Published online Jan 25, 2016. doi: 10.4253/wjge.v8.i2.40
Peer-review started: June 30, 2015
First decision: August 25, 2015
Revised: November 1, 2015
Accepted: November 24, 2015
Article in press: November 25, 2015
Published online: January 25, 2016
Processing time: 204 Days and 15 Hours
Over the last few years, endoscopic submucosal dissection (ESD) has shown to be effective in the management of early colorectal neoplasms, particularly in Asian countries where the technique was born. In the Western world, its implementation has been slow and laborious. In this paper, the indications for ESD, its learning model, the available methods to predict the presence of deep submucosal invasion before the procedure and the published outcomes from Asia and Europe will be reviewed. Since ESD has several limitations in terms of learning achievement in the West, and completion of the procedure for the first cases is difficult in our part of the world, a short review on colorectal assisted ESD has been included. Finally, other endoscopic and surgical treatment modalities that are in competition with colorectal ESD will be summarized.
Core tip: In the Western world, endoscopic submucosal dissection (ESD) implementation is slow and laborious. In this paper, the indications for ESD, its learning model, the available methods to predict the presence of deep submucosal invasion before the procedure and the published outcomes from Asia and Europe will be reviewed. Additionally, a short review on colorectal assisted ESD has been included. Finally, other endoscopic and surgical treatment modalities that are in competition with colorectal ESD will be summarized.
- Citation: Marín-Gabriel JC, Fernández-Esparrach G, Díaz-Tasende J, Herreros de Tejada A. Colorectal endoscopic submucosal dissection from a Western perspective: Today’s promises and future challenges. World J Gastrointest Endosc 2016; 8(2): 40-55
- URL: https://www.wjgnet.com/1948-5190/full/v8/i2/40.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i2.40
Current colorectal cancer (CRC) screening population-based programs[1,2] will increase the detection of early neoplastic lesions suitable for endoscopic resection[3]. Although endoscopic mucosal resection (EMR) is appropriate to resect large flat or sessile colorectal lesions[4-8], recurrence after piecemeal resection is still a limitation[9,10]. In recent years, endoscopic submucosal dissection (ESD) has been endorsed as an ideal technique for en bloc resection of large colorectal neoplasms with high risk of focal adenocarcinoma or submucosal fibrosis[11]. Nevertheless, the optimal outcomes of colorectal ESD (CR-ESD) achieved in Japanese series[12,13] are constrained by the long learning curve and high complication rate when trying to introduce it in Western countries[14-16]. Thus, there is some controversy regarding the best approach to the management of large early neoplastic lesions in the colon[17]. Some authors advocate for the refinement of piecemeal EMR or a hybrid method of combined submucosal incision and EMR as a more realistic option for Western endoscopists[7,10,18], whereas others support progressive embracement of CR-ESD through a well-defined training strategy[19-21]. Different topics related to CR-ESD, including training, indications, outcomes, adjunctive devices to simplify the procedure and results when ESD is compared to alternative techniques, will be reviewed.
ESD is a complex procedure and the mastery of technical skills by new trainees has been based on a traditional mentor-pupil close teaching relationship in Japan since the introduction of the technique[22,23]. However, recent expansion of ESD in Western countries has been led by a small group of experienced endoscopists that have usually performed a self-learning process based on observation and animal model training[24-27]. Obvious reasons for this different approach are the lack of ESD experts in Western countries and the low detection rate of early gastric cancer as the ideal setting for beginners.
In Japan, the traditional model of teaching ESD has consisted of senior experts in large referral centers directly supervising new trainees in a step-by-step scheme[28-30]. Firstly, there is a selection of potential candidates based on prior achievement of good skills on endoscopic diagnosis of early gastrointestinal cancer and therapeutic maneuvers[28]. Secondly, the apprentice has to observe a certain number of ESD procedures performed by the mentor, occasionally participating as an assistant to become familiar with the special devices used. If possible, the trainee should complete this initial training period with some hands-on exposure to animal models[31]. The trainee is then invited to perform some partial phase of the ESD (marking, initial circumferential cutting, final dissection, preventive coagulation…) under close supervision by the mentor[32]. The ideal setting that has been suggested to begin with is performing ESD in selected lesions at an easily accessible gastric location[30]. When considered ready, the trainee is finally encouraged to perform a complete gastric ESD. Increasing number of cases completed eventually grant enough skills to move on to more difficult locations in the stomach. Several Japanese authors have suggested a number ranging from 20 to 80 cases to be considered proficient in gastric ESD[29,30,32]. Afterwards, the trainee may continue with other areas of the GI tract: esophagus, rectum and colon. Difficult colonic cases are generally restricted to experts with outstanding skills and extensive experience[33].
CR-ESD represents the last step in the natural evolution of ESD training. Colonic lesions are commonly located in difficult areas, where positioning of the endoscope may be extremely challenging, and there is general agreement that prior experience with gastric ESD is needed[34,35]. Several studies have investigated the appropriate number of CR-ESD to achieve proficiency. Some authors have proposed a minimum number of 20-30 cases under close supervision to achieve a certain level of competence[36], and it is advisable to begin with rectal and smaller lesions[37]. Nevertheless, the numbers needed to secure a high profile of successful R0 resection with few complications are closer to 80-100, according to some reports[38].
Small groups of endoscopists with particular interest in the technique have commonly promoted initiation of ESD in Western countries. The typical profile is that of an experienced attending gastroenterologist with extensive background in interventional endoscopy (EUS, ERCP, EMR…)[39]. Preliminaries could be either self-study based on articles and videos of procedures, attending ESD courses with hands-on training in animal models, etc. It is of particular interest to complete a visit to Japanese centers, where the trainee can benefit from first-hand experience observing experts performing ESD cases[25,26]. This is a good opportunity to learn the basics of chromoendoscopy and magnification for lesion assessment, different knives and ancillary devices used, steps of the ESD procedure including management of early and late complications, as well as specimen fixation and pathological assessment[27]. Additional extensive hands-on training using animal models is essential for the next steps in skills acquirement, up to the point when main outcomes are good enough to encourage completion of the first human ESD cases[19,24,25]. The fulfillment of the first human ESD cases should be based on a careful selection with preference for small gastric or rectal lesions. All these steps have been recommended in a European Society of Gastrointestinal Endoscopy position statement[40], and a training algorithm comprising most of them has been recently proposed[19].
Unfortunately, in many centers this training pathway must be self-teaching and is limited by the unfeasibility to obtain access to animal laboratory resources. Frustration from technical struggle or frequent complications may lead the process to a premature dead end. In addition, the bulk of potential candidates for ESD according to current recommendations are colorectal lesions[11,39], which makes it ever more arduous and disheartening. There are some approaches to overcome these limitations: proposing a Japanese expert to come to your institution for direct supervision during the first ESD cases[24,27,41] or attending hands-on courses in animal models in Japan to practice ESD under expert supervision have been suggested[42].
There are several studies in Europe focused on CR-ESD training. Initial reports showed suboptimal en bloc and R0 resection rates at the beginning[15,43], but rapid progression was observed within a relatively a short time[14,20,21,41]. The majority of endoscopists begin with selected small rectal lesions, to later move on to other colonic locations.
Some authors have proposed that a minimal intensive training may be sufficient for expert Western endoscopists to complete a sequential learning curve in rectal and colonic ESD, with a minimum of 20 untutored cases each after a short initial animal hands-on period (< 10 cases)[41]. Nevertheless, such an approach should be carefully considered since reports from high volume Japanese centers recommend a minimum of 80 cases to obtain adequate skills, both in terms of speed (< 15 min/cm2), perforation (< 6%), en bloc (> 95%) and R0 (> 90%) resection rates[38]. These numbers must be considered in light of the well-established scenario of close expert supervision in Japanese centers, which is frequently not the case in Europe[16]. Some experts have recommended for inexperienced Western endoscopists to complete at least 40 cases before attempting large or fibrotic CR lesions[44], two characteristics commonly present in the eligible population for CR-ESD[11].
In summary, it has been shown that the ESD training process in Europe in a prevalence-based approach will be undoubtedly shaped by a significant number of colonic and rectal cases[39]. Untutored ESD training can achieve good outcomes in CR-ESD, but it is encouraged that initial cases are early neoplastic lesions with a low risk of invasion due to the fact that R1 resection is common in inexperienced endoscopists[39]. Western reports have generally considered a resection rate > 80% acceptable; however, if Western endoscopists wish to pursue excellence in ESD, target outcome standards should probably not be less than those established in Japan, i.e., en bloc and R0 resection rate > 90%.
Intramucosal lesions and those well or moderately differentiated T1 adenocarcinomas with submucosal invasion less than 1000 μm and no lymphovascular infiltration, have little or no risk of metastasis[45] and therefore constitute a typical indication for endoscopic treatment and especially for ESD. In a retrospective series of patients treated at the National Cancer Center Hospital (NCCH) in Tokyo, it was noted that the mucosal morphological pattern accurately predicted the risk of submucosal invasion. In this study, the laterally spreading tumor non granular (LST-NG) type lesions showed a higher risk of submucosal invasion compared with granular (LST-G) type lesions with a statistically significant difference (14% vs 7%; P < 0.01)[46]. On the other hand, the presence of large nodules in LST-G type lesions, the finding of an invasive pit-pattern, “sclerotic” changes in the colorectal wall and a larger size in LST-NG type neoplasms, were also predictors of submucosal invasion. In this series, whereas submucosal invasion in LST-G most often occurs beneath the largest nodules and less frequently under depressed areas, 28% of LST-NG showed multifocal submucosal invasion in areas where there was no endoscopic warning signs. These findings were recognized as evidence of a different biological behaviour and drew attention to the need for an en bloc resection of these neoplasms.
The development of magnification chromoendoscopy (MCE) allowed Japanese endoscopists to describe different pit-patterns[47] as well as microvascular structures[48] in early CRC, increasing the accuracy of the histopathological prediction and improving the therapeutic decision-making process. When performed by Japanese expert endoscopists, MCE achieved a diagnostic accuracy of 98.8% in differentiating intramucosal or submucosal sm1 superficial invasion from sm2-sm3 deep submucosal invasion[49]. In another seminal study, the identification of a type IIIA microvascular pattern by Narrow Band Imaging was predictive of intramucosal or sm1 neoplasia in 94.5% of cases, while a type IIIB pattern was associated with sm2-3 carcinomas in 72% of cases[50].
We have fewer data from European or American centers, but a major Australian series of colorectal tumors treated by EMR[7] found that LST-NG type with a Paris 0-IIa + IIc morphology and a Kudo crypt pattern V had a risk of submucosal invasion of 55.5%. On the other hand, LST-G homogeneous type tumors presented submucosal invasion in only 1.5% of cases. These figures reflect that superficial colorectal neoplasms behave similarly to those described in Japanese series and therefore morphological pattern and epithelial crypt analysis can be used for histological prediction and treatment decision-making in Western patients.
Many studies confirm the accuracy of Western endoscopists in differentiating between neoplastic and non-neoplastic polyps, but few reports have focused specifically on their ability to predict the presence of deep submucosal invasion prior to an endoscopic resection attempt. A study from the United Kingdom[51] showed that Western endoscopists achieved a diagnostic accuracy of 78% in predicting deep submucosal invasion in Paris 0-II lesions by analyzing epithelial crypts and vascular patterns with MCE. In this study, high frequency miniprobe ultrasound examination improved the accuracy up to 94%.
Nevertheless, the limited data available from surgical series, including lesions deemed as endoscopically non-resectable, have demonstrated that between 10% and 20% of the specimens showed deep colonic wall invasion (stages T2-T4) or lymph node metastases that had not been suspected in the endoscopic assessment[52-55], indicating a lower than expected accuracy in real life conditions.
The role of endoscopic ultrasonography (EUS) in the diagnosis of submucosal invasion or nodal involvement has been controversial. In one small study from Western Europe, endoscopic ultrasound with a 20 MHz probe was better than MCE in determining the depth of invasion and nodal staging[56], but these results have not been consistently observed in other series of patients. In a study including more than 430 neoplasms treated in a single center in Japan, no significant differences were noted in the diagnostic accuracy between MCE and EUS[57].
In general terms, ESD is indicated for the treatment of colorectal neoplasms that show no suspicion of deep submucosal invasion assessed by MCE and that cannot be resected en bloc by EMR. Given the technical characteristics of ESD, the size of the lesion is not a limitation, although circumferential lesions are generally considered a contraindication given the high risk of stenosis.
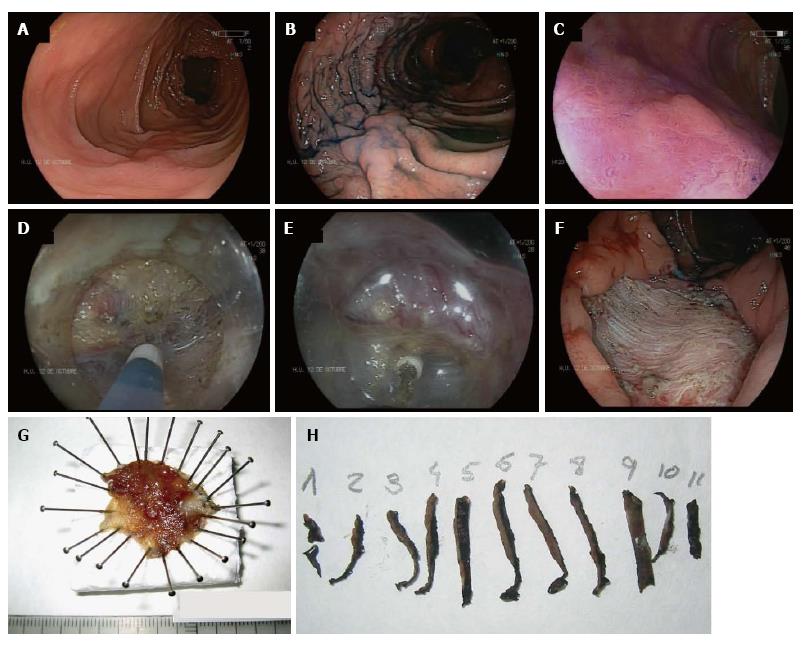
In the absence of local evidence, most Western endoscopists performing ESD have traditionally followed Japanese guidelines. Table 1 shows ESD indications of the Japan Gastroenterological Endoscopy Society[11]. In Europe, the Spanish Society of Digestive Endoscopy[58] and the European Association of Endoscopic Surgery[59] have adopted most of the Japanese indications for ESD as a standard treatment for superficial neoplasms larger than 20 mm in which en bloc EMR is difficult. These statements include mixed type LST-G, LST-NG, especially the pseudo-depressed type (Figure 1), large depressed lesions with a noninvasive pattern as assessed by MCE and neoplasia with fibrosis in the context of prior biopsy, attempts of resection or chronic inflammation.
| Lesions for which endoscopic en bloc resection is required |
| (1) Lesions for which en bloc resection with snare EMR is difficult to apply |
| LST-NG, particularly LST-NG pseudo-depressed type |
| Lesions showing a Vi-type pit pattern |
| Carcinoma with shallow T1 submucosal invasion |
| Large depressed-type tumors |
| Large protruded-type lesions suspected to be carcinoma. Including LST- G, nodular mixed type |
| (2) Mucosal tumors with submucosal fibrosis as a result of a previous biopsy or prolapse caused by intestinal peristalsis |
| (3) Sporadic localized tumors in conditions of chronic inflammation such as ulcerative colitis |
| (4) Local residual or recurrent early carcinomas after endoscopic resection |
Despite the gradual incorporation of Japanese knowledge about diagnosis and prediction of histological findings into European and American practices, major differences exist between Eastern and Western viewpoints on the endoscopic treatment of colorectal neoplasms. While ESD is widely accepted in Japan, and Japanese National Health Insurance has been covering its cost since 2012, in most Western hospitals a significant number of patients with endoscopically treatable lesions are still referred for surgery. In our part of the world, EMR is the preferred technique for the treatment of superficial neoplasms. As an alternative modality, ESD is still in the early steps of development, with a lack of a clear definition of its place in the treatment algorithms and significant uncertainties about the coverage of its costs.
These differences are clearly due to the greater experience of Japanese endoscopists, but also and significantly, because in Japan there has been little controversy about the importance of en bloc resection of tumors in which a risk of submucosal invasion is foreseeable. On the contrary, many Western endoscopists would contend that since most T1 adenocarcinomas with deep submucosal invasion can be identified in the histopathological study of piecemeal EMR, the benefits of en bloc resections are limited to a relatively small number of lesions with sm1 infiltration which, in the case of R0 resections, could avoid surgery. Others would however argue that even intramucosal large LST-NG that can be difficult to resect by EMR because of partial non-lifting, could itself justify the implementation of the procedure.
The tortuous morphology of the large intestine, a thinner wall when compared with the stomach, and the strong peristaltic motion of the colon, leads to a higher likelihood of complications during the procedure. It is very likely that the ESD learning curve is slower in the colon than in the stomach, and it has been overcome for many years in the experienced Asian centers.
As mentioned before, the experience with colorectal ESD out of the Asian countries is scarce. In European countries, our limited experience has shown less favourable results than those coming from the East, with lower en bloc and R0 resection rates and higher perforation rates.
Tables 2 and 3 summarize the most relevant data of the published series. Many of them have methodological limitations and an intention-to-treat analysis is lacking. Although it is commonly reported that the cases are consecutively enrolled, other information is often not provided. In most cases, they are cross-sectional studies and when follow-up is included, this is usually for a period less than 3 years. More importantly, considering that we are talking about oncological outcomes, the 5-year survival rate has been assessed in only one study[60].
| Ref. | Patients, n(% rectal) | Study design | Enrollmentperiod | Size(mm) | Time(min) | En bloc(%) | R0(%) | Perforationrate(%) | Delayedbleeding(%) | Hospitalstay(d) | Follow-up |
| Fujishiro et al[63], 2006 | 35 (100) | Prospective | Feb 2001 Feb 2005 | 32.8 | NS | 88.6 | 62.9 | 5.7 | 28.6 | - | Missing rate: n = 0; 0% Mean: 36 mo (12-60) Recurrence rate at 2 mo: 2.8% 31/32 (96.8%) recurrence-free at 3 yr |
| Tamegai et al[64], 2007 | 71 (23.9) | NS | Jan 2003 Dec 2005 | 32.7 | 61.1 | 98.6 | 95.6 | NS | 1.4 | - | Missing rate: n = 7; 9.86% Mean: 12.2 mo (range 3-34) Recurrences: 0% |
| Hurlstone et al[70], 2007 | 42 (33.3) | Prospective | Mar 2004 Aug 2006 | 31 | 48 | 78.6 | 73.8 | 2.4 | 2.4 | 22 | Missing rate: n = 6; 14.3% Median: 6 mo (range: 3-18) Recurrences 4/36 (11%) Curative resections at 6 mo: 34/42 (81%) |
| Fujishiro et al[112], 2007 | 200 (26) | NS | Jul 2000 Mar 2006 | 29.9 | - | 91.5 | 70.5 | 6 | 0.5 | - | Median: 18 mo (range 12-60) Recurrences: 1.8% |
| Saito et al[65], 2007 | 200 (30.5) | NS | Oct 2003 Jul 2006 | 35 | 90 | 84 | 70 | 5 | 2 | 5 | Median: 7 mo Missing rate: 10% Recurrences: 0.5% |
| Tanaka et al[68], 2007 | 70 (48.6) | NS | < Dec 2005 | 28 | 70.5 | 80 | - | 10 | 1.4 | - | In curative resections, 0% recurrence rate Other information not provided |
| Zhou et al[113], 2009 | 74 (56.7) | NS | Jul 2006 Dec 2007 | 32.6 | 110 | 93.2 | 89.2 | 8.1 | 1.4 | - | Missing rate: n = 0; 0% Median: 14.3 mo (range 3-22) Recurrences: 0% |
| Isomoto et al[114], 2009 | 292 (26.7) | NS | May 2001 Dec 2008 | 26.8 | - | 90.1 | 79.8 | 7.9 | 0.7 | - | Missing rate: 24.6% Median: 33 mo in R0 36 mo in non-R0 resections R0: 0% recurrences 1 recurrence in non-R0 resections |
| Saito et al[115], 2009 | 405 (27.4) | NS | NS | 40 | 90 | 86.9 | - | 3.5 | 1 | - | Mean ± SD: 20 ± 13 mo 2% recurrences |
| Iizuka et al[62], 2009 | 44 (59) | Retrospective | Jan 2000 Dec 2004 | 39 | 110 | 61 | 58 | 8 | - | - | - |
| Niimi et al[60], 2010 | 310 (26.1) | Retrospective Monocentric | Jul 2000 Dec 2008 | 28.9 | - | 90.3 | 74.5 | 4.8 | 1.3 | - | Median: 38.7 mo (12.8-104.2) 2% recurrences 3-yr overall/disease-specific survivals: 97.1%/100% 5-yr overall/disease-specific survivals: 95.3%/100% 8 died of other coexisting diseases 0 died of CRC |
| Yoshida et al[116], 2010 | 250 (31.6) | NS | Apr 2005 Mar 2010 | 29.6 | 106 | 86.8 | 81.2 | 6 | 2.4 | - | - |
| Saito et al[81], 2010 | 145 (50.3) | Retrospective | Jan 2003 Dec 2006 | 37 | 108 | 84 | - | 6.2 | 1.4 | - | Median: 20 mo 2.1% recurrences |
| Hotta et al[38], 2010 | 120 (27.5) | NS | Jun 2003 Sep 2008 | > 30 | 141 | 93.3 | 85 | 7.5 | - | - | - |
| Saito et al[12], 2010 | 1111 (30.3) | Multicentric Prospective | Jun 1998 Feb 2008 | 35 | 116 | 88 | 89 | 5.3 | 1.5 | - | - |
| Toyonaga et al[117], 2010 | 268 (25.7) | Retrospective | May 2002 May 2007 | 40.3 | 64.5 | 99.2 | 98.1 | 2.2 | 0.37 | - | Median: 32.2 mo (6.5-85.2) Follow-up: 227 out of the 241 curative resections (94.2%) Missing rate: 5.8% Recurrences: 0% |
| Matsumoto et al[118], 2010 | 203 (NS) | NS | Nov 2002 Jun 2009 | 33 | - | 85.7 | - | 6.9 | - | - | - |
| Uraoka et al[119], 2011 | 202 (32.7) | NS | Apr 2006 Mar 2010 | 40 | - | 91.6 | 87.1 | 2.4 | 0.5 | - | Median: 11.4 mo Missing rate: 14% 0% disease specific mortality 1.5% overall mortality |
| Shono et al[61], 2011 | 137 (26.2) | NS | Apr 2007 Oct 2010 | 29.2 | 79.2 | 89.1 | 85.4 | 3.6 | 3.6 | - | 0% recurrences No other information provided |
| Kim et al[120], 2011 | 108 (44) | Retrospective | Mar 2007 Feb 2009 | 27.6 | 61.9 | - | 78.7 | 20.4 | - | - | - |
| Lee et al[84], 2012 | 314 (19.1) | Retrospective | Jan 2004 Nov 2009 | 28.9 | 54.7 | 92.7 | 87.6 | 8 | 0.64 | 3.6 | - |
| Lee et al[121], 2012 | 499 (18.1) | Retrospective | Oct 2006 Nov 2010 | 28.9 | 61.3 | 95 | - | 7.4 | - | 3.6 | - |
| Hisabe et al[122], 2012 | 200 (30) | NS | Jun 2003 Jun 2011 | 32.7 | 108.9 | 86 | - | 7 | 1 | - | - |
| Saito et al[123], 2012 | 1321 (25.6) | Multicentric | - | 34.2 | 90 | 95.4 | 87.2 | 2.9 | 2.5 | - | - |
| Okamoto et al[71], 2013 | 30 (50) | NS | Dec 2010 Aug 2012 | 36 | 61 | - | 100 | 0 | 0 | - | - |
| Lee et al[72], 2013 | 874 (20.7) | Retrospective | > Oct 2006 | 26.5 | 53.8 | 97.1 | 90.5 | 6.1 | 0.5 | 3.5 | - |
| Nakajima et al[80], 2013 | 816 (36.3) | Prospective Multicentric | Oct 2007 Dec 2010 | 39.4 | 96 | 94.5 | 90.6 | 2 | 2.2 | - | - |
| Nawata et al[124], 2014 | 150 (20.6) | Retrospective 2 groups: A < 50 mm/B ≥ 50 mm | Apr 2010 Jul 2013 | 26/59 | 38/86 | 98.7 | 97.3 | 0 | 0 | - | - |
| Sakamoto et al[66], 2014 | 164 (38) | Retrospective | Apr 2005 Mar 2012 | 30 | 95 | 95 | 92 | 4 | 3 | - | - |
| Saito et al[109], 2014 | 900 (NS) | NS | NS | 40 | 100 | 91 | 87 | 2.7 | 1.7 | - | - |
| Lee et al[125], 2015 | 173 (24.3) | Retrospective | Jan 2010 Dec 2013 | 25.95 | - | 88.4 | 81.5 | 11 | 3.4 | - | - |
| Rahmi et al[67], 2015 | 28 (25) | Retrospective 100% recurrences | Dec 2008 Jul 2013 | 17.5 | 63 | 96.4 | 92.9 | 3.5 | 0 | 7 | Median: 22 mo Missing rate: 35.7% Recurrences: 0% |
| Ref. | Patients, n (% rectal) | Study design | Enrollment period | Size (mm) | Time (min) | En bloc (%) | R0 (%) | Perforation rate (%) | Delayed bleeding (%) | Hospital stay (d) | Follow-up |
| Farhat et al[16], 2011 | 85 (84.7) | Prospective | Jan 2008 Aug 2010 | - | - | 67 | 62.3 | - | - | - | - |
| Probst et al[14], 2012 | 76 (86.6) | NS | Oct 2004 Sep 2011 | 45.8 | 176 | 81.6 | 69.7 | 6.6 | 10.5 | - | Median: 23.6 mo (2-83) Included in follow-up: n = 65 9.2% residual neoplasms (5 piecemeal and 1 en bloc R1 lateral) |
| Repici et al[20], 2013 | 40 (100) | Prospective | Apr 2010 Jan 2011 | 46.8 | 86.1 | 90 | 80 | 5 | 2.5 | - | Recurrences: 2.5% |
| Thorlacius et al[126], 2013 | 29 (59) | NS | Jan 2012 Mar 2013 | 28 | 142 | 72 | 69 | 6.9 | 0 | - | Recurrences: 0% at 3-6 mo Missing rate: 82.7% |
| Spychalski et al[127], 2015 | 70 (56) | NS | Jun 2013 Jun 2014 | 30 | 110 | 66 | - | 8 | 6 | - | Missing rate: 14.6% Recurrences: 4.9% Follow-up < 12 mo |
| Rahmi et al[128], 2014 | 45 (100) | NS | Feb 2010 Jun 2012 | 35 | 110 | 64 | 53 | 18 | 13 | 3.4 | For curative resections at 12 mo: 88% |
| Bialek et al[129], 2014 | 37 (67.6) | Prospective | 2007 2013 | 37 | 70 | 86.5 | 81.1 | 0 | 5.7 | - | At 1-yr follow-up: 1.7% recurrences |
The percentage of non-curative resections oscillates between 3.6%[61] and 22.7%[62]. Furthermore, it is noteworthy that the percentage of aborted procedures is scarcely reported. This is particularly striking when a complex procedure, with a prolonged learning curve, comes into focus. Reviewing the published series, aborted ESD procedures of between 3.6% and 15.9% have been described[14,61,62].
Additionally, regarding complications, the perforation rate requiring surgery is seldom described, within a range of between 0%[61-67] and 2.8%[68]. Similarly, the need for transfusion or urgent endoscopic therapy due to severe gastrointestinal bleeding are, fortunately, rare, between 0%[12,64,65,69-71] and 2.2%[72].
Since ESD is accompanied by risk of delayed perforation and bleeding the postoperative course needs to be monitored carefully. However, no recommendations have been established for patient discharge after the procedure. Some Japanese authors have suggested a 5-d hospital stay for ESD[73]. In South Korea and some European countries, duration of the hospital stay is 2-3 d unless complications develop[16,72]. Recently, a Japanese group has published a clinical pathway to shorten hospital stays after the procedure. The authors concluded in the study that a three-day stay may be sufficient when no abnormalities occurred during ESD or on the first day after the endoscopic resection[74]. In our center, a stay that lasts 3 d is typically the case when no complications are observed. No delayed perforations have been identified after those 3 d in our experience; indeed, this complication is more likely to happen during the first 24 h after the procedure.
A good visualization of the submucosal layer is one of the key factors for performing an effective and safe CR-ESD, and this can only be achieved by proper traction of the tissue.
Benefits of applying traction during ESD are the following: (1) It can provide better submucosal exposure and consequently decreases the risk of perforation; and (2) Traction decreases the contact area between the tissue and the endoknife, enabling a more effective cut[75].
However, achieving good traction using only one knife through the scope is not easy. Unlike surgeons, who maintain tension and visibility by the hands of assistants, or by more than one device, the endoscopist who performs ESD can be considered as a one-armed person. In order to improve this disadvantage, a number of adjunctive devices have been designed.
A sinker-assisted ESD for colorectal neoplasms was reported by Saito et al[76]. A 1 g sinker is attached to a metallic clip by a nylon thread. After the initial dissection of the submucosa, the clip is deployed to the edge of the mucosa. The sinker will then pull down the partly resected tumor. Finally, changing the position of the patient, will allow gravity to expose the submucosal layer in order to enhance visibility for the remaining dissection.
The S-O clip (Sakamoto and Osada clip) consists of a metal clip attached to the end of a spring. A double nylon loop is connected at its other end. This system passes easily through the working channel of the endoscope. The device is attached to the mucosal flap and a second clip grasps the distal nylon loop to insert this end of the S-O clip to the wall opposite the lesion[77].
A double-scope method for large LSTs in the distal sigmoid colon or rectum has been reported by Uraoka et al[78]. When partial dissection of the submucosa has been performed, a clip is attached to the edge of the mucosal flap. A thinner endoscope is then passed through the anus and the primary endoscope is removed. At that point, a snare grasps the clip and pulls the lesion away from the muscle layer. This maneuver allows retraction of the submucosa and improves visualization. The primary scope is inserted again to resume the dissection. A limitation of this method is that the thin endoscope is not stiff enough to achieve deep intubation and using it for proximal lesions is not possible.
Yamamoto et al[79] reported recently a simple procedure requiring only common clips. After the mucosal flap has been created and the submucosal layer partially dissected, the edge of the mucosa is grasped with an endoclip. The cap attached to the tip of the endoscope is slipped under the clip and the dissection can be resumed as normal. One endoclip can be used for one region and other endoclips can be deployed in additional regions as needed. It is also possible to use two clips crossing one another. However, this method has several limitations. When the colonic lumen narrows or the position of the endoscope becomes unstable, it may be difficult to grasp the mucosa with the clip and slip the cap under the device.
Currently, ESD is the only technique that allows en bloc resection of colorectal mucosal or submucosal neoplasms of any size except for the full-thickness resection procedures. In the Western world, however, most lesions larger than 20 mm are still treated by piecemeal EMR.
In a prospective study of a large series of patients treated in 18 Japanese reference centers, it was observed that the rate of en bloc EMR was significantly reduced as the diameter of the lesion increased, reaching 66.5% in lesions of 20-29 mm, but was only 12.3% in lesions larger than 40 mm. Conversely, ESD en bloc resection rates remained above 90% regardless of the size of the lesion[80].
The first study comparing retrospectively the results of colorectal EMR and ESD included 373 (145 ESD/228 EMR) resected tumors between 2003 and 2006 by expert endoscopists at the NCCH in Tokyo[81]. As a result of differences in the indications of both procedures, the ESD group included larger lesions (37 ± 14 mm vs 28 ± 8 mm; P = 0.0006). However, the en bloc resection rate was significantly higher when performing ESD (84% vs 33%; P < 0.0001). An increased risk of tumor recurrence at follow-up colonoscopies was observed after EMR when compared with ESD (2% vs 14%; P < 0.0001). It is worth noting that, in this study, all recurrences detected in the ESD group occurred after treatment of lesions previously treated by piecemeal EMR. The mean procedure time was nevertheless more than three times longer in patients treated with ESD (108 min vs 29 min; P < 0.0001) and perforations were almost five times higher (6.2% vs 1.3%), although differences were not statistically significant.
Some Japanese and South Korean studies[73,82-85] have shown better outcomes for ESD in terms of en bloc and R0 resections and lower recurrence rates. In addition, higher perforation rates and an increased length of the procedure time have been also observed. Some of these studies, however, excluded patients who underwent surgery because of submucosal invasion, which could represent an overestimation of the clinical effectiveness of this procedure[73,82]. Furthermore, it has been shown in both Eastern and Western series of ESD that its benefits on a lower rate of local recurrence rely on the ability of the procedure to achieve en bloc resections and only become evident in those procedures performed by endoscopists with a high proportion of R0 resections[14].
The endoscopic resection of recurrent adenomas is another matter of concern. Although ESD may be used in endoscopic salvage procedures for recurrent lesions, performing this procedure is extremely difficult because of the presence of submucosal fibrosis attributable to previous resection. For this reason, in the Western world, the most commonly endoscopic procedure for treating recurrent adenomas after EMR is one additional EMR, although fibrosis after a previous resection often prevents lifting of the lesion after submucosal injection and causes the snare to slip off the tumor. There are very limited published data on the results for this strategy, with more than 10% of the patients needing surgery in this scenario[7].
In a retrospective case series that included 67 cases of a second endoscopic resection for recurrent neoplasias, ESD achieved a 56% en bloc resection rate compared with 39% in the EMR group. Both of these results are lower than expected for primary colorectal tumors[86]. In contrast, another study observed that 27 out of 28 patients were successfully treated using ESD for residual or recurrent colorectal of tumors[67].
More recently, underwater EMR (UEMR) has been evaluated for the treatment of these recurrences. When colon water distension is used instead of gas, the mucosa and submucosa involute, keeping the muscle layer in place, and there is no need for submucosal injection. Thus, the tumor can be snared easier than with conventional EMR. In a retrospective study, the en bloc resection and endoscopic complete removal rates were higher in the UEMR group when compared with the EMR group, and these differences were statistically significant. In addition, argon plasma coagulation ablation of residual tumor was lower in the UEMR group[87]. Although the study had several limitations, UEMR appears to be useful for salvage endoscopic management of recurrent lesions after a previous EMR.
Finally, some aspects remain to be clarified concerning the use of these endoscopic procedures for the treatment of defiant colorectal polyps. Thus, isolated cases of submucosal recurrences after piecemeal resection for intraepithelial or intramucosal neoplasms have been reported[73,81]. This complication has been attributed to staging errors derived from the histopathological study of a piecemeal resection. Additionally, there is no data concerning the impact of perforations that occur during ESD on oncological prognosis.
The two main surgical options at present are laparoscopic-assisted colorectal surgery (LACS) and transanal endoscopic microsurgery (TEM). Several non-randomized controlled studies have compared ESD and surgical modalities for management of colorectal lesions, but good quality evidence is lacking to allow substantial recommendations. ESD has been shown to be a good option for early colorectal neoplastic lesion with absent or shallow submucosal invasion[12,20,72], but diverse results have been reported when compared with alternative surgical modalities. Recent European guidelines for early rectal cancer do recommend either ESD or TEM, both with optimal curative resection rate, and discourage against conventional transanal excision unless both ESD and TEM are not feasible[59].
LACS has widely succeeded as a less invasive technique compared with conventional open surgery[88,89]. One retrospective study performed at the NCCH in Tokyo compared ESD with LACS for colorectal early carcinoma[90]. The study population comprised T1m/T1sm1 in the ESD group and T1sm2 in the LACS group. Lesions were located from cecum to rectum, with double the proportion of rectal lesions in the ESD group (38% vs 17%). Results showed that ESD was associated with a shorter procedure time (106 min vs 206 min), shorter hospital stay (5 d vs 13 d) and lower complication rates (6.4% vs 13.6%). Nevertheless, en bloc and curative resection rates were lower in the ESD group (87.2% and 80.4%, respectively), compared to 100% for surgical patients. Similarly, a recent retrospective study comparing a series of 300 colorectal ESD to 190 LACS revealed high en bloc and curative resection rates for ESD (> 90%), with a shorter procedure time and hospital stay, and a lower complication rate compared with LACS (90 min vs 185 min; 5 d vs 10 d; 7% vs 15%, respectively)[91]. It should be noted, however, that this report might be shaped by selection bias since a significant proportion of cases in the LACS group (35%) were post-EMR “lesions/scars”vs no cases in the ESD group, and apparently different from what was defined as local recurrence on ESD. Additionally, the ESD group included more than 75% of the lesions as LSTs vs only 10% in the LAC group.
In terms of hospital stay, five days or longer in LACS groups are common in Japanese studies. However, other studies have reported shorter periods when an elective surgery has been performed, ranging from 3[52] to 6 d[92-94].
TEM is a technique for en bloc full-thickness rectal wall excision up to the level of the perirectal fat that can be applied for lesions located as far as 18-20 cm from the anal verge. The minimal distance from the anal verge is 5 cm due to the rigid structure of the rectoscope, making it troublesome to approach a lesion next to the anal verge[95]. Developed more than 25 years ago as an alternative to standard transanal surgery[96], TEM has become one of the gold standards for early rectal cancers, whenever available[59]. There is increasing evidence that TEM is superior to conventional transanal resection (TAR) in terms of en bloc and R0 resection rates, and thus, lower recurrence, together with lower complication rates[97-99]. Some of the limitations of TEM include the long learning curve[100], similar to ESD, and the need for quite expensive special equipment.
Contradictory results have been obtained when comparing TEM with ESD. Whereas a recent meta-analysis showed better outcomes for TEM[101], single center-based studies, either head-to-head between both techniques, or only limited to rectal location, showed better outcomes for ESD, with fewer complications or a shorter length of the hospital stay. In the aforementioned meta-analysis, TEM appeared more effective than ESD in terms of en bloc and R0 resection rates (98.7% vs 87.8% and 88.5% vs 74.6%, respectively), with a shorter procedure time (67 min vs 96 min) and with no significant differences in the complication rate or the need for additional surgery due to adverse events. Adenoma recurrence rate was, however, higher in the TEM group (5.2% vs 2.6%). Nevertheless, this study included small ESD series, most of them with less than 50 cases and published before 2010[101]. A report with a small study population in both groups of ESD and TEM (< 20) showed comparable en bloc (91%-84%) and R0 (81%-84%) resection rates, with no differences in complications or length of hospital stay[102]. A South Korean single center retrospective study included patients with flat lesions with suspected high grade dysplasia or submucosal invasive carcinoma who underwent ESD or TEM[103]. En bloc and R0 resection rates were similar in both groups (ESD vs TEM: 96.7% vs 100% and 96.7% vs 97.0%, respectively), with no statistically significant differences in complication rate (3.3% vs 6.1%, respectively). Hospital stay was significantly lower after ESD (3.6 d vs 6.6 d). It should be noted that over 20% of patients in both groups required additional treatment, mostly due to histological risk factors for lymph node metastasis.
Regarding hospital stay with TEM, this outcome may vary significantly across centers. Thus, some authors have reported a median hospital stay of 2-3 d[104-107], while other studies suggest even shorter stays and have reported a 24 h discharging policy[108]. To our knowledge, prospective direct comparisons between TEM and ESD that address the question of superiority in terms of length of hospital stay have yet to be published.
In summary, in an ideal scenario of a well-trained endoscopist, ESD might be the best option for early colorectal neoplasia as it combines a high rate of curative resection, similar to surgical procedures, while maintaining a low profile of invasiveness and less need for general anesthesia[109]. But frequently this is not the case in most institutions in Western countries, and standard surgical techniques are commonly more accessible to physicians. Favorable results for ESD compared to surgical procedures published recently were only based in retrospective analysis studies, with significant risk of selection bias. There is a lack of randomized controlled trials to establish good quality evidence regarding both techniques. Nevertheless, it seems that if colorectal ESD expansion backed with encouraging outcomes continues, it might be difficult to complete such ideal head-to-head randomized studies since less invasive procedures with good results frequently gain spontaneous acceptance by patients and physicians.
Since CR-ESD is a technically demanding procedure, with a long learning curve and requires more time for its completion when compared with other resection techniques, simpler and more standardized methods are required for the treatment of colorectal neoplasms. Furthermore, performing CR-ESD is challenging in the presence of technically difficult lesions with severe fibrosis, recurrent lesions, or difficult locations (at the bottom of the cecum, near the terminal ileum, and in the appendix). The advantage of the full thickness resection is the ability to easily and quickly resect the main lesion and quickly close the colon wall defect. However, large-sized lesions are difficult to resect when using only a device that depends on a snare to achieve the resection[110]. To date, endoscopic treatment for this type of lesion requires additional devices to support closure and suturing. Unfortunately, most of them are not commercially available for widespread use.
Recently, a novel over-the-scope (OTSC) device has been developed for colorectal endoscopic full-thickness resection (eFTR). Although, colonic eFTR is not widely available in clinical practice, the initial results of this procedure have been published recently[111].
The full-thickness resection device (FTRD) consists of an OTSC System cap with a preloaded clip and a snare integrated into cap’s distal end. The lesion that has been previously marked with a marking probe included in the kit is then identified with the colonoscope. The tumor is then pulled into the cap using a grasping forceps. After ensuring that all the marked tissue is completely included into the cap, the OTSC is deployed. Finally, the lesion is resected after closing the snare, applying electrosurgical current, and retrieved from the colon with the endoscope.
In the study mentioned above the main indication for eFTR was the presence of residual or recurrent neoplasm after a previous endoscopic resection. The median time to complete the procedure was 50 min. The mean diameter of the resection specimen was 24 mm within a range of 12 to 40 mm. The en bloc resection rate, R0 resection rate and the percentage of histologically confirmed full-thickness resection were 83.3%, 75.0% and 87.5%, respectively. There were no perforations or severe bleeding episodes. A postpolypectomy electrocoagulation syndrome was observed in 8% of the patients that was successfully treated with antibiotics.
The FTRD has, however, several limitations. The diameter of the outer cap does not allow the system to pass through the oral route. Consequently, it cannot be used for resection in the upper gastrointestinal tract. In the colon, the main limiting factors are the size of the lesion and the presence of submucosal fibrosis. Tumors over 25 millimeters in diameter might not easily fit into the cap and lack of elasticity of the colonic wall because of severe fibrosis often makes the resection difficult. Additionally, in the rectum, the perirectal tissue that fixes it prevents the achievement of a full-thickness resection.
CR-ESD is a major advance for the treatment of colorectal neoplasms: it has well-established indications, achieves higher en bloc resection rates when compared with EMR and is less invasive and costly than surgery.
Nevertheless, ESD also has several disadvantages: It has a long learning curve and the training process is not well established outside of Asian countries. These problems still have to be resolved in Europe. Additionally, complications in terms of bleeding and perforation rates are higher than those associated with EMR, a more established endoscopic procedure in Western countries.
Despite the many devices commercially available to perform the technique, standardization of CR-ESD still needs to be defined. Indeed, to date, the skill of the endoscopist seems to be the determining factor to achieve excellent outcomes.
Finally, simpler and more time-efficient methods for the treatment of colorectal tumors are required and new developments in this area are very likely to appear in the next few years. Probably, in the near future, methods such as FTRD will be competing treatments for CR-ESD in selected patients. More importantly, innovative methods and new devices for eFTR and suturing are evolving and may change the way the colorectal neoplasms are managed, blurring the boundaries between advanced endoscopy and surgery.
P- Reviewer: Friedland S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 720] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 2. | Shaukat A, Mongin SJ, Geisser MS, Lederle FA, Bond JH, Mandel JS, Church TR. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 646] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 3. | Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas Á, Andreu M, Carballo F, Morillas JD, Hernández C. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 658] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 4. | Lim TR, Mahesh V, Singh S, Tan BH, Elsadig M, Radhakrishnan N, Conlong P, Babbs C, George R. Endoscopic mucosal resection of colorectal polyps in typical UK hospitals. World J Gastroenterol. 2010;16:5324-5328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Heresbach D, Kornhauser R, Seyrig JA, Coumaros D, Claviere C, Bury A, Cottereau J, Canard JM, Chaussade S, Baudet A. A national survey of endoscopic mucosal resection for superficial gastrointestinal neoplasia. Endoscopy. 2010;42:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Luigiano C, Consolo P, Scaffidi MG, Strangio G, Giacobbe G, Alibrandi A, Pallio S, Tortora A, Melita G, Familiari L. Endoscopic mucosal resection for large and giant sessile and flat colorectal polyps: a single-center experience with long-term follow-up. Endoscopy. 2009;41:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Chen RY, Byth K. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1909-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 439] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 8. | Conio M, Repici A, Demarquay JF, Blanchi S, Dumas R, Filiberti R. EMR of large sessile colorectal polyps. Gastrointest Endosc. 2004;60:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014;46:388-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (2)] |
| 10. | Moss A, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Burgess NG, Sonson R, Byth K. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 367] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 11. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 436] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 12. | Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 593] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 13. | Repici A, Hassan C, De Paula Pessoa D, Pagano N, Arezzo A, Zullo A, Lorenzetti R, Marmo R. Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: a systematic review. Endoscopy. 2012;44:137-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 14. | Probst A, Golger D, Anthuber M, Märkl B, Messmann H. Endoscopic submucosal dissection in large sessile lesions of the rectosigmoid: learning curve in a European center. Endoscopy. 2012;44:660-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Repici A, Conio M, De Angelis C, Sapino A, Malesci A, Arezzo A, Hervoso C, Pellicano R, Comunale S, Rizzetto M. Insulated-tip knife endoscopic mucosal resection of large colorectal polyps unsuitable for standard polypectomy. Am J Gastroenterol. 2007;102:1617-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Farhat S, Chaussade S, Ponchon T, Coumaros D, Charachon A, Barrioz T, Koch S, Houcke P, Cellier C, Heresbach D. Endoscopic submucosal dissection in a European setting. A multi-institutional report of a technique in development. Endoscopy. 2011;43:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 17. | Bourke M. Current status of colonic endoscopic mucosal resection in the west and the interface with endoscopic submucosal dissection. Dig Endosc. 2009;21 Suppl 1:S22-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Moss A, Bourke MJ, Tran K, Godfrey C, McKay G, Chandra AP, Sharma S. Lesion isolation by circumferential submucosal incision prior to endoscopic mucosal resection (CSI-EMR) substantially improves en bloc resection rates for 40-mm colonic lesions. Endoscopy. 2010;42:400-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Draganov PV, Coman RM, Gotoda T. Training for complex endoscopic procedures: how to incorporate endoscopic submucosal dissection skills in the West? Expert Rev Gastroenterol Hepatol. 2014;8:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Repici A, Hassan C, Pagano N, Rando G, Romeo F, Spaggiari P, Roncalli M, Ferrara E, Malesci A. High efficacy of endoscopic submucosal dissection for rectal laterally spreading tumors larger than 3 cm. Gastrointest Endosc. 2013;77:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Hülagü S, Şentürk Ö, Korkmaz U, Şirin G, Duman AE, Dindar G, Çelebi A, Koç DÖ, Bozkurt N, Yılmaz H. Endoscopic submucosal dissection for colorectal laterally spreading tumors. Turk J Gastroenterol. 2013;24:532-540. [PubMed] |
| 22. | Kakushima N, Fujishiro M, Kodashima S, Muraki Y, Tateishi A, Omata M. A learning curve for endoscopic submucosal dissection of gastric epithelial neoplasms. Endoscopy. 2006;38:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Yamamoto S, Uedo N, Ishihara R, Kajimoto N, Ogiyama H, Fukushima Y, Yamamoto S, Takeuchi Y, Higashino K, Iishi H. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy. 2009;41:923-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Berr F, Ponchon T, Neureiter D, Kiesslich T, Haringsma J, Kaehler GF, Schmoll F, Messmann H, Yahagi N, Oyama T. Experimental endoscopic submucosal dissection training in a porcine model: learning experience of skilled Western endoscopists. Dig Endosc. 2011;23:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Parra-Blanco A, Gonzalez N, Arnau MR. Ex vivo and in vivo models for endoscopic submucosal dissection training. Clin Endosc. 2012;45:350-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Draganov PV, Chang M, Coman RM, Wagh MS, An Q, Gotoda T. Role of observation of live cases done by Japanese experts in the acquisition of ESD skills by a western endoscopist. World J Gastroenterol. 2014;20:4675-4680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Herreros de Tejada A. ESD training: A challenging path to excellence. World J Gastrointest Endosc. 2014;6:112-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 28. | Fujishiro M, Jung HY, Goda K, Hirasawa K, Kakushima N, Lee IL, Morita Y, Oda I, Takeuchi M, Yamamoto Y. Desirable training and roles of Japanese endoscopists towards the further penetration of endoscopic submucosal dissection in Asia. Dig Endosc. 2012;24 Suppl 1:121-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Yamamoto Y, Fujisaki J, Ishiyama A, Hirasawa T, Igarashi M. Current status of training for endoscopic submucosal dissection for gastric epithelial neoplasm at Cancer Institute Hospital, Japanese Foundation for Cancer Research, a famous Japanese hospital. Dig Endosc. 2012;24 Suppl 1:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Oda I, Odagaki T, Suzuki H, Nonaka S, Yoshinaga S. Learning curve for endoscopic submucosal dissection of early gastric cancer based on trainee experience. Dig Endosc. 2012;24 Suppl 1:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Yoshida N, Yagi N, Inada Y, Kugai M, Kamada K, Katada K, Uchiyama K, Ishikawa T, Takagi T, Handa O. Possibility of ex vivo animal training model for colorectal endoscopic submucosal dissection. Int J Colorectal Dis. 2013;28:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Tsuji Y, Ohata K, Sekiguchi M, Ito T, Chiba H, Gunji T, Yamamichi N, Fujishiro M, Matsuhashi N, Koike K. An effective training system for endoscopic submucosal dissection of gastric neoplasm. Endoscopy. 2011;43:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Saito Y, Otake Y, Sakamoto T, Nakajima T, Yamada M, Haruyama S, So E, Abe S, Matsuda T. Indications for and technical aspects of colorectal endoscopic submucosal dissection. Gut Liver. 2013;7:263-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Uraoka T, Kawahara Y, Kato J, Saito Y, Yamamoto K. Endoscopic submucosal dissection in the colorectum: present status and future prospects. Dig Endosc. 2009;21 Suppl 1:S13-S16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | Niimi K, Fujishiro M, Goto O, Kodashima S, Koike K. Safety and efficacy of colorectal endoscopic submucosal dissection by the trainee endoscopists. Dig Endosc. 2012;24 Suppl 1:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum. 2011;54:1307-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Shiga H, Endo K, Kuroha M, Kakuta Y, Takahashi S, Kinouchi Y, Shimosegawa T. Endoscopic submucosal dissection for colorectal neoplasia during the clinical learning curve. Surg Endosc. 2014;28:2120-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 38. | Hotta K, Oyama T, Shinohara T, Miyata Y, Takahashi A, Kitamura Y, Tomori A. Learning curve for endoscopic submucosal dissection of large colorectal tumors. Dig Endosc. 2010;22:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Berr F, Wagner A, Kiesslich T, Friesenbichler P, Neureiter D. Untutored learning curve to establish endoscopic submucosal dissection on competence level. Digestion. 2014;89:184-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Deprez PH, Bergman JJ, Meisner S, Ponchon T, Repici A, Dinis-Ribeiro M, Haringsma J. Current practice with endoscopic submucosal dissection in Europe: position statement from a panel of experts. Endoscopy. 2010;42:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Iacopini F, Bella A, Costamagna G, Gotoda T, Saito Y, Elisei W, Grossi C, Rigato P, Scozzarro A. Stepwise training in rectal and colonic endoscopic submucosal dissection with differentiated learning curves. Gastrointest Endosc. 2012;76:1188-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 42. | Kaltenbach T, Soetikno R, Kusano C, Gotoda T. Development of expertise in endoscopic mucosal resection and endoscopic submucosal dissection. Tech Gastrointest Endosc. 2011;13:100-104. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Hulagu S, Senturk O, Aygun C, Kocaman O, Celebi A, Konduk T, Koc D, Sirin G, Korkmaz U, Duman AE. Endoscopic submucosal dissection for premalignant lesions and noninvasive early gastrointestinal cancers. World J Gastroenterol. 2011;17:1701-1709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Uraoka T, Parra-Blanco A, Yahagi N. Colorectal endoscopic submucosal dissection: is it suitable in western countries? J Gastroenterol Hepatol. 2013;28:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 45. | Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 486] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 46. | Uraoka T, Saito Y, Matsuda T, Ikehara H, Gotoda T, Saito D, Fujii T. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55:1592-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 47. | Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880-885. [PubMed] |
| 48. | Sano Y, Ikematsu H, Fu KI, Emura F, Katagiri A, Horimatsu T, Kaneko K, Soetikno R, Yoshida S. Meshed capillary vessels by use of narrow-band imaging for differential diagnosis of small colorectal polyps. Gastrointest Endosc. 2009;69:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 49. | Matsuda T, Fujii T, Saito Y, Nakajima T, Uraoka T, Kobayashi N, Ikehara H, Ikematsu H, Fu KI, Emura F. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol. 2008;103:2700-2706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 50. | Ikematsu H, Matsuda T, Emura F, Saito Y, Uraoka T, Fu KI, Kaneko K, Ochiai A, Fujimori T, Sano Y. Efficacy of capillary pattern type IIIA/IIIB by magnifying narrow band imaging for estimating depth of invasion of early colorectal neoplasms. BMC Gastroenterol. 2010;10:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 51. | Hurlstone DP, Hunter MD, Sanders DS, Thomson M, Cross SS. Olympus Lucera high-resolution vascular ectasia mapping in combination with the type V crypt pattern for the invasive depth estimation and nodal disease estimation in Paris type II colorectal cancers: a comparative prospective analysis to 20 MHz ultrasound. J Clin Gastroenterol. 2007;41:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 52. | Pokala N, Delaney CP, Kiran RP, Brady K, Senagore AJ. Outcome of laparoscopic colectomy for polyps not suitable for endoscopic resection. Surg Endosc. 2007;21:400-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 53. | Brozovich M, Read TE, Salgado J, Akbari RP, McCormick JT, Caushaj PF. Laparoscopic colectomy for apparently benign colorectal neoplasia: A word of caution. Surg Endosc. 2008;22:506-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Dulskas A, Samalavicius NE, Gupta RK, Zabulis V. Laparoscopic colorectal surgery for colorectal polyps: single institution experience. Wideochir Inne Tech Maloinwazyjne. 2015;10:73-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Itah R, Greenberg R, Nir S, Karin E, Skornick Y, Avital S. Laparoscopic surgery for colorectal polyps. JSLS. 2009;13:555-559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Hurlstone DP, Brown S, Cross SS, Shorthouse AJ, Sanders DS. High magnification chromoscopic colonoscopy or high frequency 20 MHz mini probe endoscopic ultrasound staging for early colorectal neoplasia: a comparative prospective analysis. Gut. 2005;54:1585-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | Fu KI, Kato S, Sano Y, Onuma EK, Saito Y, Matsuda T, Koba I, Yoshida S, Fujii T. Staging of early colorectal cancers: magnifying colonoscopy versus endoscopic ultrasonography for estimation of depth of invasion. Dig Dis Sci. 2008;53:1886-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Fernández-Esparrach G, Calderón Á, De-la-Peña J, Díaz-Tasende JB, Esteban JM, Gimeno-García AZ, Herreros-de-Tejada A, Martínez-Ares D, Nicolás-Pérez D, Nogales Ó. Endoscopic submucosal dissection. Sociedad Española de Endoscopia Digestiva (SEED) clinical guideline. Rev Esp Enferm Dig. 2014;106:120-132. [PubMed] |
| 59. | Morino M, Risio M, Bach S, Beets-Tan R, Bujko K, Panis Y, Quirke P, Rembacken B, Rullier E, Saito Y. Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg Endosc. 2015;29:755-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 60. | Niimi K, Fujishiro M, Kodashima S, Goto O, Ono S, Hirano K, Minatsuki C, Yamamichi N, Koike K. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2010;42:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 61. | Shono T, Ishikawa K, Ochiai Y, Nakao M, Togawa O, Nishimura M, Arai S, Nonaka K, Sasaki Y, Kita H. Feasibility of endoscopic submucosal dissection: a new technique for en bloc resection of a large superficial tumor in the colon and rectum. Int J Surg Oncol. 2011;2011:948293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Iizuka H, Okamura S, Onozato Y, Ishihara H, Kakizaki S, Mori M. Endoscopic submucosal dissection for colorectal tumors. Gastroenterol Clin Biol. 2009;33:1004-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Fujishiro M, Yahagi N, Nakamura M, Kakushima N, Kodashima S, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A. Endoscopic submucosal dissection for rectal epithelial neoplasia. Endoscopy. 2006;38:493-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | Tamegai Y, Saito Y, Masaki N, Hinohara C, Oshima T, Kogure E, Liu Y, Uemura N, Saito K. Endoscopic submucosal dissection: a safe technique for colorectal tumors. Endoscopy. 2007;39:418-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 65. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc. 2007;66:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 291] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 66. | Sakamoto T, Sato C, Makazu M, Sekiguchi M, Mori G, Yamada M, Kinjo Y, Turuki E, Abe S, Otake Y. Short-term outcomes of colorectal endoscopic submucosal dissection performed by trainees. Digestion. 2014;89:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Rahmi G, Tanaka S, Ohara Y, Ishida T, Yoshizaki T, Morita Y, Toyonaga T, Azuma T. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis. 2015;16:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 69. | Sakamoto T, Takamaru H, Mori G, Yamada M, Kinjo Y, So E, Abe S, Otake Y, Nakajima T, Matsuda T. Endoscopic submucosal dissection for colorectal neoplasms. Ann Transl Med. 2014;2:26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 70. | Hurlstone DP, Atkinson R, Sanders DS, Thomson M, Cross SS, Brown S. Achieving R0 resection in the colorectum using endoscopic submucosal dissection. Br J Surg. 2007;94:1536-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 71. | Okamoto K, Kitamura S, Muguruma N, Takaoka T, Fujino Y, Kawahara Y, Okahisa T, Takayama T. Mucosectom2-short blade for safe and efficient endoscopic submucosal dissection of colorectal tumors. Endoscopy. 2013;45:928-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Lee EJ, Lee JB, Lee SH, Kim do S, Lee DH, Lee DS, Youk EG. Endoscopic submucosal dissection for colorectal tumors--1,000 colorectal ESD cases: one specialized institute’s experiences. Surg Endosc. 2013;27:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 73. | Kobayashi N, Yoshitake N, Hirahara Y, Konishi J, Saito Y, Matsuda T, Ishikawa T, Sekiguchi R, Fujimori T. Matched case-control study comparing endoscopic submucosal dissection and endoscopic mucosal resection for colorectal tumors. J Gastroenterol Hepatol. 2012;27:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 74. | Tomiki Y, Kawai M, Takehara K, Tashiro Y, Munakata S, Kure K, Ishiyama S, Sugimoto K, Kamiyama H, Takahashi M. Clinical pathway to discharge 3 days after colorectal endoscopic submucosal dissection. Dig Endosc. 2015;27:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Lee BI. Debates on colorectal endoscopic submucosal dissection - traction for effective dissection: gravity is enough. Clin Endosc. 2013;46:467-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Saito Y, Emura F, Matsuda T, Uraoka T, Nakajima T, Ikematsu H, Gotoda T, Saito D, Fujii T. A new sinker-assisted endoscopic submucosal dissection for colorectal cancer. Gastrointest Endosc. 2005;62:297-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Mori H, Kawabe M, Nagahara A, Otaka M, Ogihara T. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest Endosc. 2009;69:1370-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 78. | Uraoka T, Ishikawa S, Kato J, Higashi R, Suzuki H, Kaji E, Kuriyama M, Saito S, Akita M, Hori K. Advantages of using thin endoscope-assisted endoscopic submucosal dissection technique for large colorectal tumors. Dig Endosc. 2010;22:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Yamamoto K, Hayashi S, Saiki H, Indo N, Nakabori T, Yamamoto M, Shibuya M, Nishida T, Ichiba M, Inada M. Endoscopic submucosal dissection for large superficial colorectal tumors using the “clip-flap method”. Endoscopy. 2015;47:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3262-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 81. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 82. | Tajika M, Niwa Y, Bhatia V, Kondo S, Tanaka T, Mizuno N, Hara K, Hijioka S, Imaoka H, Ogura T. Comparison of endoscopic submucosal dissection and endoscopic mucosal resection for large colorectal tumors. Eur J Gastroenterol Hepatol. 2011;23:1042-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 83. | Terasaki M, Tanaka S, Oka S, Nakadoi K, Takata S, Kanao H, Yoshida S, Chayama K. Clinical outcomes of endoscopic submucosal dissection and endoscopic mucosal resection for laterally spreading tumors larger than 20 mm. J Gastroenterol Hepatol. 2012;27:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 84. | Lee EJ, Lee JB, Lee SH, Youk EG. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 2012;26:2220-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 85. | Kim YJ, Kim ES, Cho KB, Park KS, Jang BK, Chung WJ, Hwang JS. Comparison of clinical outcomes among different endoscopic resection methods for treating colorectal neoplasia. Dig Dis Sci. 2013;58:1727-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 86. | Sakamoto T, Saito Y, Matsuda T, Fukunaga S, Nakajima T, Fujii T. Treatment strategy for recurrent or residual colorectal tumors after endoscopic resection. Surg Endosc. 2011;25:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 87. | Kim HG, Thosani N, Banerjee S, Chen A, Friedland S. Underwater endoscopic mucosal resection for recurrences after previous piecemeal resection of colorectal polyps (with video). Gastrointest Endosc. 2014;80:1094-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 88. | Spanjersberg WR, van Sambeeck JD, Bremers A, Rosman C, van Laarhoven CJ. Systematic review and meta-analysis for laparoscopic versus open colon surgery with or without an ERAS programme. Surg Endosc. 2015;29:3443-3453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 140] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 89. | Kennedy RH, Francis A, Dutton S, Love S, Pearson S, Blazeby JM, Quirke P, Franks PJ, Kerr DJ. EnROL: a multicentre randomised trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme. BMC Cancer. 2012;12:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 90. | Kiriyama S, Saito Y, Yamamoto S, Soetikno R, Matsuda T, Nakajima T, Kuwano H. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis. Endoscopy. 2012;44:1024-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 91. | Nakamura F, Saito Y, Sakamoto T, Otake Y, Nakajima T, Yamamoto S, Murakami Y, Ishikawa H, Matsuda T. Potential perioperative advantage of colorectal endoscopic submucosal dissection versus laparoscopy-assisted colectomy. Surg Endosc. 2015;29:596-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 92. | Wilson MZ, Hollenbeak CS, Stewart DB. Laparoscopic colectomy is associated with a lower incidence of postoperative complications than open colectomy: a propensity score-matched cohort analysis. Colorectal Dis. 2014;16:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 93. | Iroatulam AJ, Chen HH, Potenti FM, Parameswaran S, Wexner SD. Laparoscopic colectomy yields similar morbidity and disability regardless of patient age. Int J Colorectal Dis. 1999;14:155-157. [PubMed] |
| 94. | Chen HH, Wexner SD, Weiss EG, Nogueras JJ, Alabaz O, Iroatulam AJ, Nessim A, Joo JS. Laparoscopic colectomy for benign colorectal disease is associated with a significant reduction in disability as compared with laparotomy. Surg Endosc. 1998;12:1397-1400. [PubMed] |
| 95. | Demartines N, von Flüe MO, Harder FH. Transanal endoscopic microsurgical excision of rectal tumors: indications and results. World J Surg. 2001;25:870-875. [PubMed] |
| 96. | Buess G, Kipfmüller K, Hack D, Grüssner R, Heintz A, Junginger T. Technique of transanal endoscopic microsurgery. Surg Endosc. 1988;2:71-75. [PubMed] |
| 97. | Moore JS, Cataldo PA, Osler T, Hyman NH. Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses. Dis Colon Rectum. 2008;51:1026-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 98. | Allaix ME, Arezzo A, Cassoni P, Famiglietti F, Morino M. Recurrence after transanal endoscopic microsurgery for large rectal adenomas. Surg Endosc. 2012;26:2594-2600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 99. | Middleton PF, Sutherland LM, Maddern GJ. Transanal endoscopic microsurgery: a systematic review. Dis Colon Rectum. 2005;48:270-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 202] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 100. | Koebrugge B, Bosscha K, Ernst MF. Transanal endoscopic microsurgery for local excision of rectal lesions: is there a learning curve? Dig Surg. 2009;26:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 101. | Arezzo A, Passera R, Saito Y, Sakamoto T, Kobayashi N, Sakamoto N, Yoshida N, Naito Y, Fujishiro M, Niimi K. Systematic review and meta-analysis of endoscopic submucosal dissection versus transanal endoscopic microsurgery for large noninvasive rectal lesions. Surg Endosc. 2014;28:427-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 102. | Kawaguti FS, Nahas CS, Marques CF, Martins BC, Retes FA, Medeiros RS, Hayashi T, Wada Y, de Lima MS, Uemura RS. Endoscopic submucosal dissection versus transanal endoscopic microsurgery for the treatment of early rectal cancer. Surg Endosc. 2014;28:1173-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 103. | Park SU, Min YW, Shin JU, Choi JH, Kim YH, Kim JJ, Cho YB, Kim HC, Yun SH, Lee WY. Endoscopic submucosal dissection or transanal endoscopic microsurgery for nonpolypoid rectal high grade dysplasia and submucosa-invading rectal cancer. Endoscopy. 2012;44:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 104. | Barendse RM, van den Broek FJ, van Schooten J, Bemelman WA, Fockens P, de Graaf EJ, Dekker E. Endoscopic mucosal resection vs transanal endoscopic microsurgery for the treatment of large rectal adenomas. Colorectal Dis. 2012;14:e191-e196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 105. | Darwood RJ, Wheeler JM, Borley NR. Transanal endoscopic microsurgery is a safe and reliable technique even for complex rectal lesions. Br J Surg. 2008;95:915-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 106. | Mihai R, Borley N. Transanal endoscopic microsurgery--impact on the practice of a colorectal surgeon in a district general hospital. Ann R Coll Surg Engl. 2005;87:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 107. | Maglio R, Muzi GM, Massimo MM, Masoni L. Transanal minimally invasive surgery (TAMIS): new treatment for early rectal cancer and large rectal polyps-experience of an Italian center. Am Surg. 2015;81:273-277. [PubMed] |
| 108. | Wright CJ, Tutton M. Early discharge following transanal endoscopic microsurgery is safe. J Laparoendosc Adv Surg Tech A. 2014;24:399-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 109. | Saito Y, Yamada M, So E, Abe S, Sakamoto T, Nakajima T, Otake Y, Ono A, Matsuda T. Colorectal endoscopic submucosal dissection: Technical advantages compared to endoscopic mucosal resection and minimally invasive surgery. Dig Endosc. 2014;26 Suppl 1:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 110. | Fujihara S, Mori H, Kobara H, Nishiyama N, Matsunaga T, Ayaki M, Yachida T, Morishita A, Izuishi K, Masaki T. Current innovations in endoscopic therapy for the management of colorectal cancer: from endoscopic submucosal dissection to endoscopic full-thickness resection. Biomed Res Int. 2014;2014:925058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 111. | Schmidt A, Bauerfeind P, Gubler C, Damm M, Bauder M, Caca K. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy. 2015;47:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 112. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678-683; quiz 645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 275] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 113. | Zhou PH, Yao LQ, Qin XY. Endoscopic submucosal dissection for colorectal epithelial neoplasm. Surg Endosc. 2009;23:1546-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 114. | Isomoto H, Nishiyama H, Yamaguchi N, Fukuda E, Ishii H, Ikeda K, Ohnita K, Nakao K, Kohno S, Shikuwa S. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2009;41:679-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 115. | Saito Y, Sakamoto T, Fukunaga S, Nakajima T, Kiriyama S, Matsuda T. Endoscopic submucosal dissection (ESD) for colorectal tumors. Dig Endosc. 2009;21 Suppl 1:S7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 116. | Yoshida N, Naito Y, Kugai M, Inoue K, Wakabayashi N, Yagi N, Yanagisawa A, Yoshikawa T. Efficient hemostatic method for endoscopic submucosal dissection of colorectal tumors. World J Gastroenterol. 2010;16:4180-4186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 117. | Toyonaga T, Man-i M, Fujita T, East JE, Nishino E, Ono W, Morita Y, Sanuki T, Yoshida M, Kutsumi H. Retrospective study of technical aspects and complications of endoscopic submucosal dissection for laterally spreading tumors of the colorectum. Endoscopy. 2010;42:714-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 118. | Matsumoto A, Tanaka S, Oba S, Kanao H, Oka S, Yoshihara M, Chayama K. Outcome of endoscopic submucosal dissection for colorectal tumors accompanied by fibrosis. Scand J Gastroenterol. 2010;45:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 119. | Uraoka T, Higashi R, Kato J, Kaji E, Suzuki H, Ishikawa S, Akita M, Hirakawa T, Saito S, Hori K. Colorectal endoscopic submucosal dissection for elderly patients at least 80 years of age. Surg Endosc. 2011;25:3000-3007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 120. | Kim ES, Cho KB, Park KS, Lee KI, Jang BK, Chung WJ, Hwang JS. Factors predictive of perforation during endoscopic submucosal dissection for the treatment of colorectal tumors. Endoscopy. 2011;43:573-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 121. | Lee EJ, Lee JB, Choi YS, Lee SH, Lee DH, Kim do S, Youk EG. Clinical risk factors for perforation during endoscopic submucosal dissection (ESD) for large-sized, nonpedunculated colorectal tumors. Surg Endosc. 2012;26:1587-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 122. | Hisabe T, Nagahama T, Hirai F, Matsui T, Iwashita A. Clinical outcomes of 200 colorectal endoscopic submucosal dissections. Dig Endosc. 2012;24 Suppl 1:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 123. | Saito Y, Kawano H, Takeuchi Y, Ohata K, Oka S, Hotta K, Okamoto K, Homma K, Uraoka T, Hisabe T. Current status of colorectal endoscopic submucosal dissection in Japan and other Asian countries: progressing towards technical standardization. Dig Endosc. 2012;24 Suppl 1:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 124. | Nawata Y, Homma K, Suzuki Y. Retrospective study of technical aspects and complications of endoscopic submucosal dissection for large superficial colorectal tumors. Dig Endosc. 2014;26:552-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 125. | Lee SP, Kim JH, Sung IK, Lee SY, Park HS, Shim CS, Han HS. Effect of submucosal fibrosis on endoscopic submucosal dissection of colorectal tumors: pathologic review of 173 cases. J Gastroenterol Hepatol. 2015;30:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 126. | Thorlacius H, Uedo N, Toth E. Implementation of endoscopic submucosal dissection for early colorectal neoplasms in Sweden. Gastroenterol Res Pract. 2013;2013:758202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 127. | Spychalski M, Dziki A. Safe and efficient colorectal endoscopic submucosal dissection in European settings: is successful implementation of the procedure possible? Dig Endosc. 2015;27:368-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 128. | Rahmi G, Hotayt B, Chaussade S, Lepilliez V, Giovannini M, Coumaros D, Charachon A, Cholet F, Laquière A, Samaha E. Endoscopic submucosal dissection for superficial rectal tumors: prospective evaluation in France. Endoscopy. 2014;46:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 129. | Białek A, Pertkiewicz J, Karpińska K, Marlicz W, Bielicki D, Starzyńska T. Treatment of large colorectal neoplasms by endoscopic submucosal dissection: a European single-center study. Eur J Gastroenterol Hepatol. 2014;26:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |