Published online Mar 25, 2016. doi: 10.4253/wjge.v8.i6.282
Peer-review started: December 7, 2015
First decision: December 22, 2015
Revised: January 5, 2016
Accepted: January 29, 2016
Article in press: January 31, 2016
Published online: March 25, 2016
Processing time: 106 Days and 20.4 Hours
Periampullary diverticulum (PAD) is duodenal outpunching defined as herniation of the mucosa or submucosa that occurs via a defect in the muscle layer within an area of 2 to 3 cm around the papilla. Although PAD is usually asymptomatic and discovered incidentally during endoscopic retrograde cholangiopancreatography (ERCP), it is associated with different pathological conditions such as common bile duct obstruction, pancreatitis, perforation, bleeding, and rarely carcinoma. ERCP has a low rate of success in patients with PAD, suggesting that this condition may complicate the technical application of the ERCP procedure. Moreover, cannulation of PAD can be challenging, time consuming, and require the higher level of skill of more experienced endoscopists. A large portion of the failures of cannulation in patients with PAD can be attributed to inability of the endoscopist to detect the papilla. In cases where the papilla is identified but does not point in a suitable direction for cannulation, different techniques have been described. Endoscopists must be aware of papilla identification in the presence of PAD and of different cannulation techniques, including their technical feasibility and safety, to allow for an informed decision and ensure the best outcome. Herein, we review the literature on this practical topic and propose an algorithm to increase the success rate of biliary cannulation.
Core tip: Presence of periampullary diverticulum (PAD) is thought to complicate the application of endoscopic retrograde cholangiopancreatography, which is already a technically difficult procedure. To improve success rates, different techniques have been developed to achieve successful biliary cannulation in patients with PAD. For patients with PAD, endoscopists must be aware of papilla identification and the different available cannulation techniques, as well as the technical feasibility and safety of each.
- Citation: Altonbary AY, Bahgat MH. Endoscopic retrograde cholangiopancreatography in periampullary diverticulum: The challenge of cannulation. World J Gastrointest Endosc 2016; 8(6): 282-287
- URL: https://www.wjgnet.com/1948-5190/full/v8/i6/282.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i6.282
Periampullary diverticulum (PAD) is duodenal outpunching defined as herniation of the mucosa or submucosa that occurs via a defect in the muscle layer within an area of 2 to 3 cm around the papilla. Prevalence of PAD increases with age, and overall prevalence among the elderly is reportedly 65%[1]. The formation of PAD is related to progression of duodenal motility disorders. Furthermore, increased intraduodenal pressure and progressive weakening of intestinal smooth muscles are known as the main underlying etiologies for this defect[2]. PAD is sub-classified into two categories according to the location of the papilla with respect to the diverticulum. In type I, or peri-diverticular papilla, the papilla is located at the edge of the diverticulum or within a radius of 2 cm from the diverticular edge. In type II, or intra-diverticular papilla (IDP), the papilla is located inside the diverticulum or lying between two adjacent diverticula[3].
Although PAD is usually asymptomatic and discovered incidentally in patients during endoscopic retrograde cholangiopancreatography (ERCP), it is associated with different pathological conditions such as common bile duct (CBD) obstruction, pancreatitis, perforation, bleeding, and rarely carcinoma[4-7]. Several hypotheses have been put forth to explain the observed higher incidence of biliary stone formation in the presence of PAD. First, it was proposed that dysfunction in the sphincter of Oddi, which in turn causes reflux of pancreatic fluid and intestinal content, can lead to biliary stone formation[8]. Second, it was proposed that diverticula cause spasm of the sphincter, thereby increasing biliary tract pressure that may in turn produce jaundice and cholangitis as well as predispose for choledocholithiasis[9]. Finally, it was proposed that PAD may compress the distal part of the CBD to cause functional biliary stasis, and this hypothesis was supported by the observation of increased incidence of pigment biliary stones[10,11].
Reported success rates of cannulation in patients with PAD have varied from 61% to 95.4%, a range that is significantly lower than that observed in patients without PAD[12]. In recent years, new techniques and new devices for successful biliary cannulation have been developed to improve rates of success in patients with PAD. For patients with PAD, endoscopists must be aware of papilla identification and the different cannulation techniques available, including the technical feasibility and safety of each, in order to make an informed decision and ensure the best outcome. Herein, we review the literature on this practical topic that was obtained through an electronic search of the literature databases of Google Scholar and PubMed using the following terms alone or in combination: ERCP, difficult cannulation, cannulation techniques, and periampullary diverticulum.
The presence of PAD is thought to complicate the application of ERCP, an already technically difficult procedure[2]. Cannulation of IDP can be challenging, time consuming and require the higher level of skill of more experienced endoscopists. A large portion of the failures of cannulation in patients with PAD has been attributed to inability of the endoscopist to detect the papilla[6]. However, in some studies, the finding of PAD during an ERCP was suggested as an indicator of an easier cannulation attempt, with a reported success rate of 94.9% compared to that of 94.8% in non-PAD patients after exclusion of cases with undetectable papillas that were considered to be likely IDPs[7]. In ERCP, identification of the papilla is the first major obstacle, especially in the presence of large diverticula. Thus, it is extremely helpful to know the following tips[13]: (1) in most cases, the papilla is located on the lower edge of the diverticulum or just inside, somewhere between the positions of 4 o’clock and 8 o’clock; (2) large diverticula are usually divided from proximal to distal by a ridge-like septum. This mostly involves the bile duct, with the ridge terminating at the papilla; (3) a catheter can be used to straighten and evert the folds to identify a hidden papilla within the diverticulum; (4) cannulation with the tip of the duodenoscope within the sac is also possible, but care must be taken to avoid perforation; and (5) in contrast to the usual papillary anatomy, the presence of PAD alters the biliary direction. It is often not acutely angulated superiorally, but runs more directly. Thus, acute angulation of the sphincterotome is not necessary.
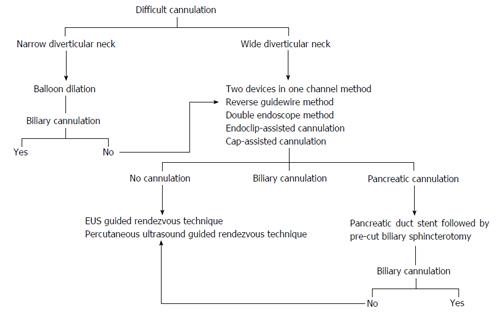
To address cases where the papilla is identified but does not point in a suitable direction for cannulation, the below-described techniques are available for consideration (Table 1).
| Two-devices in one-channel method |
| Reversed guidewire method |
| Double endoscope method |
| Balloon dilation of the narrow diverticular neck |
| Endoclip-assisted cannulation |
| Cap-assisted cannulation |
| Pancreatic duct stent placement followed by pre-cut biliary sphincterotomy |
| Percutaneous ultrasound-guided rendezvous technique |
| EUS-guided rendezvous technique |
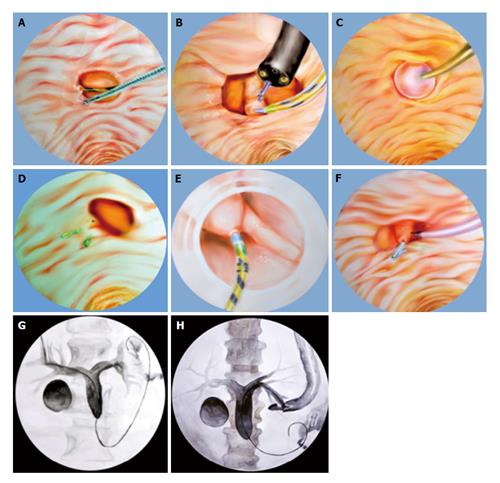
A biopsy forceps is used to pull the duodenal mucosa adjacent to the papilla, bringing the papillary orifice out of the diverticulum. Another instrument, either a cannula or sphincterotome, is then inserted into the working channel of the endoscope together with the biopsy forceps. With coordination of the two instruments, biliary cannulation can be attempted (Figure 1A). A report of this technique applied to two PAD cases showed successful cannulation for both and with no complications in either (success rate 100%)[14].
A second guidewire is advanced in reverse (stiff end forward) through the working channel of the duodenoscope, alongside the sphincterotome. This wire is then used to push the mucosa adjacent to the papilla toward the lumen of the duodenum and to straighten the folds, anchoring the papilla in a better configuration and creating a suitable direction for cannulation. A report of this technique applied to one PAD case showed successful cannulation with no complication (success rate 100%)[15].
A forward-viewing gastroscope is inserted inside the diverticulum for better visualization of the papilla. A foreign body forceps is used to grasp the tissue just beside the papilla in order to bring it into a better orientation. The gastroscope holding the papilla is left in place, to avoid backsliding after opening of the forceps. A side-viewing duodenoscope is inserted alongside the gastroscope. With both endoscopes positioned simultaneously in the duodenum, the CBD can be cannulated (Figure 1B). A report of this technique applied to one PAD case showed successful cannulation with no complication (success rate 100%)[16].
In narrow-necked papillary diverticula with the papilla located in the fundus of the diverticulum, endoscopic balloon dilation of the narrow diverticular neck, using a 15-mm stone retrieval balloon, can be done safely, bringing the papillary orifice into view. Cannulation of the bile duct can be attempted without any complications (Figure 1C). A report of this technique applied to three PAD cases showed successful cannulation and no complications (success rate 100%)[17].
One or more endoclips can be used to rotate the IDP externally and to fix it on the outside rim of the diverticulum. This manipulation can successfully evert and fix the papilla on the diverticular margin in a better position, resulting in successful biliary cannulation (Figure 1D). A report of this technique applied to two PAD cases showed successful cannulation with no complications (success rate 100%)[18].
A transparent cap is attached to the tip of a forward-viewing endoscope. At first, selective biliary cannulation can be attempted through the papillary orifice. If selective biliary cannulation fails, endoscopic fistulotomy can be attempted. Fistulotomy is performed between the lower two-thirds and the upper one-third of the papillary roof. To gain biliary access after the fistulotomy, needle puncture is made and a soft-tipped guidewire is advanced (Figure 1E). A report of this technique applied to twelve PAD cases showed successful cannulation in all cases (success rate 100%) and a minor complication (bleeding at the site of fistulotomy) in two patients (complications rate 16.5%); primary hemostasis was achieved by hemoclipping in one patient and by saline-epinephrine mixture spray in the other[19].
In the case of pancreatic duct cannulation, placement of a main pancreatic duct stent keeps the papilla out of the diverticulum, thereby facilitating pre-cut needle knife sphincterotomy and selective cannulation of the CBD (Figure 1F). A report of this technique applied to eight cases showed successful cannulation in seven of the patients (success rate 87.5%), with two of those requiring a second ERCP for success. In addition, two patients developed post-ERCP pancreatitis (complication rate 25%)[20].
After the percutaneous ultrasound-guided transhepatic biliary puncture is performed a sterile guidewire is inserted into the CBD, then into the papilla. A snare or forceps is then used to grasp the guidewire and pull it back through the working channel of the duodenoscope for subsequent over-the-wire cannulation (Figure 1G)[21]. However, it is sometimes difficult to grasp the guidewire, which may be damaged or kinked, during the withdrawal through the working channel of the duodenoscope; thus, passing a catheter over it is difficult or sometimes impossible[22]. A study on the percutaneous-ultrasound guided rendezvous technique applied to a total of fourteen patients showed success in 13 (success rate 93%) with complication (retroperitoneal perforation) experienced in only 1 (complication rate 7%)[21].
When the echoendoscope is positioned in the stomach or duodenum, and the bile ducts can be visualized by the endoscopic ultrasound (EUS), a 19-gauge or 22-gauge needle are used to puncture the bile ducts. After aspiration of bile, contrast is injected through the EUS needle to facilitate display the intra- and extra-hepatic bile ducts. After confirmation of bile duct puncture, a guidewire is advanced distally through the CBD and across the papilla under fluoroscopic guidance. The endoscope exchange is performed after passage of the guidewire through the papilla into the duodenum. In this process, the echoendoscope is removed, leaving the guidewire in place, after which a duodenoscope is passed up to the papilla alongside the EUS-placed guidewire. Finally, a snare or forceps is used to grasp the guidewire and pull it back out of the working channel of the duodenoscope for subsequent over-the-wire cannulation. After access to the CBD is achieved, a standard ERCP can be performed (Figure 1H). A study on the EUS-guided rendezvous technique applied to a total of 45 patients showed success in 36 (success rate 80%) with complications (bile leakage and pneumoperitoneum) experienced in only 2 (complication rate 4%)[23].
We propose an algorithm based on the previous techniques to increase the success rate of cannulation (Figure 2). It is important to note, however, that this algorithm has several limitations. First, it is based on a small number of published cases for most of the techniques. Second, the success rates are comparable in most of the techniques and the choice depends on the endoscopist’s preference and experience. Finally, percutaneous ultrasound-guided and EUS-guided rendezvous techniques are not available in all centers.
When therapeutic maneuvers are performed in patients with PAD the potential risks of complications are a concern, primarily because of the thin mucosa and the absence of sphincter muscle present in the ampullary area[24]. Currently, endoscopic papillary large balloon dilation (EPLBD) combined with limited endoscopic sphincterotomy (ES) (EPLBD + ES) is regarded as an effective maneuver for treating difficult CBD stones. It has been reported that perforation and hemorrhage are less frequent in cases treated with EPLBD + ES than in those treated with standard ES alone[25,26]. The tendency toward a shorter ballooning time in patients with PAD can be explained by the lack of sphincter muscle and the ease of ampullary widening facilitated by EPLBD, which suggest that EPLBD is a safe method for retrieval of CBD stones in patients with PAD[24]. Moreover, the complication rates of ERCP are similar in patients with or without PAD and the therapeutic outcome is not affected by the presence of PAD[3,7].
PAD represents a technical barrier to the successful application of ERCP. Cannulation of IDP can be challenging, time consuming and require the skill of more experienced endoscopists. In cases where the papilla is identified but does not point in a suitable direction for cannulation, a number of feasible techniques are available for consideration. Moreover, complication rates of ERCP are similar in patients with and without PAD, and therapeutic outcome is not affected by the presence of PAD.
P- Reviewer: Gkekas I, Kitamura K, Oner OZ S- Editor: Qi Y L- Editor: A E- Editor: Liu SQ
| 1. | Shemesh E, Klein E, Czerniak A, Coret A, Bat L. Endoscopic sphincterotomy in patients with gallbladder in situ: the influence of periampullary duodenal diverticula. Surgery. 1990;107:163-166. [PubMed] |
| 2. | Lobo DN, Balfour TW, Iftikhar SY. Periampullary diverticula: consequences of failed ERCP. Ann R Coll Surg Engl. 1998;80:326-331. [PubMed] |
| 3. | Boix J, Lorenzo-Zúñiga V, Añaños F, Domènech E, Morillas RM, Gassull MA. Impact of periampullary duodenal diverticula at endoscopic retrograde cholangiopancreatography: a proposed classification of periampullary duodenal diverticula. Surg Laparosc Endosc Percutan Tech. 2006;16:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Oddo F, Chevallier P, Souci J, Baque J, Buckley MJ, Fabiani P, Diaine B, Coussement A. [Radiologic aspects of the complications of duodenal diverticula]. J Radiol. 1999;80:134-140. [PubMed] |
| 5. | Yoneyama F, Miyata K, Ohta H, Takeuchi E, Yamada T, Kobayashi Y. Excision of a juxtapapillary duodenal diverticulum causing biliary obstruction: report of three cases. J Hepatobiliary Pancreat Surg. 2004;11:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Tyagi P, Sharma P, Sharma BC, Puri AS. Periampullary diverticula and technical success of endoscopic retrograde cholangiopancreatography. Surg Endosc. 2009;23:1342-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 7. | Panteris V, Vezakis A, Filippou G, Filippou D, Karamanolis D, Rizos S. Influence of juxtapapillary diverticula on the success or difficulty of cannulation and complication rate. Gastrointest Endosc. 2008;68:903-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Yildirgan MI, Başoğlu M, Yilmaz I, Atamanalp SS, Balik AA, Aydinli B, Oztürk G. Periampullary diverticula causing pancreaticobiliary disease. Dig Dis Sci. 2004;49:1943-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Hagège H, Berson A, Pelletier G, Fritsch J, Choury A, Liguory C, Etienne JP. Association of juxtapapillary diverticula with choledocholithiasis but not with cholecystolithiasis. Endoscopy. 1992;24:248-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Miyazaki S, Sakamoto T, Miyata M, Yamasaki Y, Yamasaki H, Kuwata K. Function of the sphincter of Oddi in patients with juxtapapillary duodenal diverticula: evaluation by intraoperative biliary manometry under a duodenal pressure load. World J Surg. 1995;19:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Shinagawa N, Fukui T, Mashita K, Kitano Y, Yura J. The relationship between juxtapapillary duodenal diverticula and the presence of bacteria in the bile. Jpn J Surg. 1991;21:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Zoepf T, Zoepf DS, Arnold JC, Benz C, Riemann JF. The relationship between juxtapapillary duodenal diverticula and disorders of the biliopancreatic system: analysis of 350 patients. Gastrointest Endosc. 2001;54:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Pohl J. Periampullary Diverticulum: Cannulation and Sphincterotomy. Video J Encyclop GI Endosc. 2013;1:516-517. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Fujita N, Noda Y, Kobayashi G, Kimura K, Yago A. ERCP for intradiverticular papilla: two-devices-in-one-channel method. Endoscopic Retrograde Cholangiopancreatography. Gastrointest Endosc. 1998;48:517-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Elmunzer BJ, Boetticher NC. Reverse guidewire anchoring of the papilla for difficult cannulation due to a periampullary diverticulum. Gastrointest Endosc. 2015;82:957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Külling D, Haskell E. Double endoscope method to access intradiverticular papilla. Gastrointest Endosc. 2005;62:811-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Tóth E, Lindström E, Fork FT. An alternative approach to the inaccessible intradiverticular papilla. Endoscopy. 1999;31:554-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Huang CH, Tsou YK, Lin CH, Tang JH. Endoscopic retrograde cholangiopancreatography (ERCP) for intradiverticular papilla: endoclip-assisted biliary cannulation. Endoscopy. 2010;42 Suppl 2:E223-E224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 19. | Myung DS, Park CH, Koh HR, Lim SU, Jun CH, Ki HS, Park SY, Rew JS. Cap-assisted ERCP in patients with difficult cannulation due to periampullary diverticulum. Endoscopy. 2014;46:352-355. [PubMed] |
| 20. | Fogel EL, Sherman S, Lehman GA. Increased selective biliary cannulation rates in the setting of periampullary diverticula: main pancreatic duct stent placement followed by pre-cut biliary sphincterotomy. Gastrointest Endosc. 1998;47:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 21. | Calvo MM, Bujanda L, Heras I, Cabriada JL, Bernal A, Orive V, Miguelez J. The rendezvous technique for the treatment of choledocholithiasis. Gastrointest Endosc. 2001;54:511-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Dickey W. Parallel cannulation technique at ERCP rendezvous. Gastrointest Endosc. 2006;63:686-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Tarantino I, Barresi L, Fabbri C, Traina M. Endoscopic ultrasound guided biliary drainage. World J Gastrointest Endosc. 2012;4:306-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Kim HG, Cheon YK, Cho YD, Moon JH, Park do H, Lee TH, Choi HJ, Park SH, Lee JS, Lee MS. Small sphincterotomy combined with endoscopic papillary large balloon dilation versus sphincterotomy. World J Gastroenterol. 2009;15:4298-4304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Kim HG, Cheon YK, Cho YD, Moon JH, Park DH, Lee TH, Choi HJ, Park SH, Lee JS, Lee MS. Small sphincterotomy combined with endoscopic papillary large balloon dilation versus sphincterotomy. World J Gastroenterol. 2009;15:4298-4304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Minami A, Hirose S, Nomoto T, Hayakawa S. Small sphincterotomy combined with papillary dilation with large balloon permits retrieval of large stones without mechanical lithotripsy. World J Gastroenterol. 2007;13:2179-2182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |