Published online Jun 16, 2017. doi: 10.4253/wjge.v9.i6.273
Peer-review started: January 19, 2017
First decision: March 13, 2017
Revised: April 4, 2017
Accepted: May 18, 2017
Article in press: May 19, 2017
Published online: June 16, 2017
Processing time: 149 Days and 22.5 Hours
To compare high definition white light endoscopy and bright narrow band imaging for colon polyps’ detection rates.
Patients were randomised to high definition white light endoscopy (HD-WLE) or the bright narrow band imaging (bNBI) during withdrawal of the colonoscope. Polyps identified in either mode were characterised using bNBI with dual focus (bNBI-DF) according to the Sano’s classification. The primary outcome was to compare adenoma detection rates (ADRs) between the two arms. The secondary outcome was to assess the negative predictive value (NPV) in differentiating adenomas from hyperplastic polyps for diminutive rectosigmoid lesions.
A total of 1006 patients were randomised to HD-WLE (n = 511) or bNBI (n = 495). The mean of adenoma per patient was 1.62 and 1.84, respectively. The ADRs in bNBI and HD-WLE group were 37.4% and 39.3%, respectively. When adjusted for withdrawal time (OR = 1.19, 95%CI: 1.15-1.24, P < 0.001), the use of bNBI was associated with a reduced ADR (OR = 0.69, 95%CI: 0.52-0.92). Nine hundred and thirty three polyps (86%) in both arms were predicted with high confidence. The sensitivity (Sn), specificity (Sp), positive predictive value and NPV in differentiating adenomatous from non-adenomatous polyps of all sizes were 95.9%, 87.2%, 94.0% and 91.1% respectively. The NPV in differentiating an adenoma from hyperplastic polyp using bNBI-DF for diminutive rectal polyps was 91.0%.
ADRs did not differ between bNBI and HD-WLE, however HD-WLE had higher ADR after adjustment of withdrawal time. bNBI surpassed the PIVI threshold for diminutive polyps.
Core tip: Adenoma detection rate (ADR) is one of the most important quality measures in colonoscopy and bright narrow band imaging (bNBI) can theoretically improve imaging and thus reconnaissance of colorectal polyps. In addition, the magnification using bNBI with dual focus (bNBI-DF) allows the prediction of the polyp’s histology. This multicenter randomised controlled trial was conceived to compare the ADR of high definition white light endoscopy (HD-WLE) vs bNBI during withdrawal of screening colonoscopies. No difference was found in ADR between HD-WLE and bNBI. The prediction of diminutive distal polyps with bNBI-DF was satisfactory according to the American Society for Gastrointestinal Endoscopy’s threshold.
- Citation: Singh R, Cheong KL, Zorron Cheng Tao Pu L, Mangira D, Koay DSC, Kee C, Ng SC, Rerknimitr R, Aniwan S, Ang TL, Goh KL, Ho SH, Lau JYW. Multicenter randomised controlled trial comparing the high definition white light endoscopy and the bright narrow band imaging for colon polyps. World J Gastrointest Endosc 2017; 9(6): 273-281
- URL: https://www.wjgnet.com/1948-5190/full/v9/i6/273.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i6.273
Colorectal cancer is a leading cause of morbidity and mortality worldwide[1]. Its incidence in Asia-Pacific region has been rising at an alarming rate[2,3]. Screening colonoscopy and polypectomy have been shown to reduce the mortality related to colorectal cancer[4]. Despite its effectiveness, the potential to miss polyps can range between 15% to 30% with screening colonoscopy[5]. Current guidelines recommend removal of all visible polyps (except benign diminutive distal polyps) and subjection to histological assessment, irrespective of their endoscopic morphological features. This could make colonoscopy a less cost effective screening strategy[6]. Novel image enhanced endoscopic technologies have the potential to overcome some of the limitations of standard while light endoscopy (WLE) by increasing the detection rate of polyps/neoplasms and providing real-time histological diagnosis.
Narrow band imaging (NBI) is one of the most widely available and convenient to use technologies developed. Narrowed bandwidth light is used to visualize superficial vasculature and mucosal pit patterns in real-time[7,8]. The light penetrates the mucosa and submucosa and is absorbed by hemoglobin in surface microvessels, which appear as linear darker structures[9]. This enables the endoscopist to differentiate thicker and more irregular vascular landmarks. Multiple classification systems based on surface pit-pattern and vascular pattern have been developed and validated to differentiate hyperplastic polyps from adenomatous polyps[10,11]. This real-time differentiation has been proposed as a part of “resect and discard” strategy in which diminutive polyps (measuring < 5 mm) are resected without histological assessment and hyperplastic polyps in rectosigmoid region are left in situ[12]. This approach could confer substantial cost savings by avoiding unwarranted histological evaluation[13] and may avoid complications related to polypectomy[14]. Few published studies showed no significant difference in adenoma detection rates (ADRs) between NBI and WLE[15-18]. Only one meta-analysis demonstrated an increased accuracy of NBI over WLE in characterising colonic polyps with hierarchical summary receiver-operating characteristic curves exceeding 0.90[19]. Dimmer images compared to WLE[20], type of endoscopes and monitors used (high vs low resolution), inconsistent color enhancement settings and endoscopists’ experience have been proposed as potential reasons for the unimpressive performance of NBI.
Recently, a newer generation NBI system has been introduced. The system appears to provide brighter NBI (bNBI) images (by 2 fold) in a high-definition (HD) mode and has the option of further magnifying a particular target with the dual focus (DF) magnification function, up to 65 times. These provide an in-depth view of desired areas of the mucosa with clear and crisp images, which potentially may improve polyp detection as well as characterisation.
In this study we hypothesized that, when compared to high definition white light endoscopy (HD-WLE), the newer generation colonoscopes with the brighter NBI capability and the bNBI with dual focus (bNBI-DF) magnification mode could improve ADR and accurately predict polyp histology.
We performed a prospective multicenter randomised controlled trial across four centers in the Asia Pacific region (The Prince of Wales Hospital, Hong Kong; The King Chulalongkorn Memorial Hospital, Thailand; Changi General Hospital, Singapore and The Lyell McEwin Hospital, Australia) from October 2010 to April 2012. Institutional medical and ethics committees of each participating hospital approved the study protocol. The study was registered with clinicaltrials.gov (NCT 01422577).
We recruited subjects who were referred for outpatient screening colonoscopy across four centers during the study period. All patients were 40 years and older with no significant medical comorbidities and met the criteria for average risk for the colorectal cancer with no previous colonoscopies in the last five years. Patients were excluded if they were on anti-platelets or anticoagulants, had any colorectal surgical resection, inflammatory bowel disease, familial colorectal cancer syndromes (familial adenomatous polyposis and hereditary non-polyposis colorectal cancer), were unable to provide written informed consent or had poor bowel preparation.
All participating subjects were informed about the study and written informed consent was obtained before initiation of the procedure. Participants were allowed to have clear fluids on the day before the procedure and were given four liters of polyethyleneglycol as bowel preparation followed by a 6-h fast (previous day preparation). Appropriate doses of conscious sedation (Fentanyl and Midazolam ± Propofol) were given prior to and during the procedure.
Eleven endoscopists participated in the study. Each of the endoscopists had extensive experience with the use of NBI in colonoscopy having performed more than 2000 procedures each using the earlier generation colonoscopes with NBI. The CF-HQ 190 or 290 series colonoscopes with the DF mode for magnification (Olympus, Tokyo Co. Ltd) were used for all patients. The colonoscope was connected to a CLV video processor with images transmitted to HD monitors (1280 × 1024 pixels). All participating endoscopists were consultants who had experience with an earlier generation of NBI scopes.
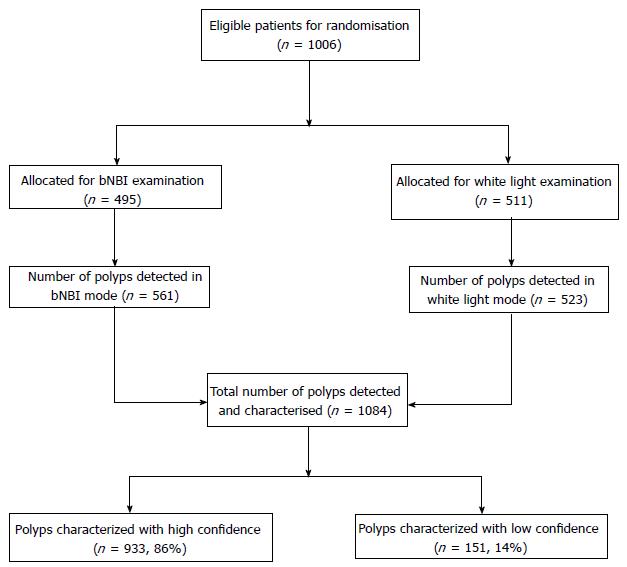
Subjects were randomised to receive the examination during withdrawal either in the HD-WLE or in the bNBI mode, followed by bNBI-DF to characterise each polyp that was identified in both arms (Figure 1). The colonoscope was inserted using HD-WLE until caecum was reached, in all subjects. Randomisation took place once the caecum or appendiceal orifice was identified and adequacy of bowel preparation was established as per the modified Aronchick scale[21]. Subjects with suboptimal bowel preparation were excluded. Patients were randomised according to a computer-generated randomisation scheme in blocks of twenty. Allocation to HD-WLE or bNBI mode of withdrawal was kept in a concealed envelope and revealed by a research assistant to the endoscopist just before withdrawal was initiated. A dedicated nurse assistant monitored both insertion (time to reach caecum from insertion) and withdrawal (time of scope removal from initiation of withdrawal) times with a stopwatch. During insertion, the stopwatch was paused during patient position change or while exerting abdominal pressure to facilitate colonoscope advancement. Similarly, the stopwatch was paused during withdrawal when biopsies or polypectomies were performed. The withdrawal time was set to a minimum of 6 min in both bNBI and HD-WLE arms and endoscopists were deliberately reminded of the time during the withdrawal phase.
Colonoscope withdrawal commenced from the caecum with the patient in the left lateral position and was carried out according to randomisation. Location of each polyp was identified using anatomical landmarks and categorised into either the right or left side of the colon. The size of each polyp was assessed using diameter of the opened biopsy forceps (7.5 mm) or the diameter of the snare used. Identified polyps were characterised by using bNBI-DF mode in both arms.
Characterisation of polyps was made by bNBI-DF using Sano’s classification, which has been found to be valid tool for predicting polyp histology[11]. The classification was based on vascular pattern on the surface of the polyp. Characterisation of polyps was made with high confidence if the polyp demonstrated endoscopic features, which were strongly suggestive of its pathology according to Sano’s classification. Polyps were characterised with low confidence if exposure was limited secondary to non-removable debris, inadequate focus or if the polyp demonstrated features of more than one Sano’s class.
The preferred mode of polypectomy was left to the endoscopists’ discretion. Once the polypectomy was performed, mucosal viewing was switched back to respective mode as per randomisation. Each identified polyp was resected and retrieved into an individual container for pathologic examination. Pathologists with experience in gastrointestinal tract, who were blinded for the endoscopic mode of the examination, evaluated all resected polyps.
The primary outcome of the study was to compare the ADR, defined as percentage of patients with one or more adenomatous polyp detected, between the two arms (HD-WLE vs bNBI). The secondary outcome was to assess if bNBI-DF could meet the American Society for Gastrointestinal Endoscopy (ASGE)’s Preservation and Incorporation of Valuable Endoscopic Innovation (PIVI) threshold in predicting histopathology of colon polyps[22]. We only assessed the second criteria from the PIVI guideline [where the technology, when used with high confidence, should provide > 90% negative predictive value (NPV) for adenomatous histology in diminutive rectosigmoid polyps] as we believed that this strategy would be more practical and generalizable.
We previously conducted a colonoscopy screening study amongst average risk Hong Kong Chinese subjects older than 50 years of age and found an ADR of 30%[23]. The prevalence of colon adenomas in asymptomatic subjects in Asia is unknown and is likely to vary across different ethnic groups. The sample size evaluation was based on assumption that improved optics and bNBI is superior to the HD-WLE mode in detection of colon polyps. We thus hypothesized that bNBI would be able to detect more adenomas than HD-WLE. A sample size of 500 per group was required to detect a relative risk of 1.28 (i.e., a difference of 38.4% vs 30%) with a power of 80% and a type 1 two-sided error of 0.05.
Continuous variables were compared using the t-test if normally distributed. Categorical variables were compared using the χ2 test or Fisher’s exact test when appropriate. The Mann-Whitney’s U test was used for skewed variables. To compare the detection of all adenomas and hyperplastic polyps (per-polyp analysis), the Poisson regression model or negative binomial regression model was used. The accuracy of bNBI-DF in examining early colorectal lesions was evaluated using Sano’s classification compared to the final histopathology and measures of sensitivity (Sn), specificity (Sp), positive predictive value (PPV), and NPV; and their correspondent 95%CIs were performed. These diagnostic tests were calculated by means of the generalized estimating equations to account for the clustering of polyps within patients.
In addition, we performed statistical analysis to examine the effect of the withdrawal time, entered as a covariate, on the ADR on a per-patient level. Specifically, a multiple logistic regression was used to estimate adjusted odds ratio (OR) and its 95%CI with all variables (see Table 1) excluding time taken to reach cecum, entered as covariates. The presence or absence of one or more adenomas was considered a response variable. Mode of withdrawal (HD-WLE or bNBI) was forced to remain into all regression models. Two-tailed P value lower than 0.05 was considered statistically significant. Multiple outcomes were tested without adjusting for the type I error rate. Statistical tests were performed with the use of SPSS software (version 19.0, SPSS, Chicago, IL, United States).
| Parameters | bNBI (n = 495) | WLE (n = 511) | P value |
| Patients | |||
| Men | 210 (42.43) | 237 (46.38) | 0.207 |
| Age, mean ± SD | 58.31 ± 6.17 | 58.36 ± 6.13 | 0.904 |
| BMI, mean ± SD | 23.75 ± 3.29 | 23.74 ± 2.99 | 0.942 |
| Current smoker | 24 (4.9) | 29 (5.7) | 0.536 |
| Current drinker | 38 (7.7) | 44 (8.7) | 0.815 |
| Current use of NSAID | 6 (1.2) | 9 (1.8) | 0.705 |
| Current use of aspirin | 13 (2.6) | 22 (4.3) | 0.345 |
| Current use of warfarin | 0 | 2 (0.4) | 0.232 |
| Comorbidities | |||
| Hypertension | 123 (24.8) | 131 (25.6) | 0.774 |
| Diabetes | 56 (11.3) | 32 (6.3) | 0.005 |
| Ischemic heart disease | 10 (2.0) | 10 (2.0) | 0.943 |
| Chronic obstructive airway disease | 2 (0.4) | 0 | 0.15 |
| Previous stroke | 1 (0.2) | 2 (0.4) | 0.582 |
| Cirrhosis | 1 (0.2) | 1 (0.2) | 0.982 |
| Gastro-esophageal reflux | 10 (2) | 8 (1.6) | 0.587 |
| Dyslipidemia | 31 (6.3) | 29 (5.7) | 0.694 |
| History of cancer | 19 (3.8) | 12 (2.3) | 0.172 |
| Examination time (min), mean ± SD | |||
| Time to cecum | 6.66 ± 4.56 | 7.06 ± 4.94 | 0.183 |
| Time for withdrawal | 11.23 ± 6.36 | 9.84 ± 5.03 | < 0.0001 |
A total of 1006 patients were enrolled during the 17-mo study period (October 2010 to April 2012), of which 44.4% were men and the mean age was 58 years. Four hundred and ninety-five participants were randomised to the bNBI arm and 511 participants to the HD-WLE arm. Table 1 demonstrates the demographics of the study population in the two arms by age, gender, body mass index (BMI), social habits, medical comorbidities and mean insertion and withdrawal times. There were no significant differences in baseline characteristics in both arms except for the prevalence of diabetes, which was seen more frequently in bNBI arm (bNBI, n = 56 (11.3%) vs HD-WLE, n = 32 (5.3%). The mean time to reach caecum was similar in both arms; however, the mean withdrawal time was 1.39 min longer in the bNBI arm (P < 0.05).
A total of 1084 polyps were detected in both arms. The overall mean polyp detection rate per colonoscopy in the bNBI arm was 1.13 and in the HD-WLE arm was 1.02 (P = 0.093). Two hundred and sixty subjects in the bNBI arm (52.53%) and 257 in the HD-WLE group (50.29%) had one or more polyp (P = 0.479). About two thirds (n = 638) of polyps were identified on left side of the colon and 92.8 % (n = 1005) of polyps were less than 10mm in size.
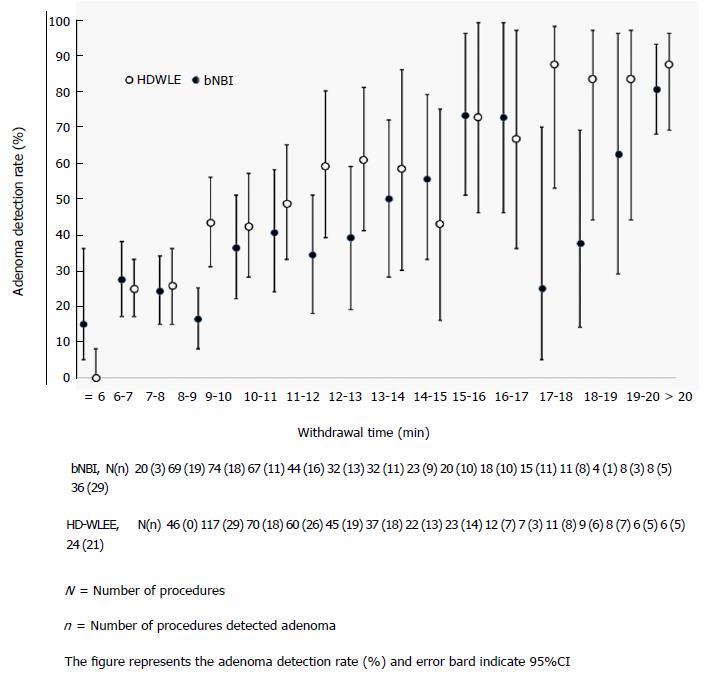
The ADR in the bNBI group and HD-WLE group was 37.4% and 39.3% respectively (185 of 495 subjects and 201 of 511 subjects had at least one adenoma) (Table 2). Table 2 demonstrates the pathological diagnosis of polyps detected in both arms. Sixty one percent (341/561) of polyps in bNBI arm and 62% (326/523) in HD-WLE arm were adenomatous (P = 0.425) in nature. ADRs were directly related to withdrawal time in both arms, as shown in Figure 2, ADR progressively increased with increasing withdrawal time. A higher number of hyperplastic polyps were identified in bNBI arm than in HD-WLE arm (bNBI, n = 178 vs HD-WLE, n = 136), which was statistically significant (P = 0.021). According to logistic regression analysis, withdrawal time (OR = 1.19, 95%CI: 1.15-1.24, P < 0.001), age (OR = 1.03, 95%CI: 1.00-1.05, P = 0.032) and male sex (OR = 1.49, 95%CI: 1.11-2.00, P = 0.008) were independently associated with an improved ADR when adjusted for differences in baseline variables. When we adjusted for withdrawal time (OR = 1.19, 95%CI: 1.15-1.24, P < 0.001), the use of bNBI was associated with a reduced ADR (OR = 0.69, 95%CI: 0.52-0.92).
| bNBI (n = 495) | HD-WLE (n = 511) | P value | |
| Adenomas | 341 | 326 | 0.425 |
| Subjects with adenomas2 (ADR) | 185 (37.4) | 201 (39.3) | 0.523 |
| Adenomas per adenoma carrier1 | 1.84 | 1.62 | 0.129 |
| Size | |||
| Subjects with 0-5 mm adenomas2 | 149 (30.1) | 162 (31.2) | 0.583 |
| Subjects with 6-9 mm adenomas2 | 52 (10.5) | 52 (10.2) | 0.864 |
| Subjects with ≥ 10 mm adenomas2 | 31 (6.3) | 31 (6.1) | 0.897 |
| Adenomas < 10 mm | 306 | 290 | 0.283 |
| Adenomas 0-5 mm | 241 | 229 | 0.334 |
| Adenomas 6-9 mm | 65 | 61 | 0.896 |
| Adenomas ≥ 10 mm | 35 | 36 | 0.837 |
| Location | |||
| Right-sided adenomas | 157 | 155 | 0.400 |
| Left-sided adenomas | 168 | 159 | 0.797 |
| Histopathology | |||
| Carcinomas | 3 | 5 | 0.418 |
| Tubular | 315 | 304 | 0.343 |
| Tubulovillious | 10 | 12 | 0.689 |
| Villious | 2 | 0 | NA |
| Adenomas with high grade dysplasia | 6 | 7 | 0.992 |
| Hyperplastic polyps | 178 | 136 | 0.020 |
| Hyperplastic polyps < 10 mm | 176 | 135 | 0.020 |
| SSA/Ps | 13 | 7 | 0.257 |
| Subjects with SSA/P, n (SSA/P-detection rate in %) | 13 (2.6) | 6 (1.2) | 0.091 |
| Inflammatory polyps | 12 | 14 | 0.572 |
| Indeterminate or non-significant | 16 | 15 | 0.987 |
| Not submitted for histologic examinations | 13 | 31 | 0.010 |
| Xanthoma | 1 | 1 | 0.961 |
Nine hundred and thirty-three polyps (86%) from both arms were categorized into various classes with high confidence according to Sano’s classification. The other 13.9% (n = 151) were classified with low confidence. Among the high confidence polyps, 308 (33%) polyps were Sano type I; 598 (64%) were type II; 20 (2.1%) were type IIIA and 7 (0.75%) were type IIIB.
The Sn, Sp, PPV and NPV in differentiating adenomatous from non-adenomatous polyps of all sizes were 95.7%, 86.5%, 93.9% and 91.0% respectively (Table 3). The Sn, Sp, PPV and NPV in differentiating an adenoma from cancer were 87.5%, 100%, 100%, and 99.8% respectively (Table 4). The Sn, Sp, PPV and NPV of bNBI-DF in the characterisation of polyps with 5mm or less in the rectosigmoid region were 94.5%, 95.4%, 94.8% and 93.7% respectively (Table 5).
| Pathology | Total | ||
| Hyperplastic/ SSA/P | Adenoma/cancer | ||
| Sano’s classification | |||
| I | 245 | 24 | 269 |
| II, IIIa, IIIb | 36 | 555 | 591 |
| Total | 281 | 579 | |
| Pathology | Total | ||
| Adenoma | Cancer | ||
| Sano’s classification | |||
| II, IIIa | 547 | 1 | 548 |
| IIIb | 0 | 7 | 7 |
| Total | 547 | 8 | |
| Pathology | Total | ||
| Hyperplastic | Adenoma/cancer | ||
| Sano’s classification | |||
| I | 146 | 10 | 156 |
| II, IIIa, IIIb | 7 | 130 | 137 |
| Total | 153 | 140 | |
This prospective multicenter randomised study compared two different modalities: HD-WLE and bNBI to assess if there was a difference in ADRs. In addition, bNBI-DF was used to characterise polyps using the Sano’s classification. We did not find a statistically significant improvement in ADR with bNBI when compared to HD-WLE. Polyp characterisation was effective with bNBI-DF in differentiating adenomas from hyperplastic polyps in diminutive distal polyps, meeting the second PIVI standard.
The study design was similar to that of Rex and Helbig, who evaluated an earlier version of NBI[24]. Their study represented a single operator experience, in contrast to this study, which involved several academic centers. In a recent tandem study, Leung et al[25] compared bNBI to HD-WLE in colonoscopy. Subjects were submitted to bNBI first and followed by HD-WLE or vice versa. The use of bNBI was associated with a higher ADR with a higher number of polyps detected per subject. However, for the HD-WLE group, the older generation 260 series colonoscopes were used. Illumination with 260 series colonoscopes is considerably less sharp when compared to the 190/290 series colonoscopes. Hence, one cannot be certain if the superiority of bNBI in finding adenomas was not a result of a “brighter processor”. In another study by Wallace et al[26], average risk subjects presenting for screening were randomised to receive the examination by a standard colonoscope (H180) or a dual focus colonoscope (HQ-190). ADR were similar between both groups (52% vs 50%). The NPV for diminutive rectosigmoid polyps were 96 and 97% respectively, which was not too dissimilar to our study.
Multiple randomised studies and a meta-analysis compared ADRs of NBI with conventional colonoscopy. The results have thus far been mixed with very few studies[27,28] demonstrating improved ADRs with NBI. Despite having endoscopists with considerable experience in using NBI and a large sample size, we were unable to demonstrate a statistically significant improvement in ADR. Actually, this study suggests that NBI could actually decrease the ADR if used exclusively for overview of the whole colon during withdrawal. The similar ADRs achieved in this study may be attributed to the fact that improved resolution could be achieved using the same high definition processor for both bNBI and HD-WLE. These findings are not too dissimilar to studies conducted in the past with older generation systems[29-31].
Previous studies conducted using bNBI to differentiate adenomatous from non-adenomatous lesions demonstrated accuracies ranging from 77% to 93%[8,32-36]. Sessile serrated adenomas/polyps (SSA/Ps), which endoscopically may resemble hyperplastic polyps but have malignant potential, were detected in 1.8% of all polyps. Previous studies have shown the prevalence of SSAs ranging from 1% to 7%[37], but a more recent study shows that the reported prevalence of SSA/Ps is raising with the years and it can get up to 15.8%[38]. This difference may be due in part to the different prevalence rate in the studied population, which included predominantly a younger Asian cohort. These polyps unfortunately do not fit into any of the available classifications at the time the study was performed.
This study adds strength to the usefulness of bNBI in characterising colonic lesions in real-time. This “endopathology” concept supports the “resect and discard” approach that carries many practical advantages. In a simulation model by Hassan et al[6], this strategy resulted in a substantial economic benefit without any impact on efficacy. Kessler et al[13] demonstrated that endoscopic diagnosis of polyp histology during colonoscopy and forgoing pathologic examination would result in substantial up-front cost savings whilst the downstream consequences of the resulting incorrect surveillance intervals appear to be negligible. bNBI-DF used in this study not only successfully met the second PIVI threshold established by the ASGE but also demonstrated the highest accuracy so far in differentiating adenomas from hyperplastic polyps[6]. More than 85% of polyps were characterised with high confidence and the overall sensitivity and specificity demonstrated was significantly higher than in other studies[18].
This study has some limitations. First, the mean withdrawal time was prolonged in both arms, but particularly in the bNBI’s arm (11.23 min vs 9.84 min). In a multiple regression model, examination of the colon in the HD-WLE mode was associated with a better ADR. Similar to this study, longer withdrawal times with bNBI were also noted in a meta-analysis by Jin et al[39] as well as by Rex et al[24]. This could be potentially explained by the lack of confidence in assessing the mucosa in an overview mode with bNBI, although endoscopists were experienced with previous versions of the modality. The ADRs in both arms of the study were higher than the target ADR set by the United States Multi-Society Task Force (men > 25% and women > 15%)[40]. Longer withdrawal times and high-definition imaging are the possible reasons for the overall higher adenoma detection rates.
In conclusion, ADR was not different between bNBI and HD-WLE. Male sex, larger withdrawal time and older age where positively correlated with ADR. When adjusted for withdrawal time, HD-WLE had higher ADR. With bNBI-DF, 85% of the polyps could be characterised with high confidence, of which more than 95% of them were predicted accurately. The most worthwhile strategy to reduce the risks associated with unwarranted polypectomies and save costs incurred with pathological assessment of polyps could be a “combination strategy” where withdrawal is performed using HD-WLE and polyp characterisation with bNBI-DF.
Colorectal polyps are the precursors of colorectal cancer and their removal through colonoscopy is effective in preventing colorectal cancer. New technologies continuously improve the imaging ability of the colonoscopes. Whether these new technologies effectively differ from each other for detection of polyps is debatable.
The development of state-of-the-art endoscopes are not always associated with better results. Technologies that enhance imaging supposedly could improve the detection of polyps. So far, the use of light filters to improve adenoma detection rate (ADR) is not recommended.
Improvement in ADR is important as it is inversely correlated with colorectal cancer risk. The improvement of old technologies has been shown beneficial for detection of polyps (i.e., HD vs non-HD imaging). However, comparison between new technologies is less studied. The authors therefore evaluated the use of two cutting-edge technologies [high definition white light endoscopy (HD-WLE) and bright narrow band imaging (bNBI)] to detect colorectal polyps.
Although virtual chromoendoscopy is useful for characterising polyps, its use for detecting them did not differ from HD-WLE in this study. Therefore, even though there is improvement in the brightness with the new light filter, it is still not recommended as standard of care for screening purposes.
Adenoma detection rate is defined as the percentage of patients that were submitted to colonoscopy and had at least one adenomatous polyp. Narrow band imaging is an optical image-enhanced technology based on specific light wavelengths, which allows enhanced visualisation of vasculature and superficial mucosal surface.
The authors compared ADR of two different modalities. They found that HD-WLE had higher ADR after adjustment of withdrawal time. bNBI had satisfactory negative predictive value in differentiating adenomatous from non-adenomatous histology in dimunitive polyps, which was above the preservation and incorporation of valuable endoscopic innovation threshold. The paper is well written.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Koksal AS, Lee YT S- Editor: Gong ZM L- Editor: A E- Editor: Wu HL
| 1. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3121] [Article Influence: 97.5] [Reference Citation Analysis (1)] |
| 2. | Sung JJ, Lau JY, Goh KL, Leung WK. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol. 2005;6:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 597] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 3. | Muto T, Kotake K, Koyama Y. Colorectal cancer statistics in Japan: data from JSCCR registration, 1974-1993. Int J Clin Oncol. 2001;6:171-176. [PubMed] |
| 4. | Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, Inamura K, Kim SA, Kuchiba A, Yamauchi M. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 1153] [Article Influence: 96.1] [Reference Citation Analysis (0)] |
| 5. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 917] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 6. | Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol. 2010;8:865-869, 869.e1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Gono K, Yamazaki K, Doguchi N, Nonami T, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S. Endoscopic observation of tissue by narrow band illumination. Opt Rev. 2003;10:211-215. [DOI] [Full Text] |
| 8. | Machida H, Sano Y, Hamamoto Y, Muto M, Kozu T, Tajiri H, Yoshida S. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004;36:1094-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 370] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 9. | Postic G, Lewin D, Bickerstaff C, Wallace MB. Colonoscopic miss rates determined by direct comparison of colonoscopy with colon resection specimens. Am J Gastroenterol. 2002;97:3182-3185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Oba S, Tanaka S, Sano Y, Oka S, Chayama K. Current status of narrow-band imaging magnifying colonoscopy for colorectal neoplasia in Japan. Digestion. 2011;83:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Uraoka T, Saito Y, Ikematsu H, Yamamoto K, Sano Y. Sano’s capillary pattern classification for narrow-band imaging of early colorectal lesions. Dig Endosc. 2011;23 Suppl 1:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Rex DK. Reducing costs of colon polyp management. Lancet Oncol. 2009;10:1135-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Kessler WR, Imperiale TF, Klein RW, Wielage RC, Rex DK. A quantitative assessment of the risks and cost savings of forgoing histologic examination of diminutive polyps. Endoscopy. 2011;43:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Levin TR, Zhao W, Conell C, Seeff LC, Manninen DL, Shapiro JA, Schulman J. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 393] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 15. | Dinesen L, Chua TJ, Kaffes AJ. Meta-analysis of narrow-band imaging versus conventional colonoscopy for adenoma detection. Gastrointest Endosc. 2012;75:604-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Nagorni A, Bjelakovic G, Petrovic B. Narrow band imaging versus conventional white light colonoscopy for the detection of colorectal polyps. Cochrane Database Syst Rev. 2012;1:CD008361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Pasha SF, Leighton JA, Das A, Harrison ME, Gurudu SR, Ramirez FC, Fleischer DE, Sharma VK. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012;107:363-370; quiz 371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | van den Broek FJ, Reitsma JB, Curvers WL, Fockens P, Dekker E. Systematic review of narrow-band imaging for the detection and differentiation of neoplastic and nonneoplastic lesions in the colon (with videos). Gastrointest Endosc. 2009;69:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | McGill SK, Evangelou E, Ioannidis JP, Soetikno RM, Kaltenbach T. Narrow band imaging to differentiate neoplastic and non-neoplastic colorectal polyps in real time: a meta-analysis of diagnostic operating characteristics. Gut. 2013;62:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Dekker E, van den Broek FJ, Reitsma JB, Hardwick JC, Offerhaus GJ, van Deventer SJ, Hommes DW, Fockens P. Narrow-band imaging compared with conventional colonoscopy for the detection of dysplasia in patients with longstanding ulcerative colitis. Endoscopy. 2007;39:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 202] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Aronchick CA, Lipshutz WH, Wright SH, DuFrayne F, Bergman G. Validation of an instrument to assess colon cleansing. Am J Gastroenterol. 1999;94:2667. |
| 22. | Rex DK, Kahi C, O’Brien M, Levin TR, Pohl H, Rastogi A, Burgart L, Imperiale T, Ladabaum U, Cohen J. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 463] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 23. | Sung JJ, Chan FK, Leung WK, Wu JC, Lau JY, Ching J, To KF, Lee YT, Luk YW, Kung NN. Screening for colorectal cancer in Chinese: comparison of fecal occult blood test, flexible sigmoidoscopy, and colonoscopy. Gastroenterology. 2003;124:608-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Rex DK, Helbig CC. High yields of small and flat adenomas with high-definition colonoscopes using either white light or narrow band imaging. Gastroenterology. 2007;133:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 25. | Leung WK, Lo OS, Liu KS, Tong T, But DY, Lam FY, Hsu AS, Wong SY, Seto WK, Hung IF. Detection of colorectal adenoma by narrow band imaging (HQ190) vs. high-definition white light colonoscopy: a randomized controlled trial. Am J Gastroenterol. 2014;109:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Wallace MB, Crook JE, Coe S, Ussui V, Staggs E, Almansa C, Patel MK, Bouras E, Cangemi J, Keaveny A. Accuracy of in vivo colorectal polyp discrimination by using dual-focus high-definition narrow-band imaging colonoscopy. Gastrointest Endosc. 2014;80:1072-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Kaltenbach T, Friedland S, Soetikno R. A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut. 2008;57:1406-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Inoue T, Murano M, Murano N, Kuramoto T, Kawakami K, Abe Y, Morita E, Toshina K, Hoshiro H, Egashira Y. Comparative study of conventional colonoscopy and pan-colonic narrow-band imaging system in the detection of neoplastic colonic polyps: a randomized, controlled trial. J Gastroenterol. 2008;43:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | Paggi S, Radaelli F, Amato A, Meucci G, Mandelli G, Imperiali G, Spinzi G, Terreni N, Lenoci N, Terruzzi V. The impact of narrow band imaging in screening colonoscopy: a randomized controlled trial. Clin Gastroenterol Hepatol. 2009;7:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Sabbagh LC, Reveiz L, Aponte D, de Aguiar S. Narrow-band imaging does not improve detection of colorectal polyps when compared to conventional colonoscopy: a randomized controlled trial and meta-analysis of published studies. BMC Gastroenterol. 2011;11:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Chung SJ, Kim D, Song JH, Kang HY, Chung GE, Choi J, Kim YS, Park MJ, Kim JS. Comparison of detection and miss rates of narrow band imaging, flexible spectral imaging chromoendoscopy and white light at screening colonoscopy: a randomised controlled back-to-back study. Gut. 2014;63:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Su MY, Hsu CM, Ho YP, Chen PC, Lin CJ, Chiu CT. Comparative study of conventional colonoscopy, chromoendoscopy, and narrow-band imaging systems in differential diagnosis of neoplastic and nonneoplastic colonic polyps. Am J Gastroenterol. 2006;101:2711-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 185] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Chiu HM, Chang CY, Chen CC, Lee YC, Wu MS, Lin JT, Shun CT, Wang HP. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 34. | East JE, Suzuki N, Saunders BP. Comparison of magnified pit pattern interpretation with narrow band imaging versus chromoendoscopy for diminutive colonic polyps: a pilot study. Gastrointest Endosc. 2007;66:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Rastogi A, Bansal A, Wani S, Callahan P, McGregor DH, Cherian R, Sharma P. Narrow-band imaging colonoscopy--a pilot feasibility study for the detection of polyps and correlation of surface patterns with polyp histologic diagnosis. Gastrointest Endosc. 2008;67:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Tischendorf JJ, Wasmuth HE, Koch A, Hecker H, Trautwein C, Winograd R. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy. 2007;39:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 37. | Simmons DT, Harewood GC, Baron TH, Petersen BT, Wang KK, Boyd-Enders F, Ott BJ. Impact of endoscopist withdrawal speed on polyp yield: implications for optimal colonoscopy withdrawal time. Aliment Pharmacol Ther. 2006;24:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Abdeljawad K, Vemulapalli KC, Kahi CJ, Cummings OW, Snover DC, Rex DK. Sessile serrated polyp prevalence determined by a colonoscopist with a high lesion detection rate and an experienced pathologist. Gastrointest Endosc. 2015;81:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 39. | Jin XF, Chai TH, Shi JW, Yang XC, Sun QY. Meta-analysis for evaluating the accuracy of endoscopy with narrow band imaging in detecting colorectal adenomas. J Gastroenterol Hepatol. 2012;27:882-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Rex DK, Bond JH, Winawer S, Levin TR, Burt RW, Johnson DA, Kirk LM, Litlin S, Lieberman DA, Waye JD. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 721] [Article Influence: 31.3] [Reference Citation Analysis (0)] |