mucosal abnormalities are critical to guide an effective work up. Our aim was to describe all abnormal histological findings and their associated endoscopic presentation in all patients undergoing colonoscopy while using MMF.
We conducted a retrospective review of all patients who were 18 years of age and older and had documented use of MMF within 6 mo of undergoing a colonoscopy or flexible sigmoidoscopy from July 2009 to September 2015. The study was conducted within the North Shore-LIJ Health System (now Northwell Health) after obtaining institutional review board approval. Only sigmoidoscopy or colonoscopy reports with abnormal histology described on the official pathology report were included in the review. Sigmoidoscopy or colonoscopy reports with normal pathology or missing pathology report were excluded. In patients with multiple eligible colonoscopies or sigmoidoscopies, the procedure report nearest to the date of MMF prescribing was the one included in the analysis. If a patient had both an eligible colonoscopy and flexible sigmoidoscopy, the colonoscopy report was used.
Demographic information, indication for colonoscopy, indication for MMF, gross and histological findings were recorded. All pathology samples were evaluated by experienced gastrointestinal pathologists. Only pathology reports specifically citing MMF use as the likely etiology were classified as “MMF-colitis”. All remaining abnormal findings were broadly classified as “other” abnormal findings, and sub-categorized according to their description in the pathology report. Abnormal histology findings proximal to the splenic flexure were defined as right colonic; findings distal to the splenic flexure were defined as left colonic. Abnormal findings occurring both proximal and distal to the splenic flexure were defined as pancolonic. Location and description of abnormal gross findings on colonoscopy/sigmoidoscopy examination also corresponded to the official procedure report. Our goals were to describe abnormal histological findings, their location within the colon, and the presence or absence of associated gross endoscopic findings.
A total of 184 colonoscopies and sigmoidoscopies from 170 patients were reviewed during the study period. Overall, screening was the most common indication for a procedure, with organ transplant as the most common indication for MMF use (Table 1). Of these, 34 colonoscopies and 5 sigmoidoscopies from 39 individual patients met inclusion criteria. Fifty-one point three percent were female. Average age at time of procedure was 51.44 years old. For this group with abnormal histology, diarrhea was the most common indication, accounting for 71.8% of the combined sigmoidoscopies and colonoscopies. Two patients had procedures for history of inflammatory bowel disease. Indications for MMF therapy were: Solid organ transplant (71.8%), hematologic disorders (17.9%) and autoimmune disease (10.3%). Of the 28 solid organ transplant patients, 27 were from renal transplants and 1 was from a lung transplant. Demographics of colitis among MMF treated patients (Table 2).
| Characteristic | |
| mean age | 57.05 |
| Female | 89 (48.4) |
| Indication for procedure2 | |
| Screening | 72 (39.1) |
| Diarrhea | 51 (27.7) |
| Bleeding | 24 (13.0) |
| Anemia | 20 (10.9) |
| Abdominal pain | 14 (7.7) |
| Constipation | 3 (1.6) |
| Weight loss | 3 (1.6) |
| Abnormal imaging | 3 (1.6) |
| Bloating | 1 (0.52) |
| Other (history of IBD, amyloid, stricture) | 9 (4.9) |
| Indication for MMF | |
| Organ transplant (kidney, liver, lung) | 116 |
| Autoimmune | 35 |
| Blood disorder | 7 |
| Unknown indication | 26 |
| Abnormal mucosa (gross) | 40 |
| With normal biopsies | 10 |
| With abnormal biopsies | 25 (2 duplicates) |
| Without biopsies1 | 5 |
| Normal mucosa (gross) | 144 |
| With normal biopsies | 44 (normal biopsy or polypectomy, 1 for mass) |
| With abnormal biopsies | 17 (1 duplicate) |
| Without biopsies1 | 83 |
| Characteristic | |
| n | 39 |
| mean age | 51.44 |
| Female | 20 (51.3) |
| Indication for procedure | |
| Diarrhea | 28 (71.8) |
| Bleeding | 2 (5.1) |
| Anemia | 1 (2.6) |
| Screening | 4 (10.3) |
| Abnormal imaging | 2 (5.1) |
| Other (history of IBD) | 2 (5.1) |
| Indication for MMF | |
| Organ transplant | 28 (71.8) |
| Autoimmune | 4 (10.3) |
| Blood disorder | 7 (17.9) |
Of the 39 patient reports meeting inclusion criteria, only 11 patient pathology reports (28.2%) indicated a specific histopathology of MMF colitis. Notably, only 9 of 39 (23.1%) specimen request forms sent to pathology provided history of MMF use. Non-specific colitis was identified in 30.8% of the reports. Four reports indicated graft vs host disease (GVHD), and all were from patients who were on MMF for leukemia or lymphoma and underwent stem cell transplant. The remaining twelve cases are included in Table 3.
| Solid organ transplant (n) | Autoimmune (n) | Blood disorder (n) | Total (n) | |
| MMF colitis | 10 | 1 | 0 | 11 |
| Graft vs host disease | 0 | 0 | 4 | 4 |
| Nonspecific colitis | 11 | 0 | 1 | 12 |
| Other | 3 (hyperplastic), 1 (lymphoid aggregate), 1 (kayexalate), 1 (ischemic), 1 (amyloid) | 1 (IBD), 1 (lymphoid aggregate), 1 (hyperplastic) | 1 (AML), 1 (reactive) | 12 |
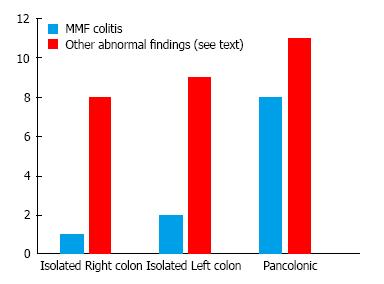
Overall 23 (59.0%) of abnormal histology corresponded to a reported gross endoscopic abnormality, while 16 (41.0%) demonstrated abnormal histology without a gross abnormality. Of the 28 procedures performed for an indication of diarrhea, 13 (46.4%) of abnormal histology corresponded to a gross endoscopic abnormality, while 15 (53.6%) demonstrated abnormal histology without a gross abnormality. Among the entire 39 cases reviewed only 23.1% of abnormal histology was isolated to the right colon. Among the subgroup of 11 MMF-colitis cases, only one (9.1%) was isolated to the right colon, Figure 1.
In our study out of 184 procedures performed on 170 patients using MMF, only 39 colonoscopies/sigmoidoscopies had abnormal pathology. Of these, only 28.2% demonstrated a specific histopathology of MMF colitis. Endoscopic and histological evidence of colitis in patients who developed diarrhea while using MMF have been previously studied[4]. Those reports, which evaluated the histopathological findings of this MMF-colitis, have shown a variety of features including prominent crypt cell apoptosis and reactive/reparative changes including enterocyte cytologic atypia, lamina propria inflammation, and crypt architectural disarray[8]. In our study, similar findings were described for MMF-colitis, with the most common feature being cell apoptosis. While the presence of apoptosis is regarded as more typical of a true MMF-related colitis[8], there is no consensus regarding the spectrum of abnormal histology related to MMF use. Our findings of frequent nonspecific colitis and other histological abnormalities suggests that a broader definition of MMF-colonopathy may be required, with a majority of our abnormal histology falling outside of the more narrowly defined MMF-colitis category.
Prior case series and reports were able to categorize abnormal histological findings in patients on MMF into IBD-like, GVHD-like, ischemic-like, acute colitis, or non-specific colitis[2,9]. In our study, 30.8% of the reports indicated non-specific colitis. This high frequency of non-specific colitis was also found by de Andrade et al[9]. In addition to non-specific colitis, there were 4 reported cases of GVHD in our study, all in patients with hematologic disease and a history of bone marrow transplant. This suggests that the transplant itself is the cause of the abnormal findings rather than the MMF, although previous studies have described GVHD-like pathology in patients on MMF following solid organ transplant[1,2,4]. Additionally, it can be difficult to histologically differential between GVHD and MMF colitis. Star et al[10] have found that high eosinophilic count, absence of neuroendocrine cell clusters and apoptotic microabscess are more suggestive of MMF colitis over GVHD. Ischemic-like pathology can also be found in patients on MMF. Johal et al[8] described MMF-induced segmental colitis mimicking ischemic colitis. There was one case of ischemic-like pathology found in our study, however, given lack of multiple biopsies, it is unknown if this patient had segmental colitis. Regardless of the pathology, most of these patients underwent colonoscopy due to diarrhea.
Gastrointestinal toxicity, usually manifested as diarrhea, is the most common side effect of MMF[8]. The reported incidence of diarrhea is variable and can range from 13% to 64%[4]. Diarrhea was the most common indication for colonoscopy in our study population (71.8%). Even though the exact mechanism of MMF induced diarrhea is unknown, different etiologies have been proposed[4]. In addition to the impact on the quality of life, diarrhea can lead to non-compliance, weight loss, and physician-directed MMF dose reduction[1].
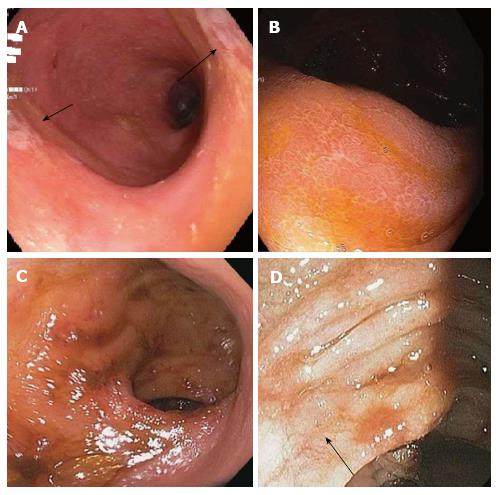
Data describing the presence of macroscopic abnormalities and distribution of findings associated with MMF-related colitis is limited. Calmet et al[4] found macroscopic findings ranging from erythema to erosions and ulcers. About half of the patients they studied had normal macroscopic findings, similar to our findings of abnormal histology without endoscopic mucosal changes in 41% patients, with others demonstrating erythema, friability, granularity, loss of vascularity, and ulcerations. These findings were found across MMF-related colitis including GVHD, AML, and MMF colitis as shown in Figure 2. Along with Calmet et al[4], our results showing such a high rate of isolated microscopic abnormalities strongly support a diagnostic protocol including random biopsies of normal appearing mucosa in patients on MMF with colitis-like complaints.
Our patients also demonstrated a low frequency of abnormal histology (23.1%) limited to the right colon, proximal to the splenic flexures. This finding was even more pronounced for the subgroup of MMF-colitis, of which only one case was isolated proximal to the splenic flexure. This appears similar to the findings of Calmet et al[4]. In a smaller sample of 20 patients they found that 25% of MMF related colitis was found in the right colon, though “right colon” was not explicitly defined[4]. Our findings suggest that a viable strategy for evaluation in this patient population could be to initially perform by a sigmoidoscopy, moving on to full colonoscopy only if distal findings are negative. While sigmoidoscopy is no longer commonly used for colon cancer screening purposes, it remains a valuable tool for the evaluation of other gastrointestinal conditions. Notably, it may be used for the diagnosis and monitoring of response to therapy in ulcerative colitis due to the near universal involvement of the rectum and left-colon typical of the disorder. Our findings suggest a similar role for sigmoidoscopy for the workup of lower gastrointestinal complaints in patients using MMF. Significant advantages to sigmoidoscopy compared to colonoscopy include avoidance of sedation and lower risk of perforation. Also, since so many of these patients have a history of renal transplantation, the avoidance of a full bowel preparation, required by colonoscopy but unnecessary for sigmoidoscopy, lowers the risk of renal compromise, which has been associated with certain colonoscopy preparations[11].
In our current series we found a relatively small number of abnormal histology reported with a diagnosis specific for MMF colitis. Notably, a similarly small number of samples that were sent to pathology directly specified usage of MMF. This implies that some pathologists, if they are not aware of MMF use, or are less familiar with MMF-related colitis, may not consider the diagnosis of MMF-related colitis, leading to decreased specificity of findings and lingering diagnostic uncertainty. It would likely be of value to pathologists to make them aware of MMF usage, so as to improve the rate at which MMF-related colitis is appreciated in pathology samples. Alternatively, as discussed, a more broadly defined MMF-colonopathy may need to be considered when evaluating and managing patients with any abnormal histology using MMF.
Our study had some limitations. The total number of patients we analyzed for abnormal histological findings, while large by comparison to other case series, was still small in absolute numbers due to the rareness of MMF use and occurrence of these findings. Also, though the electronic medical record (EMR) allowed us to track MMF prescribing, we could not confirm patient compliance. In addition, though we did analyze a significant amount of demographic information, there were limits to the EMR, such as our inability to consider patient race/ethnicity as part of our analysis.
In summary, ours is the largest study correlating all abnormal pathology associated with MMF use with both gross endoscopic findings and disease distribution. Our findings reinforce the importance of random biopsies of grossly normal appearing colonic mucosa towards making an accurate diagnosis. Importantly, our findings also support a first line diagnostic approach using a less invasive, lower risk sigmoidoscopy coupled with routine biopsy in selected MMF patients with appropriate complaints. Moving forward, valuable avenues of research would include outcomes analysis of patients diagnosed with MMF-related colonic abnormalities after intervention, whether that be via dose modification or discontinuation of MMF or other means of treatment. This would be valuable in further confirming the clinical significance of these findings, as well as the therapeutic benefit of accurately confirming such a diagnosis
Gastrointestinal complaints are common among patients using mycophenolate mofetil (MMF). Abnormal biopsy findings have been described in this population, but little information exits to guide an effective endoscopic workup of those on MMF such as the presence or absence of gross endoscopic findings and their anatomic distribution.
Abnormal biopsy findings have been described in patients treated with MMF. The current research focus is to evaluate the endoscopic findings and the proper endoscopic workup in patients with gastrointestinal complaints on MMF.
Prior studies have only described the histology in a limited number of patient on MMF. Most of these studies were limited to renal transplant patients. The authors looked at all patients treated with MMF for a variety of illnesses. Also, there is very limited information describing gross mucosal findings and anatomic distribution of abnormal findings within the MMF population. The authors results demonstrate a high rate of left sided disease and microscopic findings without gross mucosal abnormalities among patients using MMF.
A broader definition of MMF-colonopathy may be appropriate, with a majority of the authors’ abnormal histology falling outside of the more narrowly defined MMF-colitis category. The authors findings support sigmoidoscopy with random biopsies may be an appropriate initial endoscopic evaluation in patients with bowel complaints using MMF.
MMF is an immunosuppressive agent that is used mainly for the prevention of organ transplant rejection. This can lead to gastrointestinal toxicity, which typically manifests as diarrhea. Right colonic findings were defined as abnormal histology findings proximal to the splenic flexure. Left colonic findings were defined as abnormal histology findings distal to the splenic flexure.
This paper study the correlation of abnormal histology with endoscopic findings among Mycophenolate Mofetil treated patients, the sample is large.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cui J S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Lee S, de Boer WB, Subramaniam K, Kumarasinghe MP. Pointers and pitfalls of mycophenolate-associated colitis. J Clin Pathol. 2013;66:8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Selbst MK, Ahrens WA, Robert ME, Friedman A, Proctor DD, Jain D. Spectrum of histologic changes in colonic biopsies in patients treated with mycophenolate mofetil. Mod Pathol. 2009;22:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Ransom JT. Mechanism of action of mycophenolate mofetil. Ther Drug Monit. 1995;17:681-684. [PubMed] |
| 4. | Calmet FH, Yarur AJ, Pukazhendhi G, Ahmad J, Bhamidimarri KR. Endoscopic and histological features of mycophenolate mofetil colitis in patients after solid organ transplantation. Ann Gastroenterol. 2015;28:366-373. [PubMed] |
| 5. | Behrend M. Adverse gastrointestinal effects of mycophenolate mofetil: aetiology, incidence and management. Drug Saf. 2001;24:645-663. [PubMed] |
| 6. | Al-Absi AI, Cooke CR, Wall BM, Sylvestre P, Ismail MK, Mya M. Patterns of injury in mycophenolate mofetil-related colitis. Transplant Proc. 2010;42:3591-3593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Papadimitriou JC, Cangro CB, Lustberg A, Khaled A, Nogueira J, Wiland A, Ramos E, Klassen DK, Drachenberg CB. Histologic features of mycophenolate mofetil-related colitis: a graft-versus-host disease-like pattern. Int J Surg Pathol. 2003;11:295-302. [PubMed] |
| 8. | Johal K, Ratuapli SK, Lam-Himlin DM, Gurudu SR. Mycophenolate mofetil-induced segmental colitis mimicking ischemic colitis. Case Rep Gastroenterol. 2014;8:95-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | de Andrade LG, Rodrigues MA, Romeiro FG, Garcia PD, Contti MM, de Carvalho MF. Clinicopathologic features and outcome of mycophenolate-induced colitis in renal transplant recipients. Clin Transplant. 2014;28:1244-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Star KV, Ho VT, Wang HH, Odze RD. Histologic features in colon biopsies can discriminate mycophenolate from GVHD-induced colitis. Am J Surg Pathol. 2013;37:1319-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE; American Society of Colon and Rectal Surgeons; American Society for Gastrointestinal Endoscopy; Society of American Gastrointestinal and Endoscopic Surgeons. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006;63:894-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |