Published online Jun 25, 2015. doi: 10.4253/wjge.v7.i7.728
Peer-review started: September 4, 2014
First decision: January 8, 2015
Revised: January 27, 2015
Accepted: March 30, 2015
Article in press: April 2, 2015
Published online: June 25, 2015
Processing time: 307 Days and 11.8 Hours
AIM: To assess the feasibility and safety of liquid nitrogen spray cryoablation at the duodenal papilla in a porcine model.
METHODS: This prospective study protocol was approved by the University of Florida Institutional Animal Care and Use Committee. Six pigs underwent liquid nitrogen spray cryotherapy at the duodenal papilla. Freeze time of 20-s was applied per cycle (4 cycles/session). Survival animals (n = 4) were monitored for adverse events. Hemoglobin, white blood count, liver tests, and lipase were obtained at baseline and post-treatment. EGD was performed on day#7 to evaluate the papilla and for histology. All animals were euthanized and necropsy was performed at the end of the one-week survival period. Feasibility was defined as successful placement of the decompression tube in the duodenum, followed by delivery of spray cryotherapy to the duodenal papilla. Safety was determined by monitoring post-treatment blood tests and clinical course. Treatment effect was defined as endoscopic and histologic changes after cryotherapy. This was established by comparing endoscopic and histologic findings from mucosal biopsies prior to cryotherapy and on post-operative day (POD)#7. Full-thickness specimen was obtained post-mortem to assess depth of injury.
RESULTS: Spray cryotherapy was feasible and successfully performed in all 6/6 (100%) animals. Cryospray with liquid nitrogen (four 20-s freeze-thaw cycles) at the duodenal papilla resulted in white frost formation at and around the target region. The mean procedural time was 54.5 min (range 50-58 min). All six animals studied had stable blood pressure, heart rate, and pulse oximetry measurements during the procedure. There were no significant intra-procedural adverse events. There were no significant differences in hemoglobin, white cell count, liver tests or lipase from baseline to post-cryotherapy. Survival animals were monitored daily post-operatively without any clinical ill effects from the cryotherapy. There was no bleeding, infection, or perforation on necropsy. Endoscopic on POD#7 showed edema and ulceration at the duodenal papilla. On histology, there was loss of crypt architecture with moderate to severe necrosis and acute mixed inflammatory infiltration in each specimen following cryotherapy. The extent of cryogen-induced tissue necrosis (depth of injury) was limited to the mucosa on full-thickness specimen evaluation.
CONCLUSION: Endoscopic liquid nitrogen spray cryotherapy is feasible and safe for ablation at the duodenal papilla in a porcine model.
Core tip: With advances in therapeutic endoscopy, endoscopic resection is commonly performed for the management of ampullary adenomas. However, endoscopic papillectomy can still carry significant morbidity, especially in elderly patients with comorbidities. Hence, less invasive effective endoscopic ablative modalities would be desirable. In this study, we demonstrate that endoscopic liquid nitrogen spray cryotherapy is feasible and safe for ablation at the duodenal papilla in a porcine model. These preliminary findings suggest a potential role of cryotherapy as an adjunct endoscopic treatment for residual/recurrent ampullary lesions or as a primary modality in patients who are not optimal candidates for surgery or endoscopic resection.
- Citation: Yang D, Reinhard MK, Wagh MS. Feasibility and safety of endoscopic cryoablation at the duodenal papilla: Porcine model. World J Gastrointest Endosc 2015; 7(7): 728-735
- URL: https://www.wjgnet.com/1948-5190/full/v7/i7/728.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i7.728
Ampullary adenomas are dysplastic glandular lesions that arise from the major duodenal papilla. These lesions can occur sporadically or arise in the context of genetic syndromes such as familial adenomatous polyposis. If not removed, ampullary adenomas can potentially undergo malignant transformation to ampullary cancer with a reported incidence from 25% to 85%[1-3]. Based on this risk, these lesions have been treated historically with pancreatoduodenectomy, a highly invasive surgical intervention associated with high morbidity and mortality[4,5]. With advances in therapeutic endoscopy, there has been a shift towards endoscopic resection, with consideration of surgery only for locally advanced lesions. While endoscopic approaches for the treatment of ampullary adenomas are less invasive than surgery, adverse events associated with endoscopic papillectomy still carry a reported morbidity and mortality rate of 23% and 0.4% respectively[6]. Hence a less invasive modality for endoscopic ablation of ampullary lesions would be helpful, especially in elderly asymptomatic patients with comorbidities.
There has been a growing interest in endoscopic mucosal ablative techniques for the management of different gastrointestinal pathologies, ranging from adenomatous lesions, dysplasia and/or intramucosal carcinoma, to bleeding mucosal lesions in the GI tract[7]. Currently, the role of endoscopic ablative techniques (argon plasma coagulation, laser therapy, monopolar o bipolar coagulation) for ampullary adenomas is limited to destruction of residual or recurrent adenomatous tissue following resection[8].
Cryotherapy is a mucosal ablative technique that employs non-contact delivery of either low-pressure liquid nitrogen or compressed carbon dioxide (CO2) gas for tissue destruction.
There are currently two commercially available endoscopic cryotherapy systems for the gastrointestinal tract. One device delivers low-pressure liquid nitrogen (at -196 °C) (CryosprayAblation, CSA Medical Inc, Baltimore, MD) whereas the other system is based on the Joule-Thompson effect, in which highly compressed CO2 gas produces cooling upon rapid expansion and decrease in pressure (Polar Wand; GI Supply, Camp Hill, PA)[9]. Most of the current clinical experience with endoscopic cryotherapy as a mucosal ablative technique is primarily related to the data on ablation of Barrett’s esophagus, where this method has been shown to be efficacious and well tolerated[10]. The use of cryotherapy in other extra-esophageal sites, besides treatment of bleeding in the stomach and colon[11], has been limited to some degree by the concern of gas expansion and high risk of barotrauma and perforation in other regions of the GI tract. We recently reported preliminary data suggesting that liquid nitrogen cryotherapy is a safe technique even in patients with altered post-surgical gastric anatomy when appropriate measures are taken for cryogen gas decompression[12]. The aim of our study was to investigate the feasibility and safety of endoscopic liquid nitrogen spray cryotherapy at the duodenal papilla in a porcine model.
This prospective study protocol was approved by the University of Florida Institutional Animal Care and Use Committee. Six female pigs (sus) weighing 80-100 lbs were obtained from the University of Florida Swine Unit. The aim of this study was to prospectively assess the feasibility and safety of endoscopic liquid nitrogen spray cryotherapy at the duodenal papilla in a porcine model.
The animal protocol in this study was designed to minimize pain or discomfort to the animals. Pigs were housed and maintained in the University of Florida Animal Care Services unit. The animals were acclimatized to laboratory conditions (23 °C, 12h/12h light/dark, 50% humidity, ad libitum access to food and water) for 7-10 d prior to experimentation. All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for necropsy.
Primary outcomes: (1) feasibility was assessed by the technical success of cryotherapy in this porcine model. Technical success was defined as successful placement of the cryotherapy decompression tube past the papilla in the second portion of the duodenum, followed by delivery of liquid nitrogen spray to the duodenal papilla; and (2) safety was determined by monitoring peri-procedural blood tests and clinical course. Endoscopic adverse events were defined based on previously established criteria[13] and post-operative signs of distress, behavior changes, and/or loss of appetite. Elevation of liver tests and lipase post-cryotherapy to more than 3 times the upper limit of normal was considered abnormal.
Secondary outcome: Treatment effect was defined as endoscopic and histologic changes after cryotherapy. This was established by comparing endoscopic and histological findings from mucosal biopsies prior to cryotherapy and on post-operative day (POD)#7. The degree of intestinal injury was graded as previously described by Park et al[14]. Full-thickness specimen was obtained post-mortem to assess depth of injury.
A single-channel gastroduodenoscope (GIF-140 Olympus Medical Systems, Tokyo, Japan) was used for the study. A pediatric colonoscope (PCF-140, Olympus Medical Systems, Tokyo, Japan) was used as needed, to overcome the J-shaped porcine gastric anatomy in order to have adequate length of the scope available to access the distal duodenum. Endoscopic biopsy forceps (Boston-Scientific, Natick, MA) were used for endoscopic tissue acquisition.
Liquid nitrogen spray cryotherapy (CryoSpray Ablation system, CSA Medical Inc, Baltimore, MD) was used for the study. This cryotherapy system, consists of (1) a console with a liquid nitrogen holding tank and a foot pedal for the release of low-pressure (3 to 6 psi) liquid nitrogen (temperature -196 °C); (2) a 7-French cryocatheter, which is inserted through the working channel of an endoscope; and (3) a modified orogastric cryode compression tube (CDT) placed alongside the endoscope prior to starting cryotherapy. This special decompression tube has two channels, one for passive venting and another for active suction, attached to a separate suction machine to evacuate the rapidly expanding evaporated cryogenic gas during the procedure.
Freeze time was defined as the time interval from the visualization of white frost (ice formation) along the entire surface of the papilla until the cryospray was stopped. Procedure time was defined as the time from endoscope insertion to withdrawal.
Animals were not fed for 24 h prior to the procedure. Animals were pre-anesthesized with intramuscular (IM) injection of 4 mg/kg Telazol, ketamine 2 mg/kg, xylazine 2 mg/kg, and atropine (0.04 mg/kg) or glycopyrrolate (0.001 mg/kg). Induction was performed with isoflurane 3%-5% via mask delivered with a precision vaporizer prior to intubation. General endotracheal anesthesia was administered with Isoflurane 1%-3.5%. An intravenous (IV) line was placed in the marginal ear vein. Pigs were intubated and placed on mechanical ventilation.
In survival studies, blood specimens to evaluate hemoglobin (Hb), white blood count (WBC), liver tests (AST, ALT, alkaline phosphatase and bilirubin), and lipase were obtained prior to endoscopic treatment (day 0) and post cryotherapy (day 1 and on day 7).
The intended treatment site (duodenal papilla) was identified by endoscopic visualization. The pediatric colonoscope (Olympus Medical Systems, Tokyo, Japan) was introduced past the second portion of the duodenum and a 0.035 inch, 350 cm length guide-wire (Jagwire, Boston Scientific, Natick, MA) was placed. The endoscope was exchanged over the guidewire and the CDT was placed distal to the papilla. Adequate positioning of the CDT (tip of tube past the papilla in the second portion of the duodenum) was confirmed by re-inserting the endoscope, and the guide-wire was removed. The CDT was connected to active high suction controlled by a foot pedal during cryotherapy.
For survival animals, two peri-ampullary endoscopic biopsies were obtained as baseline prior to initial cryotherapy. The cryocatheter was introduced through the accessory channel of the endoscope and oriented to directly target the duodenal papilla. The freeze time was established as the period from ice formation along the entire surface of the papilla until the cryospray was stopped. The liquid nitrogen dosimetry of 4 cycles of 20-s freeze time was based on published dosing from previous animal studies[15,16]. There was complete thawing of ice between freeze cycles. Expanding liquid nitrogen gas was continuously suctioned via the CDT and through the endoscope between freeze cycles. Continuous monitoring for abdominal distention was performed by anesthesia personnel during the procedure.
The first two animals were used exclusively to evaluate the feasibility of liquid nitrogen cryotherapy at the duodenal papilla, and thus, were not survived. The animals were euthanized upon completion of the endoscopy, and necropsy performed. At necropsy, the peritoneal cavity and cryotherapy site was visually inspected for perforation, bleeding or damage to surrounding structures.
In survival experiments, pigs were extubated and recovered from general anesthesia. The pigs were monitored daily for any post-treatment adverse events, based on signs of distress, behavior changes, and/or loss of appetite. Oral feedings with standard chow were started the same day after recovering from anesthesia (day 0). Blood specimens were obtained at baseline (day 0), post-cryotherapy day 1 and day 7. Endoscopy was repeated on day 7 after cryotherapy to assess treatment effect at the duodenal papilla and biopsies were obtained at the cryotherapy site. At the end of the procedure, animals were euthanized and necropsy performed to rule out intra-abdominal adverse events associated with cryotherapy, such as transmural injury, bleeding, bowel perforation or abscess formation. Full-thickness duodenal specimen was obtained for histologic examination from one of the survival animals following necropsy.
Biopsy specimens from all survival animals were obtained at the cryotherapy site on day 7 to assess the degree of intestinal injury. To evaluate the depth of the treatment effect, a full-thickness specimen containing the duodenal papilla was harvested from one of the survival animals following necropsy. All specimens were fixed in 10% neutral buffered formalin for at least 48 h, embedded in paraffin and stained with standard hematoxylin and eosin. The degree and depth of intestinal injury was graded by a single experienced pathologist as previously described in the text.
Summary data was expressed as the mean ± SD, and range. One-way analysis of variance (analysis of numerical data) was performed (GraphPad Prism version 6.00 for Windows, GraphPad Software, San Diego California, United States). The statistical methods of this study were reviewed by [Name, division, organization].
Endoscopic liquid nitrogen spray cryotherapy was performed at the duodenal papilla in 6 animals (2 non-survival and 4 survival studies).
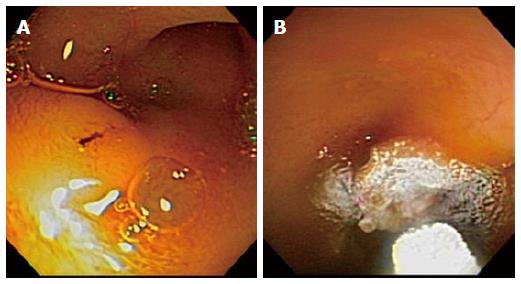
The duodenal papilla was identified in all 6 cases. The distal end of the CDT was successfully placed past the papilla into the second portion of the duodenum (Figure 1A) in all 6 animals. Cryospray with liquid nitrogen at the duodenal papilla resulted in white frost formation at and around the target region (Figure 1B). Four 20-s freeze-thaw cycles were applied with thawing between each session. Technical success was initially confirmed in the two non-survival animals and also subsequently achieved in all four survival swine studies (6/6; 100%). The mean procedural time was 54.5 min (range 50-58 min). All six animals studied had stable blood pressure, heart rate and pulse oximetry measurements during the procedure and there were no intra-procedural adverse events.
In survival studies, all 4 animals were recovered from general anesthesia and transferred to their housing facility, where they resumed regular feeding the same day of the procedure. The swine were monitored daily without any clinical ill effects from the cryotherapy (no change in activity, feeding habits and bowel and bladder elimination functions). There were no significant changes in Hb, WBC, liver tests or lipase on day 1 and 7 following cryoablation when compared to baseline (Table 1). There was no evidence of bleeding, infection (abscess), or bowel perforation on necropsy in any of the animals.
| Lab | Mean (range) | P value | ||
| Baseline | Day 1 | Day 7 | ||
| Hemogloobin, g/dL | 11.2 (10.2-12.3) | 11.8 (11.1-12.3) | 11 (9.9-11.6) | 0.36 |
| White blood count, K/µL | 16.6 (12.9-17.4) | 19.8 (14.1-22) | 17.4 (15-21.9) | 0.53 |
| Alkaline phosphatase, U/L | 98.8 (90-112) | 102 (93-112) | 84.5 (72-100) | 0.15 |
| Alanine transaminase, U/L | 44.3 (32-52) | 57.5 (47-80) | 53.5 (45-57) | 0.25 |
| Aspartate transaminase, U/L | 22.8 (16-31) | 117.3 (24-385) | 22.3 (17-29) | 0.37 |
| Total bilirubin, mg/dL | 0.2 (0.1-0.3) | 0.2 (0.1-0.2) | 0.1 (0.1-0.2) | 0.53 |
| Lipase, U/L | 5.5 (4-8) | 9.3 (6-11) | 4.8 (2-9) | 0.06 |
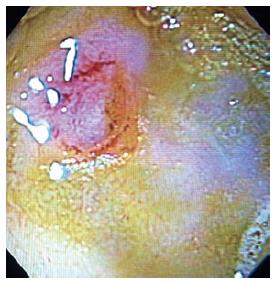
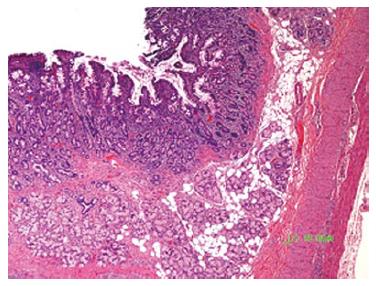
At 7 days after cryotherapy, survival animals underwent a follow up endoscopy for evaluation of treatment effect on the duodenal papilla. Edema, erythema and ulceration of the papilla were seen on endoscopy (Figure 2). Mucosal biopsies from the papilla were obtained and compared to baseline. The histologic findings of each animal are summarized in Table 2. There was loss of crypt architecture with moderate to severe necrosis and acute mixed inflammatory infiltration in each specimen obtained from the duodenal papilla on day 7 following cryotherapy. The extent of cryogen-induced tissue necrosis (depth of injury) was limited to the mucosa, with the application of four 20-s freeze-thaw cycles of liquid nitrogen (Figure 3).
| Swine | Baseline1 | Day 72 | ||
| Crypt architecture | Inflammation | Crypt Architecture | Inflammation | |
| 1 | Normal | None | Moderate necrosis | Moderate |
| 2 | Normal | None | Moderate necrosis and extensive debris | Moderate to severe |
| 3 | Normal | None | Severe necrosis and moderate debris | Severe with dense fibrosis |
| 4 | Normal | None | Moderate necrosis and extensive debris | Moderate |
Endoscopic papillectomy has been increasingly used for the treatment of ampullary adenomas and early cancers. While this approach is less invasive than surgery, it is still associated with significant risks and is mainly performed by experienced endoscopists. As such, alternative endoscopic ablative therapies would be helpful, especially for the treatment of elderly asymptomatic patients with multiple comorbidities.
Endoscopic cryotherapy has been primarily used for the management of dysplastic Barrett’s esophagus and early esophageal cancer. The clinical application of this mucosal ablative technique in extra-esophageal gastrointestinal tract has been limited by the potential risk of perforation from barotrauma. When liquid nitrogen is delivered to the lumen, it undergoes phase transformation into a gaseous state as energy is delivered to the target tissue[16]. If not evacuated properly, the rapidly expanding nitrogen gas can be a risk for gastrointestinal perforation. Previous reports have evaluated the feasibility of liquid nitrogen cryotherapy in the stomach[17]; however, its application in the small intestine has not been evaluated.
The present study demonstrates the feasibility and safety of endoscopic liquid nitrogen spray cryotherapy at the duodenal papilla in a porcine model. The commercially available CDT could be successfully placed, with the tip of the tube in the second portion of the duodenum in all 6 animals in this study. This step was crucial as it allowed for adequate suction of the nitrogen gas during cryospray application at the duodenal papilla. There were no apparent adverse effects associated with the cryotherapy based on daily post-treatment monitoring, lab data and necropsy 7 d following treatment. There was no evidence of changes in hemoglobin, WBC, liver tests or lipase from ablation at the duodenal papilla.
Our study validated cryogen treatment effect at the duodenal papilla on endoscopy and histology 7 d following treatment. The dosimetry of 4 cycles of 20-s freeze in this study was based on previous reports in animal studies as well as the commonly applied dosimetry for liquid nitrogen spray cryotherapy used in the treatment of BE with high-grade dysplasia or adenocarcinoma[18-20]. Endoscopic appearance of lesions at the duodenal papilla on day 7 ranged from mild erythema and edema to superficial erosions. These results are similar to the endoscopic findings reported by Johnston et al[15] in swine esophagus on day 7 following liquid nitrogen cryospray application. Our results demonstrate the effect of cryospray on histology based on the mucosal biopsies from the duodenal papilla on day 7. Histology on day 7 (4/4 animals) revealed loss or blunting of villous tissue and tips, crypt architectural distortion with necrosis and debris, and a mixed moderate to severe inflammatory infiltrate. This is a marked change from the normal histology obtained at baseline from the duodenal papilla and confirms treatment effect from the liquid nitrogen cryospray. We also demonstrate from a full-thickness specimen that the depth of injury was reserved to the mucosa, with inflammation and necrosis limited to the lamina propria and intact submucosa and muscularis propria. Previous animal studies have demonstrated dose-dependent injury to the esophagus, with necrosis involving the submucosa and even transmural damage with short exposures (15-30 s)[15,21]. In contrast, Shin and colleagues revealed that average grades of injury in the stomach across various doses were lower when compared with the esophagus[7]. We can speculate that the depth of tissue injury at the duodenal papilla from 4 cycles of 20-s of freeze time followed by thawing was associated with less injury compared to other studies because of differences in anatomical location in the gastrointestinal tract and mode of delivery (liquid nitrogen vs carbon dioxide). Further studies are needed to evaluate the relationship of cryogen dosimetry and depth of tissue injury in the small bowel.
We acknowledge the limitations of this study. First, our experiments were performed in a porcine model. While this animal model has been commonly used for experimental endoscopic studies, there are some important differences between human and porcine GI tract anatomy. The distal common bile duct and pancreatic duct in swine are not confluent at the duodenal papilla in pigs. In fact, while the biliary orifice is situated proximally in the duodenum at the papilla the pancreatic duct orifice is located separately several centimeters distal to the site of the biliary orifice. Since the primary aim of this pilot study was to evaluate the feasibility and safety of cryoablation at the duodenal papilla, we did not investigate treatment effect at the separate pancreatic duct orifice. This anatomical difference theoretically confers a decreased risk for pancreatic adverse events, including pancreatitis, when the duodenal papilla or biliary tract is manipulated in the porcine model. Thus, while there were no significant changes in serum lipase levels in our study to suggest pancreatitis following cryotherapy, we cannot definitively assess for this adverse event. Future studies are needed to evaluate the treatment effect and safety of cryotherapy at the pancreatic orifice. For this same reason, a pancreatic stent was also not placed after cryotherapy at the biliary orifice/papilla in this study, though this would need to be placed in human patients as is routinely done after endoscopic ampullectomy.
Second, as opposed to a side-viewing endoscope that is used in humans for endoscopy therapy at the papilla, a forward viewing endoscope (or pediatric colonoscope to overcome the J-shaped porcine gastric anatomy) was used to advance to the distal duodenum for adequate placement of the cryotherapy decompression tube. This technical hurdle may not be an issue with the human anatomy. Interestingly, the side-viewer may pose another challenge not encountered with the forward viewing endoscope used in this study. Use of the elevator of the side-viewing duodenoscope may theoretically kink the cryo catheter and not allow cryotherapy. However, the recently available, second generation of the cryotherapy device (truFreeze G2 spray cryotherapy device, CSA Medical Inc, Baltimore, MD) has a stainless steel reinforced catheter that enables 180 degree retroflexion and this upgrade may overcome the potential problem with the elevator. Additional studies in humans would be needed to evaluate the technical feasibility of placing the decompression tube side-by-side with a duodenoscope and performing cryotherapy with use of the elevator.
Third, in the absence of an animal model for ampullary lesions, cryotherapy was performed on normal porcine tissue. Thus, the extent of cryogen-induced effects may differ in humans with pathology (adenoma/carcinoma) at the duodenal papilla. Furthermore, our study was limited to small numbers of animals and findings on depth of injury need to be confirmed in larger human protocols. Lastly, the study follow-up was relatively short (7 d); therefore, long term cryo ablative effects at the duodenal papilla were not evaluated.
Despite these shortcomings, our feasibility data shows that there were no adverse events from cryotherapy at the papilla. Specifically, there was no evidence of GI tract perforation, bleeding, cholangitis or bile duct injury despite directly spraying liquid nitrogen (that transforms to gaseous state with major increase in volume) at the open biliary orifice and therefore up the bile duct. Hence it appears that cryotherapy may be a viable option for treating ampullary lesions but further studies are needed to examine optimal dosimetry, effects on the pancreas and ablation of actual neoplastic tissue.
Our preliminary findings suggest that endoscopic liquid nitrogen cryotherapy at the porcine duodenal papilla is both feasible and safe. This data may serve as starting point for assessing the potential role of cryotherapy as an adjunct endoscopic treatment for residual/recurrent ampullary lesions or as a primary modality in patients who are not optimal candidates for surgery or endoscopic resection. Further studies are needed to determine the relationship between dosimetry and tissue injury at the duodenal papilla, with specific testing for effects on the pancreatic duct.
There is a growing interest in endoscopic mucosal ablative techniques for the management of different gastrointestinal pathologies, ranging from adenomatous lesions, dysplasia and/or intramucosal carcinoma. The role of endoscopic ablative techniques for the management of ampullary adenomas has not been fully elucidated.
Cryotherapy is an emerging endoscopic mucosal ablative technique. Most of the current clinical experience with endoscopic cryotherapy is primarily related to data on ablation of Barrett’s esophagus. The use of cryotherapy in other extra-esophageal sites has been limited to some degree by the concern of gas expansion and high risk of barotrauma and perforation in other regions of the GI tract.
The authors had previously reported preliminary data suggesting that liquid nitrogen cryotherapy is a safe technique even in patients with altered post-surgical gastric anatomy when appropriate measures are taken for cryogen gas decompression. This is the first study that has evaluated the feasibility and safety of endoscopic liquid nitrogen spray cryotherapy at the duodenal papilla in a porcine model.
This study demonstrates that spray cryotherapy was feasible and successfully performed in all animals. Survival animals thrived without adverse events. Follow-up evaluation one week post-treatment confirmed cryotherapy-induced tissue necrosis limited to the mucosa. These preliminary findings suggest a potential role for cyrotherapy as a primary or adjunct modality for patients who are not optimal candidates for surgery/endoscopic resection or in those with residual/recurrent disease.
Endoscopic cryotherapy: mucosal ablative technique that employs non-contact deliver of either low-pressure liquid nitrogen or compressed carbon dioxide gas for tissue destruction. Technical success was defined as the successful placement of the cryotherapy decompression tube past the papilla followed by the delivery of liquid nitrogen spray to the target. Treatment effect was defined as endoscopic and histologic changes after cryotherapy. Freeze time was defined as the time interval from the visualization of white frost (ice formation) along the entire surface of the papilla until the cryospray was stopped.
Well designed, elegant animal study. The primary aim of the study was to assess the safety of a new therapeutic modality. The first acquired data proved the feasibility and safety during the short term follow-up period. The data are important and could be used in human studies.
P- Reviewer: Jonaitis L, Koulaouzidis A S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Hirota WK, Zuckerman MJ, Adler DG, Davila RE, Egan J, Leighton JA, Qureshi WA, Rajan E, Fanelli R, Wheeler-Harbaugh J. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc. 2006;63:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 315] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 2. | Takashima M, Ueki T, Nagai E, Yao T, Yamaguchi K, Tanaka M, Tsuneyoshi M. Carcinoma of the ampulla of Vater associated with or without adenoma: a clinicopathologic analysis of 198 cases with reference to p53 and Ki-67 immunohistochemical expressions. Mod Pathol. 2000;13:1300-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Yamaguchi K, Enjoji M. Adenoma of the ampulla of Vater: putative precancerous lesion. Gut. 1991;32:1558-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Cahen DL, Fockens P, de Wit LT, Offerhaus GJ, Obertop H, Gouma DJ. Local resection or pancreaticoduodenectomy for villous adenoma of the ampulla of Vater diagnosed before operation. Br J Surg. 1997;84:948-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Tran TC, Vitale GC. Ampullary tumors: endoscopic versus operative management. Surg Innov. 2004;11:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Han J, Kim MH. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video). Gastrointest Endosc. 2006;63:292-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Shin EJ, Amateau SK, Kim Y, Gabrielson KL, Montgomery EA, Khashab MA, Chandrasekhara V, Rolshud D, Giday SA, Canto MI. Dose-dependent depth of tissue injury with carbon dioxide cryotherapy in porcine GI tract. Gastrointest Endosc. 2012;75:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Adler DG, Qureshi W, Davila R, Gan SI, Lichtenstein D, Rajan E, Shen B, Zuckerman MJ, Fanelli RD, Van Guilder T. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006;64:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Yang D, Wagh MS. Barrett’s Esophagus: Risk Factors, Diagnosis and Management: Cryotherapy An Endoscopic Ablative Technique for the Management of Barrett’s Esophagus and Early Esophageal Cancer. Hauppauge, NY: Nova Science Publishers 2013; . |
| 10. | Gosain S, Mercer K, Twaddell WS, Uradomo L, Greenwald BD. Liquid nitrogen spray cryotherapy in Barrett’s esophagus with high-grade dysplasia: long-term results. Gastrointest Endosc. 2013;78:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Kantsevoy SV, Cruz-Correa MR, Vaughn CA, Jagannath SB, Pasricha PJ, Kalloo AN. Endoscopic cryotherapy for the treatment of bleeding mucosal vascular lesions of the GI tract: a pilot study. Gastrointest Endosc. 2003;57:403-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Suarez AL, Collins DP, Joshi V, Gross SA, Diehl DL, Horwhat JD, Wagh MS. Endoscopic liquid nitrogen spray cryotherapy in patients with post-surgical gastric anatomy: A multicenter cryotherapy users group report. Am J Gastroenterol. 2012;107:S766. |
| 13. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A, Petersen BT, Petrini JL. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1852] [Article Influence: 123.5] [Reference Citation Analysis (1)] |
| 14. | Park PO, Haglund U, Bulkley GB, Fält K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery. 1990;107:574-580. [PubMed] |
| 15. | Johnston CM, Schoenfeld LP, Mysore JV, Dubois A. Endoscopic spray cryotherapy: a new technique for mucosal ablation in the esophagus. Gastrointest Endosc. 1999;50:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Browning R, Parrish S, Sarkar S, Turner JF. First report of a novel liquid nitrogen adjustable flow spray cryotherapy (SCT) device in the bronchoscopic treatment of disease of the central tracheo-bronchial airways. J Thorac Dis. 2013;5:E103-E106. [PubMed] |
| 17. | Pasricha PJ, Hill S, Wadwa KS, Gislason GT, Okolo PI, Magee CA, Canto MI, Kuo WH, Baust JG, Kalloo AN. Endoscopic cryotherapy: experimental results and first clinical use. Gastrointest Endosc. 1999;49:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Johnston LR, Johnston MH. Cryospray ablation (CSA) in the esophagus: optimization of dosimetry. Am J Gastroenterol. 2006;101:S532-S533. |
| 19. | Shaheen NJ, Greenwald BD, Peery AF, Dumot JA, Nishioka NS, Wolfsen HC, Burdick JS, Abrams JA, Wang KK, Mallat D. Safety and efficacy of endoscopic spray cryotherapy for Barrett’s esophagus with high-grade dysplasia. Gastrointest Endosc. 2010;71:680-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Greenwald BD, Dumot JA, Abrams JA, Lightdale CJ, David DS, Nishioka NS, Yachimski P, Johnston MH, Shaheen NJ, Zfass AM. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc. 2010;71:686-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Raju GS, Ahmed I, Xiao SY, Brining D, Bhutani MS, Pasricha PJ. Graded esophageal mucosal ablation with cryotherapy, and the protective effects of submucosal saline. Endoscopy. 2005;37:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |