Published online Oct 16, 2017. doi: 10.4253/wjge.v9.i10.521
Peer-review started: January 11, 2017
First decision: February 20, 2017
Revised: May 20, 2017
Accepted: June 12, 2017
Article in press: June 13, 2017
Published online: October 16, 2017
Processing time: 277 Days and 0.6 Hours
The diagnosis and opportunity for endoscopic therapy of gastric or duodenal lesions may be missed at esophagogastroduodenoscopy (EGD) because of technical difficulty in intubating at EGD the postoperatively excluded stomach and proximal duodenum in patients status post Roux-en-Y gastric bypass (RYGB). Two cases are reported of acute upper gastrointestinal bleeding 10 or 11 years status post RYGB, performed for morbid obesity, in which the EGD was non-diagnostic due to failure to intubate the excluded stomach and proximal duodenum, whereas subsequent push enteroscopy or single balloon enteroscopy were diagnostic and revealed 4-cm-wide or 5-mm-wide bulbar ulcers and even permitted application of endoscopic therapy. These case reports suggest consideration of push enteroscopy, or single balloon enteroscopy, where available, in the endoscopic evaluation of acute UGI bleeding in patients status post RYGB surgery when the EGD was non-diagnostic because of failure to intubate these excluded segments.
Core tip: After Roux-en-Y-gastric-bypass (RYGB) surgery, the surgically excluded distal stomach/duodenum may be difficult to intubate and examine during esophagogastroduodenoscopy (EGD). Two cases are reported of acute upper gastrointestinal (UGI) bleeding many years after RYGB surgery, in which EGD was non-diagnostic due to failure to intubate these excluded segments. However, single balloon or push enteroscopy successfully permitted this intubation, enabling endoscopic diagnosis and therapy of bulbar ulcers at high risk of rebleeding. These case reports suggest using single balloon or push enteroscopy to endoscopically evaluate acute UGI bleeding in patients status-post-RYGB-surgery when EGD was non-diagnostic because of failure to intubate the excluded gastroduodenal segments.
- Citation: Hakim S, Reddy SRR, Batke M, Polidori G, Cappell MS. Two case reports of acute upper gastrointestinal bleeding from duodenal ulcers after Roux-en-Y gastric bypass surgery: Endoscopic diagnosis and therapy by single balloon or push enteroscopy after missed diagnosis by standard esophagogastroduodenoscopy. World J Gastrointest Endosc 2017; 9(10): 521-528
- URL: https://www.wjgnet.com/1948-5190/full/v9/i10/521.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i10.521
Anastomotic ulcers are the most common cause of upper gastrointestinal (UGI) bleeding after Roux-en-Y gastric bypass (RYGB), the most popular form of bariatric surgery[1], but such patients can also bleed from ordinary gastric or duodenal (peptic) ulcers[2]. Ulcers in the proximal duodenum or stomach may, however, be missed at esophagogastroduodenoscopy (EGD) after RYGB surgery because deep intubation of the proximal afferent limb is technically difficult at EGD. Two patients with UGI bleeding status post RYGB surgery are reported who had non-diagnostic EGDs because of this technical difficulty, but then had 4-cm-wide or 5-mm-wide bulbar ulcers diagnosed and endoscopically treated after successfully intubating the proximal duodenum by push enteroscopy or single balloon enteroscopy. This work alerts physicians about this potential limitation of EGD in patients status post RYGB surgery, and suggests use of push enteroscopy or single balloon enteroscopy as alternatives for endoscopic diagnosis and therapy.
The literature was systematically reviewed via PubMed using the following medical subject headings or keys words: (“Roux-en-Y gastric bypass”) AND (“duodenal ulcer” or “gastric ulcer” or “missed ulcer” or “peptic ulcer” or “esophagogastroduodenoscopy” or “push enteroscopy” or “single balloon enteroscopy” or “double balloon enteroscopy” or “therapeutic endoscopy”) OR (“excluded segment” and “esophagogastroduodenoscopy”) OR (“bariatric surgery” and “endoscopy” and “upper gastrointestinal bleeding”). The term esophagogastroduodenoscopy (EGD) is used to describe what is technically esophagogastrojejunoscopy (EGJ) in patients status post RYGB, in accordance with common usage. The IRB at William Beaumont Hospital, Royal Oak, approved/exempted this study on December 16, 2016.
A 44-year-old woman with prior RYGB 11 years earlier for morbid obesity presented to a community hospital with recurrent melena, weakness, and orthostatic dizziness for 1 wk. She had been taking non-steroidal anti-inflammatory drugs (NSAIDs) about two days per month for several months, but was not taking proton pump inhibitors (PPIs). She was a non-smoker and non-alcoholic. Physical examination revealed stable vital signs, pallor, and a benign abdominal examination. Rectal examination revealed melena and no visible hemorrhoids. On admission the hemoglobin (Hgb) was 4.1 g/dL. She was intravenously infused crystalloid solutions, transfused 4 units of packed erythrocytes, and intravenously administered PPI. EGD did not reveal any source of UGI bleeding, but the excluded proximal duodenum and stomach were not intubated and viewed. Colonoscopy did not reveal any etiology of lower GI bleeding. The patient was discharged with Hgb = 8.0 g/dL.
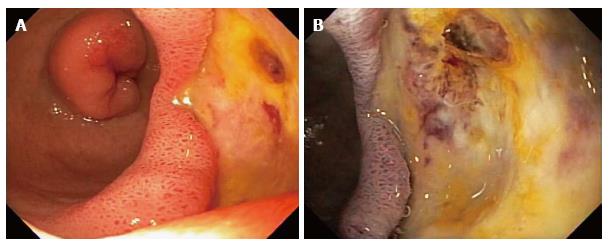
The patient was readmitted 1 d later to the same hospital, and then transferred to William Beaumont Hospital, a tertiary care hospital, for recurrent GI bleeding, with Hgb decline to 5.8 g/dL. Physical examination revealed stable vital signs, pallor, and a benign abdominal examination. Rectal examination revealed dark red blood and no visible hemorrhoids. Laboratory tests revealed a normal mean corpuscular volume, platelet count, and coagulation panel. Serum blood urea nitrogen:creatinine (BUN:Cr) ratio = 36. Liver function tests were within normal limits, except for albumin = 1.6 g/dL. She was medically stabilized with transfusions of packed erythrocytes. Abdominal computed tomography (CT) with intravenous contrast was within normal limits. Single balloon enteroscopy revealed a non-bleeding, 4-cm-wide bulbar ulcer with a non-bleeding visible vessel in the afferent limb and no other lesions (Figure 1A). The visible vessel was ablated by injection of 10 mL of dilute epinephrine 1:10000 and by heater probe thermocoagulation using coaptation with 13 pulses of 25 W for one second each (Figure 1B). The visible vessel was almost completely flattened by the thermocoagulation. After 72 h the patient had another episode of melena and the Hgb acutely declined from 9.5 to 6.5 g/dL. A technetium-labeled erythrocyte (bleeding) scan and an abdominal arteriogram did not reveal an actively bleeding source. Exploratory laparotomy revealed a giant, posterior, bulbar ulcer, which was oversewn. A Warthin-Starry stain of gastric biopsies did not reveal Helicobacter pylori (H. pylori). Serology for IgG antibodies against H. pylori was negative (low titer). No significant postoperative complications occurred. The patient is doing well at 3 mo follow-up, with no further GI bleeding.
A 64-year-old woman with prior RYGB for morbid obesity 10 years earlier presented acutely with melena and hematochezia, associated with dyspnea, fatigue, and syncope. She was taking ibuprofen about 800 mg/d for about 2 d per week for arthralgia, but had stopped about 3 mo ago. She was not taking PPIs, did not drink alcohol, and had stopped smoking cigarettes (1 pack/d) 12 years earlier. Physical examination on admission revealed stable vital signs, pallor, and a normal abdominal examination. Rectal examination revealed melena and no visible hemorrhoids. Laboratory analysis revealed Hgb = 7.4 g/dL (baseline recent Hgb = 13.3 g/dL), evident iron deficiency anemia with ferritin = 25 ng/mL, platelet count = 62000/L, and serum BUN:Cr ratio = 100. Liver function tests and coagulation panel were within normal limits. She was intravenously infused crystalloid solutions, transfused 4 units of packed erythrocytes, and intravenously administered a PPI. EGD revealed no lesions, including no anastomotic ulcers, but the afferent limb was not intubated. Colonoscopy revealed no lesions. Both computed tomographic enterography and capsule endoscopy were within normal limits. Bleeding slowly resolved and she was discharged with Hgb = 8.0 gm/dL.
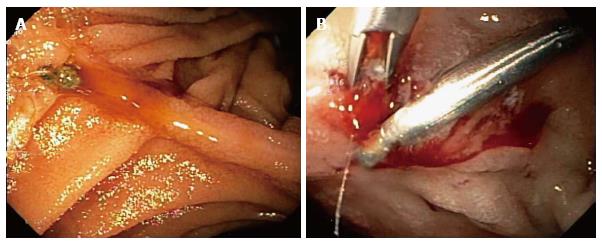
The patient was readmitted 3 d later for recurrent melena. Laboratory analysis revealed Hgb = 5.1 g/dL, platelets = 201000/L, and BUN:Cr ratio = 40. A technetium labeled erythrocyte (bleeding) scan did not reveal active GI bleeding. Push enteroscopy with intubation of the afferent limb revealed a 5-mm-wide acute bulbar ulcer with a visible vessel that was oozing blood, and an otherwise normal examination (Figure 2A). The visible vessel was endoscopically treated with dilute epinephrine injection, hemoclips, and argon plasma coagulation (APC) (Figure 2B). Serologic tests for IgG antibodies against H. pylori were negative (low titer). The patient had one further, minor, episode of melena, after which the bleeding resolved, and the patient was discharged. She has had no further GI bleeding during 3 mo of follow-up.
The prevalence of obesity in adults is currently 13% worldwide, and 34%-36% in the United States[3]. From 1960 to 2008 the prevalence of morbid obesity [body mass index (BMI) > 40 kg/m2 or > 35 kg/m2 with significant obesity-related disorders) increased from 0.9% to 6%, and the prevalence of obesity (BMI > 30 kg/m2) increased from 13.4% to 34.3% in the United States[4]. This epidemic of obesity has led to a surge in the number of bariatric surgeries, from 13365 annually in 1998 to 205000 annually in 2008 in the United States[5,6]. The direct annual costs of bariatric surgery are > 10 billion dollars per annum in the United States[7]. RYGB is the most common and most effective bariatric surgery performed in the United States[5,8].
Anastomotic ulcers are a common, important cause of UGI bleeding after RYGB, which most commonly occur in patients actively smoking or taking NSAIDs[9]. Such ulcers are usually easily detected and readily treated at EGD[10]. Contrariwise, bleeding from ordinary duodenal or gastric ulcers is rarely reported after RYGB, with only 22 reported cases[2,11-13]. Proposed mechanisms for these ulcers after RYGB include: An acidic environment within the excluded segment[14,15], deprivation of the buffering effect of ingested food in the excluded segment[16], bile acid reflux into the excluded stomach and duodenal bulb, excessive alcohol consumption, frequent NSAID use, and H. pylori infection[5,12]. In both reported cases, excessive alcohol consumption, frequent NSAID use, recent cigarette smoking, and H. pylori infection were excluded as risk factors for the peptic ulcers or GI bleeding by patient history and laboratory tests.
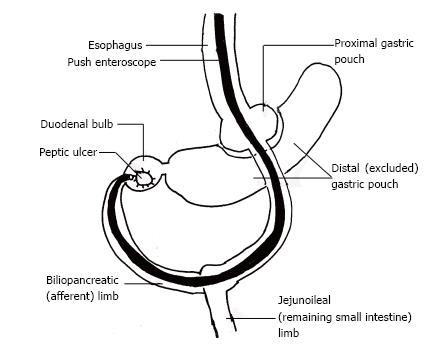
Diagnosis of ulcers in the excluded segment after RYGB is challenging because intubating the excluded stomach and proximal duodenum (afferent limb) is technically difficult due to sharp bowel angulation, deep intubation to reach the excluded segment (afferent limb) through the jejunum, endoscopic looping, and inability to adequately distend the gastric remnant with air (Figure 3). However, intubation of only the efferent limb at EGD would result in missing lesions in the afferent limb. CT or magnetic resonance imaging (MRI) virtual gastroduodenoscopy can provide excellent intra-luminal views, but cannot provide a tissue diagnosis[17]. Percutaneous endoscopic gastrostomy has been successfully used to endoscopically access the excluded segments[1]. Alternatively, patients have undergone intraoperative endoscopy or gastrostomy to identify and treat bleeding duodenal ulcers after RYGB surgery. Some authors have even suggested placing a gastrostomy tube within a radiopaque silastic ring during primary RYGB surgery to facilitate subsequent percutaneous endoscopic access to the excluded stomach[1].
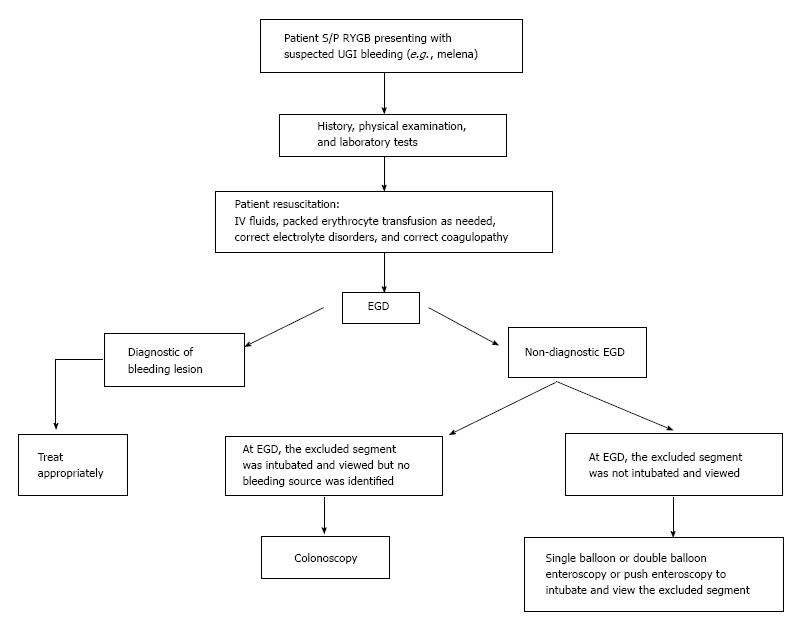
There are no standard recommendations in RYGB patients who present with obscure GI bleeding. Eid et al[9] recommended that examination of the bypassed stomach and duodenum should be attempted before performing colonoscopy to evaluate for a lower GI source and before performing capsule endoscopy to evaluate for a small bowel source. This work adds to the literature by suggesting the diagnostic and therapeutic benefits of push enteroscopy or single balloon enteroscopy if EGD was non-diagnostic and the excluded segments were not intubated at EGD in patients status post RYGB surgery (Figure 4). These alternative endoscopies can be performed either before or after colonoscopy and capsule endoscopy, depending on the likelihood of the patient having had an UGI bleed. Push enteroscopy or single balloon enteroscopy can even be done initially instead of EGD in patients status post RYGP to increase the diagnostic yield and therapeutic efficacy of the initial endoscopy. Push enteroscopy, however, can sometimes fail to reach the desired area. Double balloon enteroscopy is a promising alternative technique that requires an experienced endoscopist and is currently offered primarily at tertiary care centers. Double balloon enteroscopy was approximately 83% successful in intubating the excluded segment in a study of 6 patients status post RYGB[18,19]. Cappell et al[20] in 1992 reported 3 cases in 2 patients of severely symptomatic gastric ulcers within the surgically excluded gastric segment in patients status post vertical banded gastroplasty that were missed at EGD and only diagnosed at laparotomy. Whereas the ulcers in the surgically excluded segment status post vertical banded gastroplasty were endoscopically inaccessible, the currently reported ulcers in the excluded segment status post RYGB are often accessible using push enteroscopy or single balloon enteroscopy.
Angiography with embolization should be considered in actively bleeding and hemodynamically unstable patients. Emergency surgery, possibly including resection of the bypassed stomach, should be considered for patients with refractory bleeding, but such surgery without prior endoscopic localization of the bleeding site increases the surgical failure rate[9]. Some authorities even suggest resection of the excluded stomach, which can be performed during the initial bypass surgery[21]. Advantages of this resection include: (1) decreased acid production because of resection of most of the gastrin-releasing part of the stomach; (2) removal of difficult to access parts of the stomach; and (3) avoiding gastro-gastric fistulization. Disadvantages of this resection include: (1) risk of duodenal stump leakage; (2) risk of intraoperative or postoperative bleeding; (3) potential abscess formation from necrosis of omental fat; (4) bacterial overgrowth in the biliopancreatic blind pouch; and (5) nutrient deficiencies, including vitamin B12 deficiency. Due to the rarity of bleeding or perforation from peptic ulcers in the excluded segment after RYGB, surgical treatment should be individualized[21,22].
RYGB patients also face potentially delayed diagnosis of GI cancers in the excluded segment due to technically difficult endoscopic access, but RYGB patients have a lower incidence of gastric cancer as compared to the general population[18,23]. Five cases have been reported in the excluded segment of gastric adenocarcinoma, most of which were advanced cancers[18,24], and several cases of lymphoma or gastrointestinal stromal tumors have been reported[25]. Another problem in diagnosing these cancers are the limitations of virtual gastroduodenoscopy, including: (1) lack of visualization of fine mucosal detail, especially vascularity; (2) missing small, flat lesions; (3) confusion of residual intragastric food with a gastric mass; and (4) inability to obtain biopsy specimens for histological diagnosis[17]. Voellimger et al[26] suggested that resection of the bypassed stomach should be considered during the primary RYGB operation in patients with precancerous gastroduodenal lesions.
This study is limited by reporting only 2 cases and the retrospective methodology.
Despite the two currently reported diagnostic successes, these advanced endoscopic techniques may be non-diagnostic because of absence of lesions in the excluded segments or potential inability to sometimes intubate the excluded segments with these enteroscopes. Furthermore, endoscopic therapy of ulcers with stigmata of recent hemorrhage (SRH) may not necessarily prevent recurrent bleeding; one of the currently reported patients required surgery for re-bleeding despite the therapeutic endoscopy. Nonetheless, endoscopic therapy of ulcers with high risk SRH significantly reduces the risk of rebleeding and is therefore recommended despite occasional therapeutic failures[27]. This work should prompt a large, prospective, study to determine the technical success rate of single or double balloon enteroscopy vs standard EGD in intubating the excluded gastroduodenal segment (afferent limb) in patients status post RYGB.
In conclusion, intubation of the excluded stomach and duodenum at EGD is technically difficult in patients status post RYGB, and therefore the diagnosis and opportunity for endoscopic therapy of gastric or duodenal lesions may be missed. Two cases are reported of UGI bleeding 10 or 11 years status post RYGB in which the EGD was non-diagnostic due to failure to intubate the excluded stomach and proximal duodenum, whereas single balloon enteroscopy or push enteroscopy successfully diagnosed duodenal ulcers and provided for endoscopic treatment of these ulcers. These case reports suggest consideration of push enteroscopy, or single balloon enteroscopy, where available, in the evaluation of acute UGI bleeding in patients status post RYGB surgery when EGD was non-diagnostic because of failure to intubate these excluded segments. The results of this study require confirmation by a large study that is preferentially prospective, randomized, and controlled.
In case 1, a 44-year-old woman with prior Roux-en-Y gastric bypass (RYGB) 11 years earlier for morbid obesity presented with melena, weakness, and orthostatic dizziness for 1 wk. She had been taking non-steroidal anti-inflammatory drugs (NSAIDs) about two days per month for several months, but was not taking proton pump inhibitors (PPIs). She was a non-smoker and non-alcoholic. Physical examination revealed stable vital signs, pallor, and a benign abdominal examination. Rectal examination revealed melena and no visible hemorrhoids. In case 2, a 64-year-old woman with prior RYGB for morbid obesity 10 years earlier presented acutely with melena and hematochezia, associated with dyspnea, fatigue, and syncope. She was taking ibuprofen about 800 mg/d for about 2 d per week for arthralgia, but had stopped about 3 mo ago. She was not taking PPIs, did not drink alcohol, and had stopped smoking cigarettes (1 pack/d) 12 years earlier. Physical examination on admission revealed stable vital signs, pallor, and a normal abdominal examination. Rectal examination revealed melena and no visible hemorrhoids.
In case 1, the clinical history of melena, weakness, and orthostatic dizziness strongly suggests acute gastrointestinal bleeding. Melena strongly suggests acute upper GI bleeding, but lower GI bleeding cannot be completely excluded by this history. The symptoms of weakness and orthostatic dizziness suggest that the bleeding caused hypovolemia and was therefore physiologically significant and severe. In case 2, the clinical history of melena suggests acute upper GI bleeding, although occasionally melena may occur secondary to lower GI bleeding. The symptoms of dyspnea, fatigue, and syncope all suggest severe GI bleeding that is causing symptoms of end-organ injury or insufficiency from hypovolemia: Syncope from insufficient cerebral blood perfusion; and dyspnea and fatigue from profound anemia with decreased oxygen-carrying capacity of the blood.
In case 1, the history of prior RYGB surgery in a patient presenting with melena suggests possible bleeding from an anastomotic (marginal) ulcer, but other common causes of upper GI bleeding are in the differential diagnosis, including ordinary peptic ulcer disease, hemorrhagic gastritis, hemorrhagic reflux gastritis, as well as relatively uncommon lesions. Bleeding from esophageal varices is unlikely because of no history of chronic liver disease, absence of stigmata of chronic liver disease, and normal biochemical parameters of liver function. Also lower GI lesions must be considered in the differential diagnosis of patients presenting with melena when the EGD is non-diagnostic. The patient lacked potential risk factors for peptic ulcers or other causes of upper GI bleeding including known H. pylori infection, frequent NSAID use, smoking cigarettes, or alcoholism. In case 2, the melena suggests likely upper GI bleeding. Most prominent in the differential diagnosis in a patient status post RYGB with melena is an anastomotic (marginal) ulcer. However, other common causes of upper GI bleeding are in the differential diagnosis, including ordinary peptic ulcer disease, hemorrhagic gastritis, hemorrhagic reflux gastritis, as well as relatively uncommon lesions. Bleeding from esophageal varices is unlikely because of no history of chronic liver disease, absence of stigmata of chronic liver disease on physical examination, and normal biochemical parameters of liver function. Also lower GI lesions must be considered in the differential diagnosis of patients presenting with melena when the EGD is non-diagnostic. The patient lacked risk factors for peptic ulcer disease or other causes of upper GI bleeding including known H. pylori infection, recent NSAID use, recently smoking cigarettes, and alcoholism.
In case 1, on admission the hemoglobin level was 4.1 g/dL. This profound anemia demonstrated the importance of relatively rapidly transfusing the patient to prevent end organ injury from hypovolemia, and the patient was transfused 4 units of packed erythrocytes. A coagulopathy was not contributing to the bleeding as the coagulation profile was normal. The blood urea nitrogen:creatinine ratio was 36, consistent with upper rather lower GI bleeding. These findings are consistent with severe, upper GI bleeding. In case 2, laboratory analysis revealed hemoglobin = 7.4 g/dL (recent baseline hemoglobin = 13.3 g/dL), evident iron deficiency anemia with ferritin = 25 ng/mL, platelet count = 62000/L, and serum BUN:Cr ratio = 100. Liver function tests and coagulation panel were within normal limits. The current very low hemoglobin level that has recently declined from a normal hemoglobin level suggests acute, severe, GI bleeding. The iron deficiency anemia suggests the bleeding has been sufficiently long and severe to deplete iron stores. Melena usually arises from an upper GI source, but can occasionally result from a lower GI source, especially when associated with hematochezia. The highly elevated BUN:creatinine ratio strongly suggests that the melena is from upper rather than lower GI bleeding.
In case 1, EGD did not reveal any source of UGI bleeding, but the excluded duodenum and distal stomach were not intubated and examined. This was due to the difficulty in intubating the afferent limb status post RYGB surgery because of the long intubation needed to reach the afferent limb and acute angulation at the anastomosis to the afferent limb. Colonoscopy was performed because the EGD was non-diagnostic and did not reveal any etiology of lower GI bleeding. The patient experienced recurrent GI bleeding, with hemoglobin decline to 5.8 g/dL for which the patient was again medically stabilized. Abdominal computerized tomography with intravenous contrast was within normal limits. Single balloon enteroscopy, with intubation of the afferent limb, revealed a non-bleeding, 4-cm-wide bulbar ulcer with a non-bleeding visible vessel in the afferent limb and no other lesions. The diagnosis of a giant duodenal ulcer by single balloon enteroscopy after a non-diagnostic EGD due to failure to intubate the afferent limb at EGD is notable. In case 2, EGD did not reveal any source of UGI bleeding, including no anastomotic ulcers, but the excluded duodenum and distal stomach (afferent limb) status post RYGB surgery was not intubated and examined. This was due to the difficulty in intubating the afferent limb status post RYGB surgery because of the long intubation needed to reach the afferent limb and acute angulation at the afferent limb anastomosis. Colonoscopy was performed because of the history of melena and a non-diagnostic EGD, but did not reveal any etiology of lower GI bleeding. Both computed tomographic enterography and capsule endoscopy were within normal limits. Bleeding slowly resolved and she was discharged with hemoglobin level of 8.0 g/dL. The patient was readmitted 3 d later for recurrent melena with a hemoglobin level of 5.1 g/dL. A technetium labeled erythrocyte (bleeding) scan did not reveal active GI bleeding. Push enteroscopy revealed a 5-mm-wide acute bulbar ulcer with a visible vessel that was oozing blood, and an otherwise normal examination. The visible vessel was endoscopically treated with dilute epinephrine injection, hemoclips, and argon plasma coagulation.
In case 1, giant duodenal ulcer in excluded gastroduodenal segment in afferent limb after RYGB surgery which was diagnosed by single-balloon enteroscopy and confirmed at surgery. The lesion was not resected and therefore the diagnosis was by endoscopic and intraoperative examination without a pathologic diagnosis. In case 2, peptic ulcer in the duodenal bulb in the excluded gastroduodenal segment (afferent limb) after RYGB surgery, as diagnosed by enteroscopy. The bulbar ulcer was not resected and therefore the diagnosis was by enteroscopy, without a pathologic diagnosis.
In case 1, the visible vessel was ablated at single-balloon enteroscopy by injection of dilute epinephrine and by heater probe thermocoagulation. However, the patient had another episode of melena and the hemoglobin acutely declined from 9.5 g/dL to 6.5 g/dL 72 h after therapeutic single balloon enteroscopy. Failure despite use of two methods of endoscopic hemostasis (dilute epinephrine injection and heater probe thermocoagulation) was most likely related to the exceedingly large size of the duodenal ulcer (4 cm diameter). A technetium-labeled erythrocyte (bleeding) scan and an abdominal arteriogram did not reveal an actively bleeding source. Exploratory laparotomy revealed a giant, posterior, bulbar ulcer (which had been detected at single-balloon enteroscopy), which was oversewn. The patient was discharged with no further bleeding during the hospitalization or for three months of follow-up. In case 2, the patient received intravenously infused crystalloid solutions, transfused 4 units of packed erythrocytes, and intravenously administered a PPI. The duodenal ulcer lesion was endoscopically treated with dilute epinephrine injection, hemoclips, and argon plasma coagulation (APC) because it was oozing blood at the endoscopy. The patient had one further, self-limited, episode of melena, after which the bleeding resolved, and the patient was discharged. She has had no further GI bleeding during 3 mo of follow-up.
This work reports two cases of duodenal ulcers occurring after RYGB, supplementing 22 prior case reports of peptic ulcers after RYGB.
RYGB is an acronym for Roux-en-Y gastric bypass surgery, a popular form of bariatric surgery, in which a large part of the stomach and the duodenum is surgically excluded from the normal stream of food. This surgery leads to weight loss from decreased assimilation and absorption of food, from early satiety because of the very small residual stomach pouch, and from decreased production of ghrelin which acts as a “hunger hormone”. The afferent limb (also called the biliopancreatic limb) is composed of the duodenum which is excluded from the alimentary track after RYGB. The gastrojejunal (alimentary) limb connects directly from the proximal gastric pouch to the jejunoileum after RYGB surgery. After RYGB surgery the distal gastric pouch, duodenal bulb and rest of the duodenum are excluded from continuity with the rest of the alimentary tract.
These two cases report bleeding giant or moderately-sized duodenal ulcers being missed at routine EGD performed for acute upper GI bleeding because of inability to intubate the afferent limb (excluded segment) due to acute angulation and the need for deep intubation. This finding has been previously reported in case reports. This work reports that the afferent limb was successfully intubated by either a single balloon enteroscope or push enteroscope. This work further confirms the findings in previous isolated case reports. Use of these specialized endoscopes (enteroscopes) permitted the diagnosis of the etiology of the acute upper GI bleeding as peptic ulcers, permitted identification of high-risk stigmata of recent hemorrhage (SRH) consisting of either a visible vessel or active oozing of blood, and permitted performance of therapeutic endoscopy for hemostasis. Although recurrent GI bleeding from only one of the two high risk peptic ulcers was prevented by the endoscopic therapy and the second patient required GI surgery for recurrent GI bleeding, these two cases illustrate the usefulness of specialized enteroscopes rather than standard esophagogastroduodenoscopes to intubate, examine, and diagnose lesions in the excluded gastroduodenal segment (afferent limb) status post RYGB surgery. This work adds to the literature by emphasizing the potential diagnostic and therapeutic benefits of push enteroscopy or single balloon enteroscopy if EGD was non-diagnostic in patients status-post RYGB surgery. This work points out the need for a prospective, large study comparing the diagnostic yield of enteroscopy vs EGD in patients with upper GI bleeding status post RYGB surgery.
The authors report two cases with acute UGIB following Roux-en-Y gastric bypass where the diagnosis and treatment were performed successfully with enteroscopy. This report treats interesting cases.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen CH, Chiba H, Thomopoulos KC S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Ceppa FA, Gagné DJ, Papasavas PK, Caushaj PF. Laparoscopic transgastric endoscopy after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2007;3:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Iskandar ME, Chory FM, Goodman ER, Surick BG. Diagnosis and Management of Perforated Duodenal Ulcers following Roux-En-Y Gastric Bypass: A Report of Two Cases and a Review of the Literature. Case Rep Surg. 2015;2015:353468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Arroyo-Johnson C, Mincey KD. Obesity Epidemiology Worldwide. Gastroenterol Clin North Am. 2016;45:571-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 366] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 4. | Fryar CD, Carroll MD, Ogden CL. Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, 1960-1962 Through 2011-2012. Available from: https: //www.cdc.gov/nchs/data/hestat/obesity_adult_11_12/obesity_adult_11_12.htm. |
| 5. | Zerey M, Sigmon LB, Kuwada TS, Heniford BT, Sing RF. Bleeding duodenal ulcer after roux-en-Y gastric bypass surgery. J Am Osteopath Assoc. 2008;108:25-27. [PubMed] |
| 6. | Trainer S, Benjamin T. Elective surgery to save my life: rethinking the “choice” in bariatric surgery. J Adv Nurs. 2017;73:894-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004;350:1075-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 644] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 9. | Eid JJ, Radecke JM, Murr MM. Gastrointestinal bleeding from the excluded stomach: a proposed algorithmic approach to management. Surg Obes Relat Dis. 2015;11:e11-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Jamil LH, Krause KR, Chengelis DL, Jury RP, Jackson CM, Cannon ME, Duffy MC. Endoscopic management of early upper gastrointestinal hemorrhage following laparoscopic Roux-en-Y gastric bypass. Am J Gastroenterol. 2008;103:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Ivanecz A, Sremec M, Ceranić D, Potrč S, Skok P. Life threatening bleeding from duodenal ulcer after Roux-en-Y gastric bypass: Case report and review of the literature. World J Gastrointest Endosc. 2014;6:625-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Mittermair R, Renz O. An unusual complication of gastric bypass: perforated duodenal ulcer. Obes Surg. 2007;17:701-703. [PubMed] |
| 13. | Macgregor AM, Pickens NE, Thoburn EK. Perforated peptic ulcer following gastric bypass for obesity. Am Surg. 1999;65:222-225. [PubMed] |
| 14. | Mason EE, Munns JR, Kealey GP, Wangler R, Clarke WR, Cheng HF, Printen KJ. Effect of gastric bypass on gastric secretion. Am J Surg. 1976;131:162-168. [PubMed] |
| 15. | Flickinger EG, Sinar DR, Pories WJ, Sloss RR, Park HK, Gibson JH. The bypassed stomach. Am J Surg. 1985;149:151-156. [PubMed] |
| 16. | Bjorkman DJ, Alexander JR, Simons MA. Perforated duodenal ulcer after gastric bypass surgery. Am J Gastroenterol. 1989;84:170-172. [PubMed] |
| 17. | Silecchia G, Catalano C, Gentileschi P, Elmore U, Restuccia A, Gagner M, Basso N. Virtual gastroduodenoscopy: a new look at the bypassed stomach and duodenum after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Obes Surg. 2002;12:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Tagaya N, Kasama K, Inamine S, Zaha O, Kanke K, Fujii Y, Kanehira E, Hiraishi H, Kubota K. Evaluation of the excluded stomach by double-balloon endoscopy after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2007;17:1165-1170. [PubMed] |
| 19. | Sakai P, Kuga R, Safatle-Ribeiro AV, Faintuch J, Gama-Rodrigues JJ, Ishida RK, Furuya CK, Yamamoto H, Ishioka S. Is it feasible to reach the bypassed stomach after Roux-en-Y gastric bypass for morbid obesity? The use of the double-balloon enteroscope. Endoscopy. 2005;37:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Cappell MS, Miller SH. Gastric lesions in the excluded gastric segment undetected by endoscopy or radiography in patients status post vertical banded gastroplasty. Am J Gastroenterol. 1992;87:639-644. [PubMed] |
| 21. | Csendes A, Burdiles P, Papapietro K, Diaz JC, Maluenda F, Burgos A, Rojas J. Results of gastric bypass plus resection of the distal excluded gastric segment in patients with morbid obesity. J Gastrointest Surg. 2005;9:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Gypen BJ, Hubens GJ, Hartman V, Balliu L, Chapelle TC, Vaneerdeweg W. Perforated duodenal ulcer after laparoscopic gastric bypass. Obes Surg. 2008;18:1644-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Wingo PA, Ries LA, Giovino GA, Miller DS, Rosenberg HM, Shopland DR, Thun MJ, Edwards BK. Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91:675-690. [PubMed] |
| 24. | Watkins BJ, Blackmun S, Kuehner ME. Gastric adenocarcinoma after Roux-en-Y gastric bypass: access and evaluation of excluded stomach. Surg Obes Relat Dis. 2007;3:644-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | de Roover A, Detry O, de Leval L, Coimbra C, Desaive C, Honoré P, Meurisse M. Report of two cases of gastric cancer after bariatric surgery: lymphoma of the bypassed stomach after Roux-en-Y gastric bypass and gastrointestinal stromal tumor (GIST) after vertical banded gastroplasty. Obes Surg. 2006;16:928-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Voellinger DC, Inabnet WB. Laparoscopic Roux-en-Y gastric bypass with remnant gastrectomy for focal intestinal metaplasia of the gastric antrum. Obes Surg. 2002;12:695-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Cappell MS. Therapeutic endoscopy for acute upper gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol. 2010;7:214-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |