Published online May 16, 2017. doi: 10.4253/wjge.v9.i5.238
Peer-review started: October 23, 2016
First decision: December 1, 2016
Revised: December 27, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: May 16, 2017
Processing time: 208 Days and 12.4 Hours
We present a case of a 76-year-old man with right upper quadrant abdominal pain and weight loss, who was found to have an intraductal papillary neoplasm of the bile duct (IPNB) of the pancreaticobiliary subtype, deemed curatively resectable. The patient declined surgery and opted for endoscopic therapy. He underwent two sessions of endoscopic retrograde cholangiopancreatography (ERCP)-guided radiofrequency ablation (RFA). Ten months later, no evidence of recurrence was identified on repeat ERCP. To our knowledge, this is the first reported case of successful use of RFA as a primary treatment modality for resectable IPNB.
Core tip: Intraductal neoplasms of the bile duct (IPNB) classically present with jaundice and/or pruritus, but nonspecific symptoms such as right upper quadrant discomfort and weight loss may also develop. The first-line treatment for these tumors is surgical resection. Endoscopic retrograde cholangiopancreatography-guided radiofrequency ablation (RFA) has historically been used as adjunctive treatment; self-expanding metal stents may be used for palliation. We report a case of successful primary treatment of an IPNB with RFA alone.
- Citation: Natov NS, Horton LC, Hegde SR. Successful endoscopic treatment of an intraductal papillary neoplasm of the bile duct. World J Gastrointest Endosc 2017; 9(5): 238-242
- URL: https://www.wjgnet.com/1948-5190/full/v9/i5/238.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i5.238
Intraductal papillary neoplasms of the bile duct were first recognized as a distinct entity by the World Health Organization in 2010[1]. These tumors may harbor varying degrees of dysplasia and even invasive malignancy[1,2]. Surgical resection is therefore recommended in patients who are operative candidates[1]. Non-surgical cases are managed with palliative biliary stenting using self-expandable metal stents (SEMSs), and radiofrequency ablation (RFA) has been employed as an adjunctive therapy for malignant biliary obstruction of several different etiologies[3-6]. RFA is safe, produces good 90-d stent luminal patency rates, and has been associated with an improvement in clinical outcomes[5,7,8]. However, its utility as a primary treatment modality has not been studied.
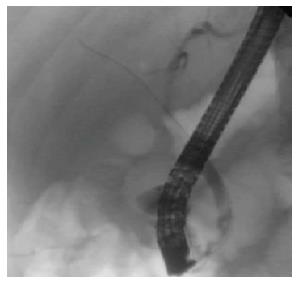
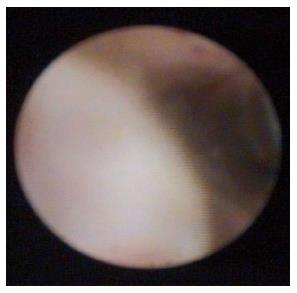
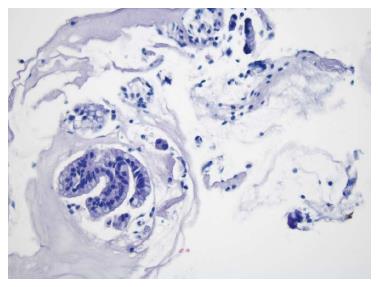
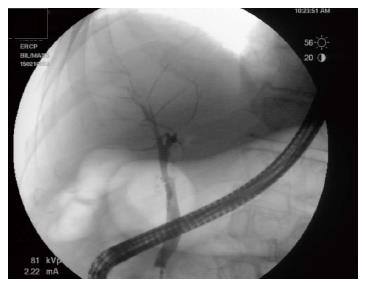
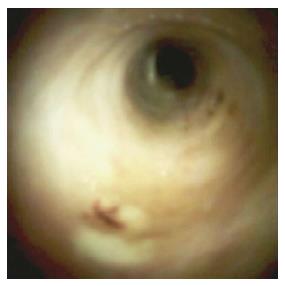
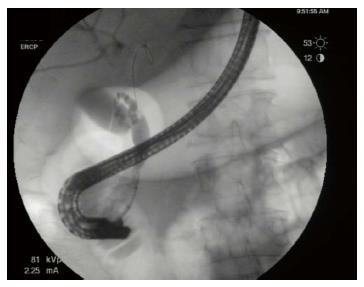
A 76-year-old man with a history of coronary artery disease and tobacco abuse was referred to our institution for evaluation of a common bile duct (CBD) stricture. Several months prior, he had presented to his primary care physician with right upper quadrant abdominal pain associated with an unintentional weight loss of 13 kg. Liver function tests (LFTs) revealed an AST of 46 IU/L (10-42), ALT of 37 IU/L (0-54), ALP of 197 IU/L (40-130), and a TB of 0.4 mg/dL (0.2-1.1). Mild intrahepatic ductal dilatation and a CBD of 10 mm were seen on right upper quadrant ultrasound. There was no choledocholithiasis. Subsequent magnetic resonance cholangiopancreatography revealed intra- and extrahepatic biliary ductal dilatation and a filling defect in the distal CBD. The pancreatic duct was not dilated. The patient was referred for endoscopic retrograde cholangiopancreatography (ERCP) at a different facility. Inspection of the ampulla of Vater was normal. The CBD was cannulated using a 0.35 inch sphincterotome and guidewire, and initial contrast injection showed dilatation of the CBD to approximately 15 mm and a saccular collection of contrast distally. A 10 mm biliary sphincterotomy was performed, and no choledocholithiasis or sludge was found after sweeping of the CBD. Cholangiogram showed a distal CBD stricture 10 mm proximal to the ampulla. Brushings for cytology were obtained, and a 10 Fr × 5 cm plastic stent was deployed across the stricture to facilitate biliary drainage. Cytology revealed atypical cells but was otherwise non-diagnostic. Due to the intraductal location of the lesion, ERCP was considered the best modality through which to obtain a tissue diagnosis, and endoscopic ultrasound was thus not performed. The patient was brought back for repeat ERCP six weeks later. Following removal of the plastic stent and balloon sphincteroplasty to 10 mm, the CBD was cannulated using a pediatric gastroscope to allow for direct visualization of the intraductal mucosa. Catheter-based cholangioscopy was not available at the outside hospital. The area of the stricture appeared nodular and erythematous. The procedure was aborted due to hypotension and hypoxemia before biopsies could be obtained. The patient was then transferred to our institution for ERCP with SpyGlass™ cholangioscopy (Boston Scientific, Natick, MA). Initial cholangiogram confirmed a distal CBD stricture (Figure 1). A 10 mm polypoid lesion was seen with SpyGlass™ cholangioscopy (Figure 2), and targeted intraductal biopsies were performed using SpyBite™ forceps (Boston Scientific, Natick, MA). Pathological findings of atypical cells, papillary configuration, and bile duct epithelium with mucinous metaplasia were consistent with intraductal papillary neoplasm of the bile duct (IPNB) without malignant transformation (Figure 3). Immunohistochemical staining was positive for MUC1 and negative for CDX2, highlighting pancreaticobiliary and lack of intestinal epithelium, respectively. The normal appearance of the ampulla, intraductal location of the neoplasm, and histology excluded an ampullary lesion. Furthermore, the most distal aspect of the CBD was normal. He was referred to hepatobiliary surgery for consideration of pancreaticoduodenectomy with curative intent, but surgical intervention was deferred due to patient preference. The decision was made to proceed with ERCP-guided RFA as primary therapy. Two sessions of RFA using an 8 Fr Habib™ EndoHPB probe were performed at 10 W for 90 s (EMcision, Montreal, Canada). A 10 mm × 4 cm WallFlex™ fully covered SEMS was deployed following the first round of RFA and exchanged at the completion of the second RFA session, three months later (Boston Scientific, Natick, MA). The procedures were uncomplicated. Four months after the second RFA session, ERCP with SpyGlass™ was repeated, the biliary stent was removed, and no residual polypoid tissue or stricture was observed on occlusion cholangiogram (Figure 4) or with Spyglass™ choledochoscopy (Figure 5). The stent was not replaced. Cytology of distal CBD brushings revealed no malignant cells. Repeat ERCP six months later (nine months after the last RFA session) demonstrated a 10 mm distal CBD stricture but no mass lesion (Figure 6). Spyglass™ choledochoscopy revealed only erythematous mucosa in the distal CBD; targeted intraductal biopsies of this area were performed using SpyBite™ forceps and revealed reactive epithelium. Cytology was negative for malignant cells, and fluorescence in situ hybridization (FISH) analysis of the distal CBD brushings showed no abnormalities. The patient underwent his last ERCP six months later, and no residual stricture or CBD lesion was seen on cholangiogram. He remained asymptomatic, and his LFTs and weight normalized.
In 2010, the World Health Organization first categorized rare bile duct tumors characterized by papillary growth as intraductal papillary neoplasms of the bile duct[1]. IPNBs are associated with mucobilia due to excessive mucin secretion, and are more commonly found in the hepatic biliary system[1]. However, mucobilia may be absent, and extrahepatic growth patterns have been described[1,9]. Four histological subtypes exist including pancreaticobiliary, oncocytic, gastric, and intestinal[10,11]. Differentiation between subtypes is made according to morphology seen on hematoxylin and eosin staining and immunohistochemical features of mucin glycoproteins[10]. Pancreaticobiliary IPNB is more frequently associated with an invasive phenotype and harbors a worse prognosis[10,11]. IPNB tumors are graded based on degree of dysplasia, from low- to high-grade and finally to invasive carcinoma, which was seen in 74% of IPNBs in one study[1,2]. Pancreatic IPMNs are classified similarly and are thought to follow the same sequence from benign to malignant[2]. The incidence of IPNBs is highest in Far Eastern countries, and particularly in patients between 50 and 70 years of age, with a slight male predominance[1]. Patients may be asymptomatic, or may present with abdominal pain, jaundice, elevated LFTs, or cholangitis[1,2]. Biliary ductal dilatation and an intraductal mass may be seen on computed tomography or magnetic resonance imaging[1]. Duodenoscopy may reveal a prominent ampulla with mucinous secretion[1]. Diagnosis is often difficult; sensitivity of brush cytology and fluoroscopically-guided biliary biopsies is low, and mixed pathologic findings may be present within a single lesion[1,12]. The majority of IPNBs contain high-grade epithelial neoplasia or carcinoma; low- or intermediate grade neoplasia is infrequent[1]. Excessive mucin secretion may impede identification of IPNB via cholangiography; cholangioscopy, however, can allow direct visualization of IPNBs[1]. Regardless of the level of dysplasia or presence of malignancy, treatment of IPNBs is warranted due to the heterogeneity of pathology within a single lesion, and to prevent complications such as obstructive jaundice and cholangitis[1]. Preoperative assessment of disease extent should be performed, and surgical resection is recommended for patients who are candidates without distant metastasis[1]. The surgical approach depends on tumor location, with pancreaticoduodenectomy being the procedure of choice for distal biliary lesions[1]. Palliative biliary stenting with SEMSs is employed in non-operative cases[12]. Over the last decade, much effort has been devoted to studying RFA as an adjunct therapy to stent placement. Using thermal energy, RFA induces coagulative necrosis of tumor tissue[13]. The procedure is safe and produces good 90-d stent luminal patency rates in patients with unresectable pancreatic carcinoma with obstructive jaundice[7,8]. RFA has also been used in the treatment of cholangiocarcinoma and biliary obstruction as a result of metastatic disease from distant primary malignancies[3-6]. These studies included only one patient with IPNB[4]. Use of RFA may result in benefits beyond local tumor ablation, and may induce secondary anti-tumor effects in patients with hepatocellular carcinoma and metastatic colorectal cancer[14]. RFA has also been associated with improved clinical outcomes, including prolonged survival[5,7]. The mechanism behind this positive effect on survival is not known but could be due to improved stent patency resulting in less infectious complications and superior biliary drainage which subsequently allows patients to receive oncologic treatment. Complications of RFA are rare but include biliary tract perforation, stricture formation, post-procedural pain, pancreatitis, cholecystitis, and bleeding[5,15]. Further investigation is needed to elucidate the effect of RFA on outcomes in patients with IPNB. This case demonstrates that, in patients who defer surgery, RFA, in combination with biliary stenting, may be used as a primary therapy for intraductal malignancies.
A 76-year-old man presenting with right upper quadrant abdominal pain and weight loss.
He had dilatation of the common bile duct (CBD), a 10 mm polypoid lesion visualized on cholangioscopy, and pathology consistent with intraductal papillary neoplasm of the bile duct.
The differential diagnosis includes a biliary stricture, choledocholithiasis, pancreatic adenocarcinoma, cholangiocarcinoma, and choledochal cyst.
Aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase were mildly elevated.
Right upper quadrant ultrasound was notable for mild intrahepatic and CBD dilatation. Magnetic resonance cholangiopancreatography showed intra- and extra-hepatic biliary ductal dilatation and a filling defect in the distal CBD. Serial endoscopic retrograde cholangiopancreatography revealed CBD dilatation and a nodular and erythematous distal CBD stricture. SpyGlass™ choledochoscopy showed a polypoid mass within the CBD.
Initial cytology from brushings showed atypical cells. Intraductal biopsies of the CBD mass revealed atypical cells, papillary configuration, and bile duct epithelium with mucinous metaplasia; immunohistochemistry was positive for MUC1 and negative for CDX2. Intraductal biopsies after treatment were negative for malignant cells.
Two sessions of radiofrequency ablation were performed.
Radiofrequency ablation (RFA) has not been previously studied as the primary treatment modality for Intraductal neoplasms of the bile duct (IPNB).
In patients who choose to defer or forego surgery, RFA may be an acceptable option for primary treatment of intraductal malignancies.
Authors describe a case of successful treatment of intraductal papillary tumor of the bile duct with endoscopic retrograde cholangiopancreatography guided RFA. The case is interesting.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Akamatsu N, Cardinale V, Imagawa A, Sperti C S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Ohtsuka M, Shimizu H, Kato A, Yoshitomi H, Furukawa K, Tsuyuguchi T, Sakai Y, Yokosuka O, Miyazaki M. Intraductal papillary neoplasms of the bile duct. Int J Hepatol. 2014;2014:459091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Rocha FG, Lee H, Katabi N, DeMatteo RP, Fong Y, D’Angelica MI, Allen PJ, Klimstra DS, Jarnagin WR. Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology. 2012;56:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (2)] |
| 3. | Tal AO, Vermehren J, Friedrich-Rust M, Bojunga J, Sarrazin C, Zeuzem S, Trojan J, Albert JG. Intraductal endoscopic radiofrequency ablation for the treatment of hilar non-resectable malignant bile duct obstruction. World J Gastrointest Endosc. 2014;6:13-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 81] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Figueroa-Barojas P, Bakhru MR, Habib NA, Ellen K, Millman J, Jamal-Kabani A, Gaidhane M, Kahaleh M. Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J Oncol. 2013;2013:910897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Sharaiha RZ, Natov N, Glockenberg KS, Widmer J, Gaidhane M, Kahaleh M. Comparison of metal stenting with radiofrequency ablation versus stenting alone for treating malignant biliary strictures: is there an added benefit? Dig Dis Sci. 2014;59:3099-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Dolak W, Schreiber F, Schwaighofer H, Gschwantler M, Plieschnegger W, Ziachehabi A, Mayer A, Kramer L, Kopecky A, Schrutka-Kölbl C. Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc. 2014;28:854-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Kallis Y, Phillips N, Steel A, Kaltsidis H, Vlavianos P, Habib N, Westaby D. Analysis of Endoscopic Radiofrequency Ablation of Biliary Malignant Strictures in Pancreatic Cancer Suggests Potential Survival Benefit. Dig Dis Sci. 2015;60:3449-3455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Steel AW, Postgate AJ, Khorsandi S, Nicholls J, Jiao L, Vlavianos P, Habib N, Westaby D. Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc. 2011;73:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 225] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 9. | Kawaguchi Y, Kawashima Y, Maruno A, Ito H, Ogawa M, Izumi H, Furukawa D, Yazawa N, Nakagori T, Hirabayashi K. An intraductal papillary neoplasm of the bile duct at the duodenal papilla. Case Rep Oncol. 2014;7:417-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Kim KM, Lee JK, Shin JU, Lee KH, Lee KT, Sung JY, Jang KT, Heo JS, Choi SH, Choi DW. Clinicopathologic features of intraductal papillary neoplasm of the bile duct according to histologic subtype. Am J Gastroenterol. 2012;107:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 11. | Gordon-Weeks AN, Jones K, Harriss E, Smith A, Silva M. Systematic Review and Meta-analysis of Current Experience in Treating IPNB: Clinical and Pathological Correlates. Ann Surg. 2016;263:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Narita M, Endo B, Mizumoto Y, Matsusue R, Hata H, Yamaguchi T, Otani T, Ikai I. Multicentric recurrence of intraductal papillary neoplasms of bile duct in the remnant intrahepatic bile duct after curative resection. Int J Surg Case Rep. 2015;12:123-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Itoi T, Isayama H, Sofuni A, Itokawa F, Tamura M, Watanabe Y, Moriyasu F, Kahaleh M, Habib N, Nagao T. Evaluation of effects of a novel endoscopically applied radiofrequency ablation biliary catheter using an ex-vivo pig liver. J Hepatobiliary Pancreat Sci. 2012;19:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Hansler J, Wissniowski TT, Schuppan D, Witte A, Bernatik T, Hahn EG, Strobel D. Activation and dramatically increased cytolytic activity of tumor specific T lymphocytes after radio-frequency ablation in patients with hepatocellular carcinoma and colorectal liver metastases. World J Gastroenterol. 2006;12:3716-3721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Zhou C, Wei B, Gao K, Zhai R. Biliary tract perforation following percutaneous endobiliary radiofrequency ablation: A report of two cases. Oncol Lett. 2016;11:3813-3816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |