Published online Jul 16, 2017. doi: 10.4253/wjge.v9.i7.304
Peer-review started: November 9, 2016
First decision: December 27, 2016
Revised: March 6, 2017
Accepted: March 23, 2017
Article in press: March 24, 2017
Published online: July 16, 2017
Processing time: 238 Days and 12.7 Hours
To evaluate the efficacy of a newly developed dilator for endoscopic ultrasound (EUS)-guided drainage (ES Dilator).
Fourteen consecutive patients who had undergone EUS-guided choledochoduodenostomy (EUS-CDS) with the ES Dilator were identified from a prospectively maintained database and enrolled in the study group. Fourteen other patients who had undergone EUS-CDS without the dilator just prior to its introduction were analyzed as the control group. A historical cohort study was carried out comparing the two groups. The main outcome measurement was the procedure time. The technical success rate and early AE rate were also compared between the two groups.
There were no significant differences in age, sex and etiology of biliary obstruction. The utilization rate of a plastic stent was higher in the control group (36% vs 0%). The technical success rate was 100% in both groups. The mean procedure time was significantly shorter in the study group than in the control group (27 ± 7 min vs 44 ± 26 min, P = 0.026). Additionally, there were no patients who required more than 40 min for the procedure in the study group. Early adverse events occurred in 29% (4/14) of the control group whereas none in the study group. The adverse events in all 4 patients was bile peritonitis, including pan-peritonitis in one patient. All patients recovered with conservative treatment by medication.
The newly developed dilator was found to be useful for shortening procedure time and would prevent adverse events related to bile leakage in EUS-CDS.
Core tip: The newly developed dilator (ES Dilator®) was useful for shortening procedure time and would prevent adverse events related to bile leakage in endoscopic ultrasound-guided choledochoduodenostomy.
- Citation: Kanno Y, Ito K, Koshita S, Ogawa T, Masu K, Masaki Y, Noda Y. Efficacy of a newly developed dilator for endoscopic ultrasound-guided biliary drainage. World J Gastrointest Endosc 2017; 9(7): 304-309
- URL: https://www.wjgnet.com/1948-5190/full/v9/i7/304.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i7.304
Endoscopic ultrasound (EUS)-guided biliary drainage (EUS-BD) is a challenging palliative treatment for biliary obstruction in patients who have unsuccessfully undergone transpapillary stenting[1]. Despite its difficulty, the technical success rate of this procedure is over 90% according to reports from high volume centers, which seems sufficiently high and acceptable[2-4]. When EUS-BD has been successfully accomplished, biliary decompression is achieved in most patients[2,5,6]. Moreover, the patency of the deployed stent is expected to be reasonably long[2,5,6].
EUS-BD can cause some adverse events (AEs) induced by bile leakage. Although bile always leaks in varying degrees in EUS-BD, a lesser amount of bile leakage may result in fewer AEs, including peritonitis and biloma formation.
Bile leakage occurs between puncture of the bile duct and stent deployment at the puncture tract, and thus shortening of the procedure time between puncture and stenting would contribute to less bile leakage. One of the most important factors resulting in longer procedure time is the difficulty of dilation of the puncture tract. When the dilation is unimpededly achieved, EUS-BD would be smoothly performed in many cases.
Recently, a new dilator for smooth dilation of the puncture tract has been developed. In this study, the efficacy of this dilator in EUS-guided choledochoduodenostomy (EUS-CDS) was evaluated.
Fourteen consecutive patients who had undergone EUS-CDS utilizing the new dilator for malignant biliary obstruction at Sendai City Medical Center (Sendai, Japan) between November 2012 and January 2016 were identified from a prospectively maintained database and enrolled in the study group. Fourteen other consecutive patients who had undergone EUS-CDS without the dilator just prior to its introduction between February 2010 and October 2012 were analyzed as a control group. This retrospective study was conducted after approval by the Sendai City Medical Center Institutional Review Board. The ID issued by UMIN was 000020772.
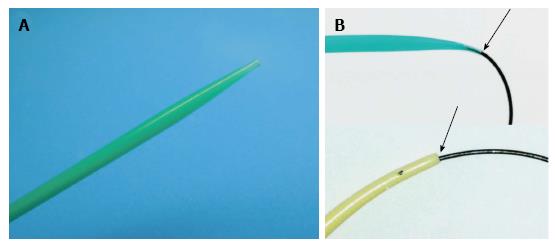
The newly developed dilator, ES Dilator® (Zeon Medical Co., Tokyo, Japan), 7 French (Fr) in diameter, is characterized by high pushability and a lesser difference in diameters of the inner lumen and the guidewire (Figure 1). It has two types of internal diameter tailored to accommodate 0.025-inch and 0.035-inch guidewires. The ES Dilator was utilized in all patients after its use was commenced in November 2012. It is commercially available in Japan.
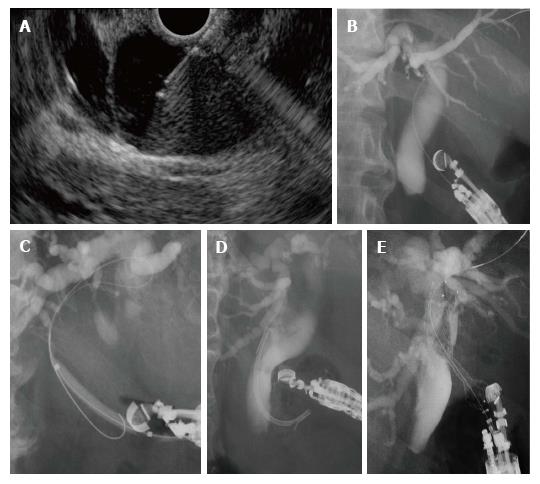
With a linear echoendoscope (UC240P or UCT260, Olympus Medical Systems Co., Tokyo, Japan), the extrahepatic bile duct was punctured from the duodenum by a 19-gauge needle for endosonography-guided fine needle aspiration (EUS-FNA) (EchoTip, Cook Co. Bloomington, Indiana; or Expect, Boston Scientific, Natick, Massachusetts). After contrast medium had been injected into the bile duct, a guidewire was advanced to the hilar side. After the puncture tract was dilated along the guidewire, a stent was finally placed at the puncture site (Figure 2).
Prior to the availability of the ES Dilator in November 2012, dilation was performed with a 5- to 7-French tapered catheter, including a Soehendra dilator (Boston Scientific), and a 4-mm balloon dilator. After November 2012, insertion of the ES Dilator was initially attempted in all patients in the study group. When dilation was not achieved with these catheters, a cautery dilator was utilized.
Procedures were performed by one of 6 expert endoscopists who had experience performing 10 or more EUS-guided drainage procedures as an operator or assistant. All of them had also experienced more than 1000 endoscopic retrograde cholangiopancreatography (ERCP) procedures and 1000 EUS examinations as an operator.
A historical cohort study was carried out, the population being divided into a control group consisting of 14 patients who received the intervention without use of the ES Dilator and a study group of 14 patients in whom the dilator was used.
Procedure time was defined as the main outcome measure. The technical success rate and early AE rate were also compared between the two groups.
Early AEs investigated included bile peritonitis, biloma formation, hemorrhage requiring endoscopic/radiological/surgical intervention or blood transfusion, stent dislocation within 7 d, and procedure-related death. Bile peritonitis was defined as a state with abdominal tenderness accompanied by peritoneal symptoms which appeared within 24 h after the intervention.
Student’s t-test was used for continuous data comparison. Fisher’s exact probability test and χ2 test were used for comparison of categorical data. A P-value of < 0.05 was considered to be significant. For analyses, SPSS software (ver.11, SPSS, Chicago, IL, United States) was used.
The patient characteristics of the two groups are shown in Table 1. There were no significant differences in age, sex and etiology of biliary obstruction. The utilization rate of a plastic stent was higher in the control group. Plastic stents used in the control group were 7-Fr Flexima (Boston Scientific, Natick, Mass, United States). Metal stents, 10-mm covered Zeostents (a delivery system 8 Fr in diameter, Zeon Medical Co.) were used in all 9 patients of the control group and in 9 patients of the study group; 10-mm X-SuiteNIR stents (a delivery system approximately 7.5 Fr in diameter, Olympus Medical Systems Co.) were used in 3 patients of the study group; and 10-mm partially covered Niti-S stents (a delivery system approximately 8.5 Fr in diameter, TaeWoong Medical Co., Wolgot-myeon, South Korea) were used in 2 patients of the study group.
| Study group (n = 14) | Control group (n = 14) | P value | |
| Age (yr), mean ± SD | 74.6 ± 16.1 | 73.5 ± 9.3 | 0.82 |
| Sex (male:female) | 9:5 | 8:6 | 1.00 |
| Etiology | 0.28 | ||
| Pancreatic cancer | 8 | 12 | |
| Biliary cancer | 3 | 1 | |
| Duodenal cancer | 2 | 0 | |
| Metastatic lymph nodes | 1 | 1 | |
| Deployed stent | 0.041 | ||
| Plastic stent | 0 | 5 | |
| Metal stent | 14 | 9 |
The technical success rate was 100% in both groups.
The procedure time was significantly shorter in the study group than in the control group (27 ± 7 min vs 44 ± 26 min, P = 0.026, Table 2). Additionally, there were no patients who required more than 40 min for the procedure in the study group.
| Study group (n = 14) | Control group (n = 14) | P value | |
| Procedure time (min), mean ± SD | 44 ± 26 | 27 ± 7 | 0.026 |
| ≤ 20 | 3 | 1 | |
| 20-40 | 11 | 8 | |
| 40-60 | 0 | 2 | |
| > 60 | 0 | 3 |
Because neither a 5-French tapered catheter nor a balloon catheter could pass through the puncture tract, a cautery catheter was used in only one patient (7%) in the control group. In the study group, the ES Dilator passed through the puncture site on the first attempt and a cautery dilator was not used in any of the patients.
The mean procedure time in the patients who received metal stent placement in the control group was 38 ± 23 min. In comparison with the study group, it was also found to be shorter although the difference was not statistically significant (P = 0.18).
Early AEs occurred in 29% (4/14) in the control group whereas no AEs occurred in the study group (Table 3). The AE in all 4 patients was bile peritonitis, including pan-peritonitis in one patient. All patients recovered with conservative treatment by medication. The procedure time in the 4 patients who developed peritonitis was 39, 45, 67, and 95 min. Among these 4 patients, a metal stent was deployed in 2 and a plastic stent in 2. The patients whose intervention required a longer procedure time (95 min) with a metal stent had severe pan-peritonitis although it did not progress to death.
| Study group (n = 14) | Control group (n = 14) | P value | |
| Overall | 0 | 4 (29%) | NA |
| Localized peritonitis | 0 | 3 | |
| Pan-peritonitis | 0 | 1 | |
| Hemorrhage | 0 | 0 | |
| Death | 0 | 0 |
Many reports on EUS-guided drainage have been published at an accelerated pace in the past decade[1-4,7], and this procedure has rapidly superseded percutaneous biliary drainage as an alternative technique after failed ERCP[4,8]. Some studies have reported that EUS-BD has a similar level of efficacy and results in fewer adverse events in comparison with percutaneous drainage[9,10]. EUS-BD seems to be the palliative intervention of choice after failed ERCP in cases with malignant distal biliary obstruction[9,10].
Due to a lack of dedicated devices for EUS-guided drainage, various devices developed for other endoscopic interventions, including EUS-FNA and ERCP, have been applied. Dilation of the puncture tract in EUS-CDS has been performed with tapered catheters and balloon catheters developed for the purpose of aspiration of bile or pancreatic juice, dilation of a biliary stricture, or endoscopic papillary balloon dilation in ERCP. However, they are inadequate for advancement into the narrow tract made by a fine needle because of their deficiency of stiffness and the difference of diameter between the inner lumen of the device and the guidewire. The ES Dilator seems to have resolved these problems.
Cautery devices would also be better in dilation of the puncture tract. It remains unknown whether physical dilation or electric dilation is more appropriate because there have been no studies comparing them. Cautery devices have not been initially used at our institution because an unexpected huge hole might be formed by electric ablation[9]. However, such a device has been used initially in some institutions with a high success rate and low AE rate[3,10]. Although there is a retrospective study reporting that electric dilation by a needle knife was the risk factor for postprocedural AEs, it is doubtful that mere needle-knife utilization was actually related to AEs because it was used only when insertion of a 6-Fr tapered catheter failed[2].
Whereas the ES Dilator shortens procedure time in EUS-CDS, such shortening would be uncertain in EUS-guided hepaticogastrostomy (EUS-HGS). EUS-HGS is considered to include other various factors relevant to longer procedure time, i.e., difficulty in puncture of an appropriate hepatic duct, difficulty in guidewire insertion into the hilar side, and an inexpedient increase of the distance between the liver and the gastric wall which move apart from each other when a metal stent is advanced. Moreover, dilation of the puncture tract is often easier because of less mobility of the intrahepatic bile duct in EUS-HGS, whereas the extrahepatic bile duct can move and separate from the gastrointestinal wall in EUS-CDS. In addition, although the liver parenchyma always intervenes in the puncture tract and prevents bile leakage in EUS-HGS as in the case of percutaneous transhepatic gallbladder/biliary drainage, there is little intervening tissue in EUS-CDS, resulting in the likelihood of bile leakage. Thus, prevention of bile leakage is considered to be more essentially important in EUS-CDS than in EUS-HGS. Therefore, especially in EUS-CDS, the ES Dilator is considered to have a favorable effect, and thus this study was limited to EUS-CDS.
The ES Dilator, unfortunately, has an extremely low visibility of the fluoroscopic image. Although it did not seem to affect the procedural success rate and the adverse events rate, it would need to be improved.
The type of deployed stent can affect the procedural outcomes. Although covered metal stents are more difficult to advance through the narrow tract than plastic stents, the procedure time was significantly shorter in the study group in which all the patients underwent intervention with a covered metal stent. Additional dilation after dilation with the 7-Fr ES Dilator was unnecessary in all patients of the study group, indicating that the most important factor related to successful EUS-CDS is dilation just up to 7-Fr, not up to the diameter of the stent which is to be inserted. On the other hand, covered metal stents could limit bile leakage after stent deployment. It is also worth noting that the 2 patients among 4 who had peritonitis in the control group received intervention with a metal stent. Metal stents are not always advantageous in preventing bile peritonitis.
This study has some limitations, namely, it was a retrospective investigation with a small population. Additionally, the proficiency level of the endoscopist may have been associated with the shorter procedure time. Despite these limitations, the present data appear to be of value because this study was carried out at a referral center which had experienced more than 30 cases of successful EUS-guided drainage before the study period. It seems to be less valuable to prospectively carry out large population studies for evaluation of a mere dilator in a field which has been rapidly evolving regardless of the low number of such patients.
In conclusion, the newly developed ES Dilator which was dedicated to EUS-BD was found to be useful for shortening procedure time and may prevent AEs relevant to bile leakage in EUS-CDS.
Dr. Naotaka Fujita, the former vice director of our center, made an enormous contribution to the development of the new dilator introduced in the present study. I would like to express my deepest gratitude to him.
Endoscopic ultrasound (EUS)-guided biliary drainage is now an alternative option when transpapillary drainage has failed. Due to the lack of dedicated devices for use in such cases, dilation of the punctured tract is often difficult, resulting in longer procedure time and adverse events.
Many new devices have been developing for EUS-guided drainage.
The newly developed dilator characterized by high pushability and a lesser difference in diameter tailored to accommodate 0.025-inch and 0.035-inch guidewires has become available.
The dilator was found to be useful.
The authors reported a novel dilator for the use of EUS-guided choledochoduodenostomy (EUS-CDS). In this paper the authors retrospectively compare the incidence of complications in patients who palliativelly underwent EUS-CDS with/without ES Dilator.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Lleo A, Teoh AYB S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Horaguchi J, Fujita N, Noda Y, Kobayashi G, Ito K, Obana T, Takasawa O, Koshita S, Kanno Y. Endosonography-guided biliary drainage in cases with difficult transpapillary endoscopic biliary drainage. Dig Endosc. 2009;21:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Park DH, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 3. | Hara K, Yamao K, Niwa Y, Sawaki A, Mizuno N, Hijioka S, Tajika M, Kawai H, Kondo S, Kobayashi Y. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol. 2011;106:1239-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Dhir V, Itoi T, Khashab MA, Park DH, Yuen Bun Teoh A, Attam R, Messallam A, Varadarajulu S, Maydeo A. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest Endosc. 2015;81:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 5. | Yamao K, Bhatia V, Mizuno N, Sawaki A, Ishikawa H, Tajika M, Hoki N, Shimizu Y, Ashida R, Fukami N. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: results of long-term follow-up. Endoscopy. 2008;40:340-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 6. | Horaguchi J, Fujita N, Noda Y, Kobayashi G, Ito K, Koshita S, Kanno Y, Ogawa T, Masu K, Hashimoto S. Metallic stent deployment in endosonography-guided biliary drainage: long-term follow-up results in patients with bilio-enteric anastomosis. Dig Endosc. 2012;24:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 480] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 8. | Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 9. | Itoi T, Itokawa F, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Moriyasu F. Endoscopic ultrasound-guided choledochoduodenostomy in patients with failed endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2008;14:6078-6082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 86] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Hara K, Yamao K, Hijioka S, Mizuno N, Imaoka H, Tajika M, Kondo S, Tanaka T, Haba S, Takeshi O. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy. 2013;45:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |