Published online Oct 16, 2016. doi: 10.4253/wjge.v8.i18.653
Peer-review started: April 18, 2016
First decision: May 19, 2016
Revised: June 15, 2016
Accepted: August 15, 2016
Article in press: August 16, 2016
Published online: October 16, 2016
Processing time: 183 Days and 15.9 Hours
To quantify the presence of villous atrophy in endoscopic images for improved automation.
There are two main categories of quantitative descriptors helpful to detect villous atrophy: (1) Statistical and (2) Syntactic. Statistical descriptors measure the small intestinal substrate in endoscope-acquired images based on mathematical methods. Texture is the most commonly used statistical descriptor to quantify villous atrophy. Syntactic descriptors comprise a syntax, or set of rules, for analyzing and parsing the substrate into a set of objects with boundaries. The syntax is designed to identify and distinguish three-dimensional structures based on their shape.
The variance texture statistical descriptor is useful to describe the average variability in image gray level representing villous atrophy, but does not determine the range in variability and the spatial relationships between regions. Improved textural descriptors will incorporate these factors, so that areas with variability gradients and regions that are orientation dependent can be distinguished. The protrusion syntactic descriptor is useful to detect three-dimensional architectural components, but is limited to identifying objects of a certain shape. Improvement in this descriptor will require incorporating flexibility to the prototypical template, so that protrusions of any shape can be detected, measured, and distinguished.
Improved quantitative descriptors of villous atrophy are being developed, which will be useful in detecting subtle, varying patterns of villous atrophy in the small intestinal mucosa of suspected and known celiac disease patients.
Core tip: Celiac disease is a relatively common ailment throughout the world, affecting approximately 1% of the population. Yet, it is little known and rarely diagnosed. Untreated, it can lead to severe intestinal disturbance, cancer, neurological problems, fertility problems, and other disorders. Villous atrophy of the small intestine is often present in untreated celiac patients. Better quantitative image analysis is important to detect areas of pathology in the small intestine endoscopically. In this study the main approaches for automatically detecting villous atrophy by computerized means are described, which can be helpful to map areas of pathology and determine disease status.
- Citation: Ciaccio EJ, Bhagat G, Lewis SK, Green PH. Recommendations to quantify villous atrophy in video capsule endoscopy images of celiac disease patients. World J Gastrointest Endosc 2016; 8(18): 653-662
- URL: https://www.wjgnet.com/1948-5190/full/v8/i18/653.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i18.653
Celiac disease is prevalent throughout the entire world, though it varies in frequency, and averages about 1% of the population[1]. An important clinical problem is that the definitive diagnosis of celiac disease is difficult and requires serologic testing and endoscopy with biopsy, which is not available with accuracy in all areas of the world. Therefore geographic regions with lesser frequencies of celiac disease may simply be regions with a lack of experience in diagnosis and or areas without the facilities necessary for definitive diagnosis[2]. The presence of villous atrophy of the small intestinal mucosa, which is determined by examining biopsy slides under light microscopy[3], may not always be evident in untreated celiac disease patients. Present in the digitized biopsy slides are villous protrusion features, which are blunted in villous atrophy as compared to those found in healthy tissue[4]. Careful orientation of the biopsy on the slides and their proper examination is crucial, since off angle villi can erroneously appear blunted, mimicking villous atrophy[5]. Thus the experience of the pathologist is very important for accurate diagnosis of celiac disease.
Typically, villous atrophy is found in untreated celiac patients at the level of the duodenal bulb and the descending duodenum[6], but may also be present at the more distal regions of the small intestine, the jejunum and ileum, and be absent more proximally. The presence of villous atrophy tends to be patchy and is interspersed with regions of normal mucosa[7]. The mucosal abnormalities may be subtle or may even be lacking in images acquired with standard or video endoscopic techniques, due to the limits of resolution and the interpretation of microscopic changes of the intestinal villi, as manifested in the macroscopic image content. Though, more recently-developed high resolution endoscopes may overcome some problems with identification of mucosal structure[8].
During the last decade video capsule endoscopy has been used to image the entire small intestine with improved spatial resolution[9]. The video capsule is convenient to use for both adult and pediatric patients suspected of having celiac disease, because it is minimally invasive[10]. The capsule is swallowed and the video camera contained within the capsule snaps images at the rate of 2 per second or more[11]. The more recent video capsules have a variable frame rate, increasing in rate as the capsule motion increases, when presumably it is moving along the lumen at a faster rate, and decreasing when the capsule motion slows[12]. This ensures a more uniform frame rate per unit distance that the capsule travels. As the spatial and temporal resolution of recently commercially available video capsules has increased, it has been proposed that the series of video images can possibly be used to map the presence of villous atrophy all along the small intestinal length.
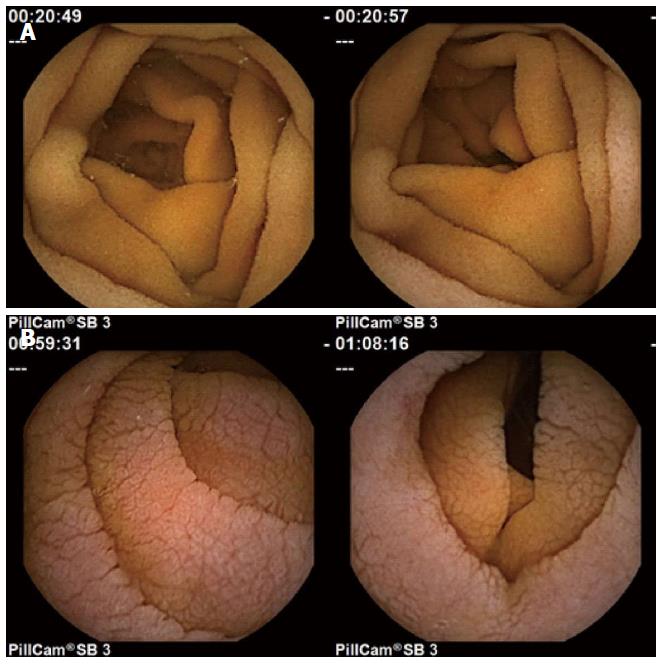
Prior research has suggested that there are differences in the video capsule endoscopy images of untreated celiac patients vs a control population[13-19]. Images from untreated celiac patients tend to be less structurally uniform both within a particular image, and across a series of images, as compared with control subjects[13-19]. In Figure 1, normal images at the top have uniform appearance and smooth folds. The celiac patient images at bottom were acquired from areas where villous atrophy was present, and have a mottled appearance, due to fissuring, and scalloping of the mucosal folds. These differences suggest the possibility that the presence of villous atrophy can be detected and mapped in a sequential series of video capsule images by computerized means. If areas of villous atrophy could be detected and mapped automatically all along the small intestinal tract, it would potentially be very helpful in the diagnosis of celiac disease. It would also be useful to monitor the progress in treatment of celiac disease. Currently, the only treatment is a lifelong gluten free diet[20], When the patient goes on the diet, the villi heal, albeit slowly[21]. Sometimes however, villous atrophy persists. Thus automated monitoring and mapping of the location and severity of villous atrophy throughout the small intestine would be very useful. In this work, we describe the main modes of quantitative detection of villous atrophy from video capsule endoscopic images, and possible avenues to improve the detection rate and to better monitor the severity and types of pathology present in endoscopic images which are abnormal due to the presence of villous atrophy. The current detection of villous atrophy is determined by an experienced observer. This introduces bias based on observer experience and knowledge and possibly fatigue. These would be obviated by computerized techniques.
For quantitative endoscopic image analysis in suspected or known celiac disease patients, it is important to detect areas of villous atrophy that may be present in the small intestinal mucosa. This is still mostly an unsolved problem. It is difficult to detect villous atrophy in part because the spatial resolution of the video capsule system from which discretized images are obtained is limited, and does not in every case clearly detect the individual villi in the small intestinal wall. The resolution in part depends on the video camera to intestinal wall distance, the camera lighting, and the camera angle, and is at best about 1 mm[17]. All of the factors for which resolution is determined are variable and tend to be random. Thus the identification of small intestinal villi, and the detection and quantification of villous atrophy, poses an important quantitative medical research problem, and a dilemma in terms of selecting the best method for recognition of the villi, and for estimation of whether or not there are normal or abnormal villi present in the image, as well as the degree and severity of areas of pathology.
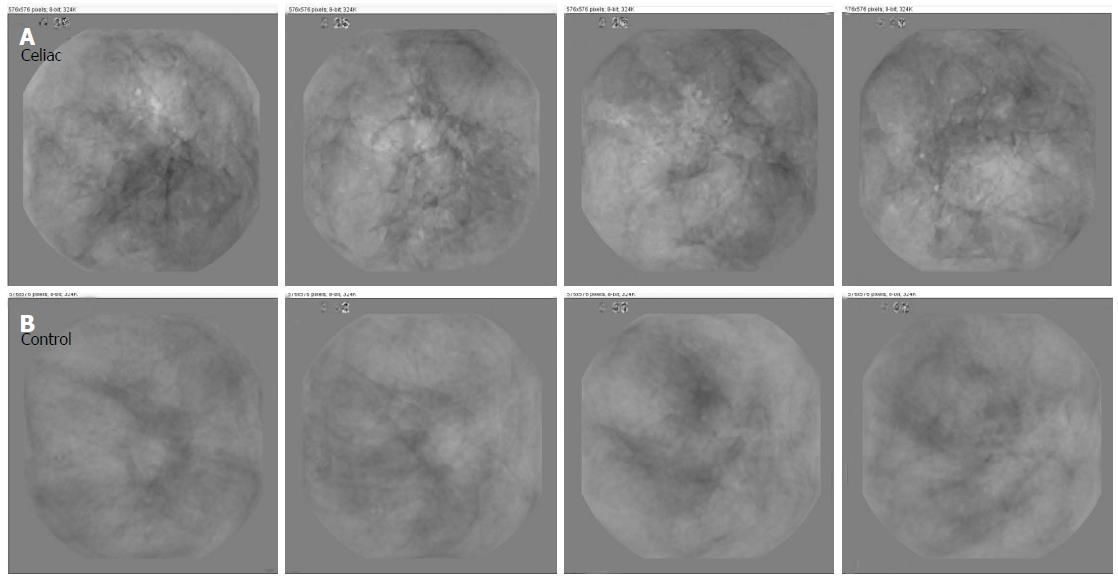
To recognize abnormalities in endoscopic images, many investigators have used textural methods in part because of their simplicity and ease of use, as well as being a tried and true method of analysis[13-19]. Several helpful methods have been developed to describe the presence of villous atrophy as a set of textural features. Image textures can be measured locally using the wavelet operator and a local binary pattern[22]. It is possible to develop a set of scale invariant texture descriptors by utilizing wavelet analysis[23]. Over a series of images, texture can be defined by the presence of salient features that tend to reappear from one image to the next. This is illustrated in Figure 2. The panels are composed of basis images - images that contain the most salient features over a series of images acquired from the same patient. At bottom is shown four basis images from a patient with normal villi at the level of the duodenum. The appearance of these basis images is mostly smooth and uniform. The upper basis images were constructed from a series of images acquired from a celiac patient with villous atrophy. Sharp lines resembling actual fissures, as well as highly varied shading and texture is present in each of the basis images. The original images used to make the celiac basis images were highly varied in terms of the number and type of features with differing texture that were present.
For automation of textural properties and their locations in endoscopic images, texture can be defined quantitatively as the value of a statistical measurement of color or grayscale digital image level. For simplicity in initial prior investigations, endoscopic color images were typically reduced to gray level images, with the gray level ranging from 0 (black) - 255 (white)[13-19]. Values between 1 and 254 inclusive are successively brighter gray shades. For endoscopic image analysis, texture is then determined by measuring and quantifying the gray level of all pixels in an image, or of a subset of pixels in the image. All image pixels are analyzed as a group if one would like to make a broad statement about the image as a whole, and/or to compare successive image frames, i.e., successive time epochs. For video capsule image analysis, successive frames will approximate the movement of the capsule along the gastrointestinal tract. However, because the capsule movement is passive and not likely to be at a constant rate, those successive images will likely represent uneven distances along the gastrointestinal system. The older imaging systems tended to have a fixed frame rate of 2 frames per second[24]. Newer systems having a variable frame rate[25] should be taken into account when considering successive image frames.
The simplest statistical measure of texture is the average or mean grayscale level (designated μ). To determine this value for the entire image, the grayscale level of all pixels is averaged. A typical digital endoscopic image will have a size of 576 × 576 pixels = 331776 pixels[4]. Thus by summing the values of grayscale levels for all pixels and dividing by 331776, the mean level is obtained. The mean level of one image can have significance in several ways. Firstly it can be compared from one patient to another or from one level of the small intestine to another in the same patient. When the images are darker, it may signify the presence of darker structures in the substrate, though it can also be due to the presence of a darker shade in the mucosal wall. If darker structures are present in the substrate, these can represent a highly variable three-dimensional topography. For example, when villous atrophy is present, there tends to be fissuring of the small intestinal mucosa. The fissures appear as dark lines in the two-dimensional images, due to the fact that they are deeper within the mucosa and further from the video camera and its light source. The fissures can be variable in length, depth, and breadth (Figure 1, lower panels). They are often random in orientation. Their presence tends to render the image darker in overall gray level. Another phenomenon that tends to signify the presence of villous atrophy is a mottled appearance in the two-dimensional images (Figure 1, lower panels). The mottled appearance can result from the presence of mucosal protrusions of varying height. These protrusions have been proposed to be clumps of villi which have become atrophied and shortened in length[18,19]. Since the three-dimensional mucosal architecture is therefore uneven, camera and light source distance are important factors for imaging mucosal protrusions. Areas of lower elevation in the images will be partly obscured and shadowed by higher areas and thus appear substantially darker. The average grayscale level may thus decrease when there is mottling of the mucosal surface. Over a short succession of image frames, a high degree of variability in the mean grayscale level would likely indicate the presence of patchy villous atrophy[26], which is common in celiac disease patients. Lesser variability at more distal regions of the small intestine would be indicative of a lesser presence of villous atrophy and a more uniform, more normal mucosal surface, which is normally the case in untreated celiac disease patients. These patients tend to have the greatest presence of villous atrophy, which is patchy, at the level of the duodenal bulb and in the distal duodenum, and lesser degrees of villous atrophy in the jejunum and in the ileum[27]. When a comparison between celiac patients is made, darker mean grayscale level would be expected to indicate the presence of a greater degree of villous atrophy, though this hypothesis has yet to be proven. Likewise, when the same patient is compared at follow-up after starting the gluten free diet, it would be anticipated that a lighter average grayscale level would signify diminishing levels of villous atrophy.
A second main measure of image texture, and perhaps the most important to current systems used for quantitative analysis, is the second central moment, or variance (σ)[13,14]. Its positive square root is the standard deviation. This moment is a measure of the spread of the distribution of grayscale levels. A larger value of σ indicates greater range of gray shading about the mean level. The standard deviation or variance from the mean pixel level has been used as a textural feature to measure the variability in brightness of image features[13,14]. When more features are present with different brightness levels, for example when fissuring is evident as a series of many dark lines in the image, the standard deviation increases. Likewise, a mottled image appearance due to villous atrophy will cause an increase in the standard deviation of grayscale image brightness.
Although not currently implemented, higher-order textural measurements that are potentially useful for quantitative analysis of villous atrophy include the third central moment or skewness (γ) and the fourth central moment or kurtosis (κ). The skewness is an estimate of the degree of lopsidedness in the pixel graylevel distribution about the mean value. It can be helpful to detect spatial non-uniformity in the image brightness. For example if clumps of villi which have atrophied are present, they will be rendered as blunted protrusions, with a large darker surface area in the image[18]. This would skew the distribution toward the darker gray level pixel values. The kurtosis is a measure of the heaviness of the tail of the distribution, i.e., how many very bright or very dark pixels are present in the image compared to the rest of the grayscale level values. The kurtosis measurement can therefore be assistive in detecting the presence of numerous very bright or very dark components of the endoscopic image space. These components can include small patches of normal tissue (bright) in areas of villous atrophy, and/or areas with fissuring (dark) among more normal villi.
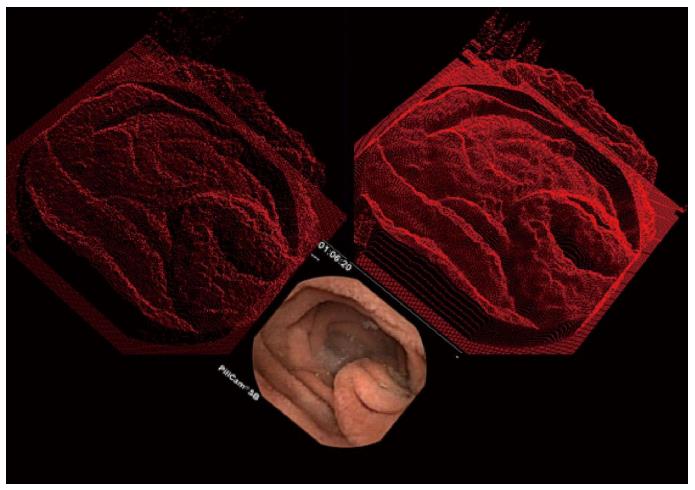
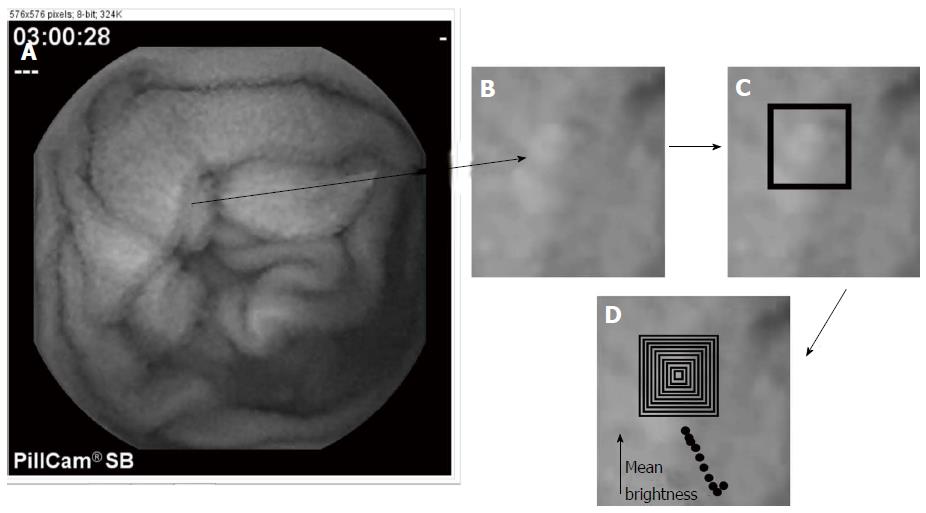
Syntactic methods are a way to model tissue structure based upon a set of prototypical or primitive features. For syntactic analysis, three-dimensional tissue structure should be generated, and can be studied by using shape-from-shading principles as shown in Figure 3. Areas of the original two-dimensional endoscopic image that are bright (lower panel) are converted into a height to render the object in three dimensions (top panels). It is evident in the top panels that the small intestinal mucosa consists of a series of mucosal protrusions. These mucosal protrusions can be modeled as a set of concentric, circular rings or squares (Figure 4). Using this syntactic model, a protrusion is detected when the average grayscale value within the ring or square is above a predefined threshold grayscale level. Based on shape-from-shading principles[18,19], the mucosal protrusion will appear as a bright spot in the endoscopic image. The outer edges of the spot will be a darker grayscale level, while the inner components will be manifested as progressively brighter pixels. At the pinnacle or center of the protrusion, the brightest grayscale level will occur. This phenomenon is based upon the camera light source to protrusion distance. The pinnacle of the protrusion extends furthest from the mucosal surface, and is therefore closest to the video camera lens, which is constrained within the small intestinal lumen as it travels distally. The inverse square law of light states that the light intensity per unit area falling on the mucosal surface will vary in inverse proportion to the square of the distance from the light source. There are nonlinearities imposed on the model, but prior findings have suggested that the approximation is sufficiently accurate to render the two-dimensional image features as three-dimensional constructs which represent actual tissue structure[18,19]. Likewise, the base of the protrusion will appear darkest since it will be furthest from the camera lens. Taller protrusions will appear in endoscopic images as having brighter central regions due to the inverse square law, and vice versa for blunter protrusions. Wider protrusions will appear as image features having longer spatial gradients, from darker pixel areas in the outer portions to brighter pixel regions at the center area. Conversely, narrower protrusions will have sharper spatial gradients, from darker pixel regions at outer edge to the brighter central core in endoscopic images.
The evident three-dimensional mucosal protrusions (Figure 3) can be modeled in the two-dimensional endoscopic images based upon a fixed or flexible template (Figure 4). The simplest modeling method is to use a fixed template. For example, a protrusion can be modeled, and its architectural parameters can be determined, using a concentric series of square or circular shapes, or rings, as alluded to earlier in the text. The algorithm can be stated as follows. Each outlying shape can be made one pixel wide as a first approximation. The width of a mucosal protrusion would then be determined as follows. The average grayscale level of the image pixels overlapping each ring is first calculated. The brightest ring will be at the center of the protrusion, since it is highest and closest to the camera lens. Successive concentric rings in the outward direction will be darker in gray shade since the protrusion falls off in amplitude there and is further from the camera lens. The base of the protrusion is syntactically defined as the outermost ring that is still diminished in average grayscale level with respect to the adjacent, more inwardly located ring. Thus, the width of the protrusion is obtained, as is shown in Figure 4. To convert the protrusion width from pixels to millimeters would require knowing the camera lens to mucosal surface distance, which can be estimated. Alternatively, mucosal protrusion width can be measured in pixel units, which is the simplest form of this syntactic model. The height of the protrusion would be the difference in average grayscale levels from the innermost to the outermost ring defining the protrusion.
To better syntactically detect mucosal protrusions, a tolerance can be added so that architectures with slightly asymmetrical features, having a less rounded or square form, depending on the model being used, would still be detected as a protrusion. So for example, if an outer ring is only slightly brighter as compared to its inner neighbor, the base of the protrusion would not yet be considered to be reached. Subsequent outer rings would be counted as part of the mucosal protrusion, until arriving at a ring at which a sharp increase in average brightness is noted. Changing the tolerance would enable more or less candidate protrusions to be detected in the image, and with greater accuracy.
Once mucosal protrusions are detected, their statistics can be analyzed[18,19]. For example, the total number of protrusions per endoscopic image can be calculated. The mean and variance in the width of the detected protrusions can be determined. And the mean and variance in the height of the detected protrusions can be computed. Greater variance in protrusion dimensions will likely indicate the presence of patchy villous atrophy. Decreased mean height and increased mean width would be suggestive of clumping of the villi, and therefore would be indicative of the presence of greater degrees of villous atrophy.
Another prominent macroscopic feature that is manifested when villous atrophy is present in the small intestinal mucosa that can be modeled syntactically is fissuring. Fissures are areas of the small intestinal mucosa that are devoid of villi. Thus architecturally, they can be described as valleys that are areas in the mucosa which are at greater depth with respect to camera lens location. They will appear in endoscopic images as dark lines of varying length and width[4,28]. Pixels of darker gray shading represent the fissured areas. One way to model the presence of fissures syntactically is to parse a linear region of darker pixels with a fixed length and width. As was described above for the modeling of mucosal protrusions, the fissures can be detected with the incorporation of a tolerance factor. The syntactic parameters in which a tolerance could be added would be the width, length, and brightness of each fissure. If the model parameters were say, width = 3 pixels, length = 10 pixels, and brightness = gray level 50/255, then tolerances could be imposed of for example ± 1 pixel and ± 10 grayscale levels. If the tolerance is made too small however, many actual fissures can be missed, and if the tolerance is made too large, structures that are not actually fissures may be detected.
Fissuring of the small intestinal mucosa due to villous atrophy does not appear to be dependent on factors such as muscle fiber orientation, and is more or less random[29]. Thus the fissure syntactic template, although it can be fixed for the length, width, and brightness parameters, needs to be flexible with respect to the orientation parameter. One way to implement this is simply to orient the model fissure at various predefined angles, at a particular location, and determine whether or not there is a satisfactory match of any orientation with the actual pixel content in the image. More orientations used for comparison would enable better detection of any fissure that is present, but at the expense of longer computation time. To reduce computation time, the image can be skeletonized. This is a standard processing technique in which image features are converted to a series of line structures which represent the central locations along each segment of the feature. The line structures in the skeletonized images can each be converted into a straight line approximation using linear regression analysis, and the angle of the straight line is then readily calculated. The prototypical template is then oriented according to the calculated angle of the structure, and a fissure is detected if the actual structure has similar length, width and brightness as the model, to within the specified tolerance.
Another structure that is often evident in images where villous atrophy is present, which can be modeled syntactically, is the scalloping of mucosal folds. This is a phenomenon in which the edges of the folds become scalloped - consisting of repeated structures with a rounded appearance, which are often of similar size[30,31]. To syntactically model the presence of scalloping, curved structures with similar brightness should be developed. The scalloping generally appears on edge as the camera viewing angle is toward distal regions along the small intestinal lumen. Thus the scalloped edges along each fold will appear to have similar brightness, as well as similar size and shape. The parameters for modeling the curved structure of each scallop would therefore be the width and height in terms of the number of pixels, and the degree of curvature.
The presence of a mottled appearance in endoscopic images can be modeled as a series of light and dark patches, with the length and width of the patches tailored to fit the observations of actual mottled areas found in exemplar images. It would be anticipated, as a first approximation, that the light and dark patches of mottled regions would be symmetric, and therefore have similar or the same length and width parameter values. The actual shape of each light and dark patch could be modeled as circular or square as a first estimate, with the use of tolerance to detect any mottled components with a more irregular shape. The construction of a small prototype would be useful to detected mottled regions, and by sliding this prototypical template about the image using a computer algorithm, and correlating template to image at each window location, the extent of the mottled region can then be determined.
In this work, several currently proposed methods were described for the detection and measurement of villous atrophy in the small intestinal mucosa by means of quantitative analysis of video capsule images. These methods can be subdivided into statistical and syntactic types of analyses. Both types of analyses seek to automatically detect abnormalities in the endoscopic images. The statistical methods are useful to analyze the entire image, or to analyze predefined portions of it, and to determine whether the statistics are substantially different with respect to control images. Statistical parameters can be compared from one segment, or subimage, to another in a particular endoscopic image, as well as from one endoscopic image to the next over a series of images, as they are obtained from the video capsule when it progresses along the small intestinal lumen. Using a threshold level for each statistical parameter, it is possible to automatically detect the presence of abnormal image regions and/or abnormal locations along the small intestinal lumen over a time lapse sequence of video capsule endoscopic images. Furthermore, the presence of gradients with varying statistics, either along a single image or across a series of video capsule images, can possibly be detected and measured, though this must be shown in future work. Such gradients would be expected to be indicative of the presence of patchy villous atrophy in celiac patients. The resolution of the statistical measures is limited only by the video camera resolution, which has been steadily improving in recent years[32].
The advantages of syntactic or structural methods to detect the presence of villous atrophy were also described herein. Syntactic methods seek to model the structure of the small intestinal lumen based upon the presence of abnormal image features, which are indicative of pathology. Although actual villi located within the small intestine are difficult to detect at the current spatial resolution of video capsule camera systems, the manifestation of villous atrophy as structures in the small intestinal mucosa includes the presence of blunted protrusions, fissures, scalloped mucosal folds and a mottled or mosaic appearance of the mucosa. These structures are macroscopic, unlike the microscopic nature of the villi themselves, and can be detected by using appropriate prototypical templates. For simplicity, prototypical templates with fixed parameters can be used, with a tolerance added to all template parameters, so that features which are slightly out of proportion can still be detected. Once abnormal features are detected, they can be analyzed in terms of their density, shape and gray level characteristics, and gradients across individual endoscopic images and along a sequence of images can be determined.
Based on the above measures, automatic detection of regions with pathology indicative of villous atrophy in untreated celiac disease patients may soon be realized, even when the pathology is subtle or variable, and patchy in appearance. By mapping these structures, it would be possible to determine the extent of the pathology, and the change in pathologic region and content during the treatment of the disease.
Although the methods described herein were limited to analysis of video capsule endoscopy images, other techniques can be used to potentially improve the detection of pathologic features. In the method of chromoendoscopy, dyes are sprayed onto the mucosal surface via a working channel of the endoscope to enable detailed evaluation of the mucosal surface at high magnification[33]. Fujinon intelligent chromoendoscopy assisted capsule endoscopy is useful to evaluate patients with obscure gastroenterology bleeding[34,35]. Furthermore, narrowband imaging is capable of predicting the histological characteristics such as those present in gastric cancer lesions[36]. Optical coherence tomography has been found useful for noninvasive cross-sectional imaging in biological systems[37]. The water-immersion technique may be utilized to minimize patient discomfort and to minimize the need for sedation in children and adults[38]. Confocal laser endomicroscopy is a technique that involves a miniaturized confocal microscope, and was initially developed and integrated in the distal tip of a conventional colonoscope[39,40]. High-resolution magnification endoscopy can reliably identify normal vs atrophic mucosal regions[41]. I-scan technology consists of three types of algorithms: Surface enhancement, contrast enhancement, and tone enhancement, and can lead to easier detection, diagnosis and treatment of gastrointestinal diseases[42].
Currently, video capsule endoscopic imaging is constrained in several respects. Firstly, the images depend upon camera angle with respect to the small intestinal lumen, as well as on the illumination by the camera light source. Poor camera angle can result in an incorrect interpretation of the presence and degree of pathology. Furthermore, for syntactic analysis, the rendering of two-dimensional endoscopic images as three-dimensional constructs depends upon the inverse square law for light illumination, but nonlinearities may be introduced during the process. The nonlinearities can distort the actual small intestinal features and their dimensions, as observed using shape-from-shading principles. The spatial resolution of each image depends on the camera lens to small intestinal mucosal surface distance, which is variable from image to image and even in a single image, whenever the camera angle to mucosal surface angle is not normal, i.e., the light source is not pointed precisely perpendicular to the mucosal surface. For quantitative analysis, color images are typically converted to grayscale level for simplicity. For improved analysis, use of the tricolor image information may be helpful to detect subtle features of villous atrophy, a subject for future investigation.
This research is of potential importance to treat celiac disease, a common malady. The main symptom used to diagnose villous atrophy is the presence of villous atrophy in the small intestine. The villous atrophy can be subtle and patchy; therefore computerized means may be better at detecting and assessing the severity.
Quantitative research on analysis of celiac disease using video capsule endoscopy is a new field. Only for the last 12 or so years has this technology been available. In recent versions, time and spatial resolution is markedly improving so that subtle details can be observed without the need for light microscopy.
The use of the video capsule is an improvement over standard endoscopy, because it travels throughout the gastrointestinal tract, not just at the proximal portions. It is also less invasive to the patient and can be used for pediatric patients.
This methodology could possibly be used with online video capsule software to detect villous atrophy as the capsule travels passively along the gastrointestinal tract. In future manifestations, should biopsy become available, a biopsy could be taken at each region in which villous atrophy is detected.
Celiac disease is an autoimmune disease in which the patient is reactive to the protein gluten, which is found in wheat, rye, and barley grains. A video capsule is a device with camera which takes images at 2 frames or more per second and transmits them via radio link as it passes through the gastrointestinal tract.
This study was peer-reviewed by both clinical specialists and bioengineering specialists. The reviews were generally quite favorable. Improvements have been made in describing the data analysis and clinical setting.
Manuscript source: Invited manuscript
Specialty Type: Gastroenterology and hepatology
Country of Origin: United States
Peer-Review Report Classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Albuquerque A, Ianiro G, Iovino P, Pavlovic M S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
| 1. | Green PH, Jabri B. Celiac disease. Annu Rev Med. 2006;57:207-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 2. | Cataldo F, Montalto G. Celiac disease in the developing countries: a new and challenging public health problem. World J Gastroenterol. 2007;13:2153-2159. [PubMed] |
| 3. | Dickson BC, Streutker CJ, Chetty R. Coeliac disease: an update for pathologists. J Clin Pathol. 2006;59:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 201] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Ciaccio EJ, Bhagat G, Tennyson CA, Lewis SK, Hernandez L, Green PH. Quantitative assessment of endoscopic images for degree of villous atrophy in celiac disease. Dig Dis Sci. 2011;56:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Corazza GR, Villanacci V, Zambelli C, Milione M, Luinetti O, Vindigni C, Chioda C, Albarello L, Bartolini D, Donato F. Comparison of the interobserver reproducibility with different histologic criteria used in celiac disease. Clin Gastroenterol Hepatol. 2007;5:838-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 6. | Gonzalez S, Gupta A, Cheng J, Tennyson C, Lewis SK, Bhagat G, Green PH. Prospective study of the role of duodenal bulb biopsies in the diagnosis of celiac disease. Gastrointest Endosc. 2010;72:758-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Lee SK, Green PH. Endoscopy in celiac disease. Curr Opin Gastroenterol. 2005;21:589-594. [PubMed] |
| 8. | Penny HA, Mooney PD, Burden M, Patel N, Johnston AJ, Wong SH, Teare J, Sanders DS. High definition endoscopy with or without IScan increases the detection of celiac disease during routine endoscopy. Dig Liver Dis. 2016;48:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Bouchard S, Ibrahim M, Van Gossum A. Video capsule endoscopy: perspectives of a revolutionary technique. World J Gastroenterol. 2014;20:17330-17344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Van Weyenberg SJ, Smits F, Jacobs MA, Van Turenhout ST, Mulder CJ. Video capsule endoscopy in patients with nonresponsive celiac disease. J Clin Gastroenterol. 2013;47:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Ibrahim M, Van Gossum A. Novel imaging enhancements in capsule endoscopy. Gastroenterol Res Pract. 2013;2013:304-723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Fisher LR, Hasler WL. New vision in video capsule endoscopy: current status and future directions. Nat Rev Gastroenterol Hepatol. 2012;9:392-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Ciaccio EJ, Tennyson CA, Lewis SK, Krishnareddy S, Bhagat G, Green PH. Distinguishing patients with celiac disease by quantitative analysis of video capsule endoscopy images. Comput Methods Programs Biomed. 2010;100:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Ciaccio EJ, Tennyson CA, Bhagat G, Lewis SK, Green PH. Classification of video capsule endoscopy image patterns: comparative analysis between patients with celiac disease and normal individuals. Biomed Eng Online. 2010;9:44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Ciaccio EJ, Tennyson CA, Bhagat G, Lewis SK, Green PH. Transformation of video capsule images to detect small bowel mucosal differences in celiac versus control patients. Comput Methods Programs Biomed. 2012;108:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Ciaccio EJ, Tennyson CA, Bhagat G, Lewis SK, Green PH. Quantitative estimates of motility from video capsule endoscopy are useful to discern celiac patients from controls. Dig Dis Sci. 2012;57:2936-2943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Ciaccio EJ, Lewis SK, Green PH. Detection of villous atrophy using endoscopic images for the diagnosis of celiac disease. Dig Dis Sci. 2013;58:1167-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Ciaccio EJ, Tennyson CA, Bhagat G, Lewis SK, Green PH. Use of shape-from-shading to estimate three-dimensional architecture in the small intestinal lumen of celiac and control patients. Comput Methods Programs Biomed. 2013;111:676-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Ciaccio EJ, Tennyson CA, Bhagat G, Lewis SK, Green PH. Implementation of a polling protocol for predicting celiac disease in video capsule analysis. World J Gastrointest Endosc. 2013;5:313-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 20. | Lee AR, Ng DL, Diamond B, Ciaccio EJ, Green PH. Living with coeliac disease: survey results from the U.S.A. J Hum Nutr Diet. 2012;25:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Lebwohl B, Granath F, Ekbom A, Montgomery SM, Murray JA, Rubio-Tapia A, Green PH, Ludvigsson JF. Mucosal healing and mortality in coeliac disease. Aliment Pharmacol Ther. 2013;37:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Vécsei A, Amann G, Hegenbart S, Liedlgruber M, Uhl A. Automated Marsh-like classification of celiac disease in children using local texture operators. Comput Biol Med. 2011;41:313-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Hegenbart S, Uhl A, Vécsei A, Wimmer G. Scale invariant texture descriptors for classifying celiac disease. Med Image Anal. 2013;17:458-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Fernandez-Urien I, Carretero C, Borobio E, Borda A, Estevez E, Galter S, GonzalezSuarez B, Gonzalez B, Lujan M, Martinez JL. Capsule endoscopy capture rate: has 4 frames-per-second any impact over 2 frames-per-second? World J Gastroenterol. 2014;20:14472-14478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Adler SN, Bjarnason I. What we have learned and what to expect from capsule endoscopy. World J Gastrointest Endosc. 2012;4:448-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Alaedini A, Green PH. Narrative review: celiac disease: understanding a complex autoimmune disorder. Ann Intern Med. 2005;142:289-298. [PubMed] |
| 27. | Kurien M, Evans KE, Hopper AD, Hale MF, Cross SS, Sanders DS. Duodenal bulb biopsies for diagnosing adult celiac disease: is there an optimal biopsy site? Gastrointest Endosc. 2012;75:1190-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Ciaccio EJ, Bhagat G, Lewis SK, Green PH. Quantitative image analysis of celiac disease. World J Gastroenterol. 2015;21:2577-2581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Goenka MK, Majumder S, Goenka U. Capsule endoscopy: Present status and future expectation. World J Gastroenterol. 2014;20:10024-10037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (2)] |
| 30. | Ianiro G, Gasbarrini A, Cammarota G. Endoscopic tools for the diagnosis and evaluation of celiac disease. World J Gastroenterol. 2013;19:8562-8570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 31. | Hegenbart S, Uhl A, Vécsei A. Survey on computer aided decision support for diagnosis of celiac disease. Comput Biol Med. 2015;65:348-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Koprowski R. Overview of technical solutions and assessment of clinical usefulness of capsule endoscopy. Biomed Eng Online. 2015;14:111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Jung M, Kiesslich R. Chromoendoscopy and intravital staining techniques. Baillieres Best Pract Res Clin Gastroenterol. 1999;13:11-19. [PubMed] |
| 34. | Pohl J, May A, Rabenstein T, Pech O, NguyenTat M, Fissler-Eckhoff A, Ell C. Comparison of computed virtual chromoendoscopy and conventional chromoendoscopy with acetic acid for detection of neoplasia in Barrett’s esophagus. Endoscopy. 2007;39:594-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Gupta T, Ibrahim M, Deviere J, Van Gossum A. Evaluation of Fujinon intelligent chromo endoscopy-assisted capsule endoscopy in patients with obscure gastroenterology bleeding. World J Gastroenterol. 2011;17:4590-4595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA. Optical coherence tomography. Science. 1991;254:1178-1181. [PubMed] |
| 38. | Leung CW, Kaltenbach T, Soetikno R, Wu KK, Leung FW, Friedland S. Water immersion versus standard colonoscopy insertion technique: randomized trial shows promise for minimal sedation. Endoscopy. 2010;42:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 39. | Hoffman A, Goetz M, Vieth M, Galle PR, Neurath MF, Kiesslich R. Confocal laser endomicroscopy: technical status and current indications. Endoscopy. 2006;38:1275-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Kiesslich R, Goetz M, Neurath MF. Confocal laser endomicroscopy for gastrointestinal diseases. Gastrointest Endosc Clin N Am. 2008;18:451-466, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Anagnostopoulos GK, Yao K, Kaye P, Fogden E, Fortun P, Shonde A, Foley S, Sunil S, Atherton JJ, Hawkey C. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pyloriassociated gastritis, and gastric atrophy. Endoscopy. 2007;39:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 42. | Kodashima S, Fujishiro M. Novel image-enhanced endoscopy with iscan technology. World J Gastroenterol. 2010;16:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 130] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |