Published online Jun 10, 2015. doi: 10.4253/wjge.v7.i6.628
Peer-review started: November 1, 2014
First decision: November 27, 2014
Revised: January 24, 2015
Accepted: February 9, 2015
Article in press: February 11, 2015
Published online: June 10, 2015
Processing time: 231 Days and 17.3 Hours
Endoscopic ultrasound (EUS) has emerged as an important diagnostic and therapeutic modality in the field of gastrointestinal endoscopy. EUS provides access to many organs and lesions which are in proximity to the gastrointestinal tract and thus giving an opportunity to target them for therapeutic and diagnostic purposes. This modality also provides a real time opportunity to target the required area while avoiding adjacent vascular and other structures. Therapeutic EUS has found role in management of pancreatic fluid collections, biliary and pancreatic duct drainage in cases of failed endoscopic retrograde cholangiopancreatography, drainage of gallbladder, celiac plexus neurolysis/blockage, drainage of mediastinal and intra-abdominal abscesses and collections and in targeted cancer chemotherapy and radiotherapy. Infact, therapeutic EUS has emerged as the therapy of choice for management of pancreatic pseudocysts and recent innovations like fully covered removable metallic stents have improved results in patients with organised necrosis. Similarly, EUS guided drainage of biliary tract and pancreatic duct helps drainage of these systems in patients with failed cannulation, inaccessible papilla as with duodenal/gastric obstruction or surgically altered anatomy. EUS guided gall bladder drainage is a useful emergent procedure in patients with acute cholecystitis who are not fit for surgery. EUS guided celiac plexus neurolysis and blockage is more effective and less morbid vis-à-vis the percutaneous technique. The field of interventional EUS is rapidly advancing and many more interventions are being continuously added. This review focuses on the current status of evidence vis-à-vis the established indications of therapeutic EUS.
Core tip: Therapeutic endoscopic ultrasound (EUS) has found role in management of pancreatic fluid collections, biliary and pancreatic duct drainage in cases of failed endoscopic retrograde cholangiopancreatography, drainage of gallbladder, celiac plexus neurolysis/blockage, drainage of mediastinal and intra-abdominal abscesses and collections and in targeted cancer chemotherapy and radiotherapy. The field of interventional EUS is rapidly advancing and many more interventions are being continuously added. This review focuses on the current status of evidence vis-à-vis the established indications of therapeutic EUS.
Endoscopic ultrasound (EUS) is an important diagnostic and therapeutic technique in the field of gastroenterology. The ability to visualise and access organs in vicinity of the gastrointestinal tract has opened this exciting field with many interventional EUS procedures now overtaking conventional approaches for treatment of various gastrointestinal diseases. While advances have been made in all aspects of diagnostic and therapeutic EUS, the present review will focus on advances in therapeutic EUS and use of EUS in drainage of pancreatic collections, celiac plexus neurolysis, biliary/pancreatic duct drainage, and in the drainage of intra-abdominal abscesses.
- Citation: Sharma V, Rana SS, Bhasin DK. Endoscopic ultrasound guided interventional procedures. World J Gastrointest Endosc 2015; 7(6): 628-642
- URL: https://www.wjgnet.com/1948-5190/full/v7/i6/628.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i6.628
Acute and chronic pancreatitis can be complicated by collections of varying nature composed of pancreatic juice and varying amounts of necrotic debris in patients with acute necrotising pancreatitis[1]. The morphological characteristics of pancreatic collections complicating acute pancreatitis seem to change with time and the amount of solid necrotic debris lessens with time[2]. Pancreatic fluid collections need to be drained if they get infected or become symptomatic and cause abdominal pain, gastric outlet obstruction or biliary obstruction. Radiological, surgical and endoscopic approaches have been used to drain pancreatic collections[3,4]. Broadly, collections needing drainage early in the course of illness when a wall has not yet formed are drained via percutaneous interventions while endoscopic drainage is feasible late in the course when wall has formed[5]. The distinction between the types of collection is important before drainage as the nature and outcome of drainage depend to a large part on the amount of solid debris present in the pancreatic fluid collections (PFCs)[6-8]. While non-necrotic collections have an excellent outcome with endoscopic drainage, the fate of necrotic collections is not as good. In one report while treatment success was 93.5% in pseudocyst drainage it was much lower at 63.2% for drainage of walled off necrosis[9]. Morphologic features like size and amount of debris predict the number of procedures needed as increasing size and amount of debris predict more number of procedures[8].
While many centres continue to perform pancreatic pseudocyst drainage endoscopically, there is some evidence to suggest that EUS-guided drainage may be preferable. Two randomised trials have indicated a higher technical success especially in non-bulging lesions (Table 1)[10,11]. EUS-guided drainage is preferable in certain other clinical scenarios like presence of portal hypertension, collaterals around the collection, and presence of calcification in the wall[14,15]. A meta-analysis of available studies suggest that the technical success rates are higher for EUS guided drainage but the short term and long term results appeared to be similar[12]. In one of the report comparing the endoscopic and EUS guided drainage, median hospital stay was reported as similar with the two modalities[11]. Both reports indicate that the procedure time was not significantly different with either of the modality[10,11].
| Ref. | Patients and methods | Results |
| Park et al[10] | Randomised trial of conventional vs EUS guided drainage of pancreatic pseudocysts (n = 60) | EUS guided drainage has higher technical success (94% vs 72%). EUS preferable in non-bulging collections. Complications and pseudocyst resolution similar |
| Varadarajulu et al[11] | RCT of conventional vs EUS guided drainage (n = 15 each) | Higher technical success in EUS guided procedure (100% vs 33%) with lesser complications |
| Kahaleh et al[12] | Conventional drainage in bulging pseudocysts and absence of portal hypertension vs EUS guided in rest (n = 99) | No differences in short term or long term success and similar complications |
| Barthet et al[13] | Algorithm based approach of transpapillary (for small), EUS guided (nonbulging) or Conventional drainage of pseudocysts | EUS guided approach needed for atleast half of the patients |
The drainage using EUS is done by using a linear echoendoscope which is advanced into the stomach or duodenum. The window is assessed using colour Doppler for any regional vascularity as well as the distance between the gastrointestinal tract wall and the cyst is measured. A 19 gauge EUS fine needle aspiration (FNA) is utilised to access the collection and contents aspirated for visual assessment as well as for analysis (cultures, amylase and carcinoembryonic antigen levels). Following this a guidewire is coiled into the cyst cavity and the tract is dilated[6,7,16]. Following this various modifications are available for the drainage of PFCs including single or multiple stentings, multistep procedure with initial nasocystic drain followed by placement of stent or insertion of fully covered self-expanding metallic stents[17]. Also, after resolution of PFC, removal of transmural stents may result in recurrence of PFCs[18]. Long term indwelling plastic stents, especially in patients with disconnected duct, is a preferred approach currently in these patients[19]. Multiple authors have reported good results of EUS guided drainage and Table 2 shows important studies reporting outcomes with EUS-guided drainage of PFCs[20-36].
| Ref. | Number | Outcome |
| Giovannini et al[20] | 35 patients: 15 pseudocyst and 20 WON | Technical success: 94.3% |
| Clinical success: 88.5% | ||
| Hookey et al21] | 116 patients (51 EUS guided transmural drainage) | Technical success: 93.8% |
| Clinical success: 90.6% | ||
| Krüger et al[22] | 35 patients (both pseudocysts and abscess) | Technical success: 94% |
| Clinical success: 88% | ||
| Antillon et al[23] | 33 patients: all pseudocysts | Technical success: 94% |
| Clinical success: 90% | ||
| Lopes et al[24] | 62 procedures: 36 pseudocysts and 26 abscesses | Technical success: 94% |
| Clinical success: 84.3% | ||
| Ardengh et al[25] | 77 patients with sterile PFCs | Technical success: 94% |
| Clinical success: 91% | ||
| Varadarajulu et al[26] | 60 patients: 36 pseudocyst and 24 with abscess/WON | Technical success: 95% |
| Clinical success: 93% | ||
| Ahn et al[27] | 47 patients with pseudocyst | Technical success: 89% |
| Clinical success: 100% | ||
| Will et al[28] | 132 patients: 31 pseudocysts (n = 32), 115 abscesses/WON | Technical success: 97% |
| Clinical success: 96% | ||
| Seewald et al[29] | 70 patients: including pseudocyst, WON, abscess | Technical success: 97.5% |
| Clinical success: 83% | ||
| Puri et al[30] | 40 patients with pseudocyst | Technical success: 100% |
| Clinical success: 97% | ||
| Kato et al[31] | 67 patients with pseudocyst | Technical success: 88% |
| Clinical success: 83% | ||
| Künzli et al[32] | 108 patients | Technical success: 97% |
| Clinical success: 84% | ||
| Siddique et al[33] | 87 patients with WON | Technical success: 99% |
| Clinical success: 73.5% | ||
| Hocke et al[34] | 30 patients with WON | Technical success: 96.7% |
| Clinical success: 83.4% | ||
| Jürgensen et al[35] | 35 patients with WON | Technical success: 100% |
| Clinical success: 97% | ||
| Yasuda et al[36] | 57 patients with WON | Technical success: 100% |
| Clinical success: 75% |
Use of metallic stents: Use of self expanding metallic stents (SEMS) has recently been advocated as they may provide a better drainage because of wider diameter and thus a quicker resolution of the symptoms[37]. Various removable stents with anti-migration features have been introduced for drainage of PFCs. Fully covered stents with dumbbell like shape have been introduced which provide lumen apposition and have lesser chances of migration[38]. Various innovations like insertion of plastic pigtail stents to prevent migration have been employed with these stents[39]. The major benefit of SEMS is likely to be in walled off necrosis (WON) as they may provide ease of repeated access for necrosectomy, however this remains to be proven in prospective studies. Table 3 depicts the studies where metallic stents were used in management of PFCs.
| Ref. | Population | Stent | Design | Outcome |
| SEMS | ||||
| Talreja et al[17] | 18 patients with PFCs | FCSEMS (biliary stent) | Prospective cohort | 95% success |
| Belle et al[40] | 4 patients with WON | PCSEMS | Case series | 100% clinical success |
| Fabbri et al[41] | 22 patients with infected PFCs | FCSEMS (biliary) | Case series | 77% clinical success |
| Penn et al[39] | 20 with PFCs | FCSEMS (biliary) with plastic pigtail | Case series | Technical success 100%, clinical success 85% |
| Weilert et al[42] | 18 patients with PFCs | FCSEMS | Case series | Clinical success in 78% |
| LACSEMS | ||||
| Shah et al[43] | Pseudocyst and WON (n = 33) | AXIOS (EUS guided in 30/33) | Prospective cohort | 91% technical success, 93% resolution of PFC |
| Walter et al[44] | 46 patients WON and 15 pseudocyst | AXIOS stent | Prospective cohort | Technical success: 98%, clinical success: 93% in pseudocyst and 81% in WON |
| Gornals et al[45] | 9 patients with PFCs | AXIOS | Case series | Technical success in 88% and 100% clinical success |
| Itoi et al[46] | 15 patients with pseudocysts | AXIOS | Retrospective case series | 100% clinical success |
| Yamamoto et al[37] | 9 PFCs, 5 pseudocyst and 4 WON | FCSEMS (Nagi stent) | Retrospective case series | 77.8% clinical success |
| ESOPHAGEAL SEMS | ||||
| Sarkaria et al[47] | 17 patients with WON | Esophageal FCSEMS | Retrospective case series | 88% clinical success |
Non-fluoroscopic drainage: It has been demonstrated that EUS-guided drainage is feasible even without fluoroscopic control[6,48]. Seicean et al[48] have demonstrated the utility of EUS in drainage of PFCs in 24 patients and documented complete resolution in 83.3% cases. However difficulty arose in PFCs with thickened wall for which fluoroscopic control was recommended by the authors. We have also demonstrated the efficacy of EUS in draining non-bulging PFCs in 20 patients in absence of fluoroscopic control. Only one patient needed percutaneous intervention amongst these 20 patients[6]. In another report of EUS guided drainage of 22 patients with PFCs, drainage was technically feasible in 19 patients even in absence of fluoroscopy. Success after single procedure was noted in 59% patients[49].
Creation of multiple drainage routes: In management of walled off necrosis, creation of a single enteral opening may not provide adequate drainage of the collection. In this regard it may be better to have multiple access sites into the cavity which may help in improving drainage and irrigation of the cavity. Dual modality drainage involving percutaneous and endoscopic drainage simultaneously has been advocated for achieving this end[50]. A purely endoscopic procedure: EUS guided multi transluminal gateway technique has been evaluated and reported to have a high success (91.7%) vis-à-vis convention drainage (52.1) in a non-randomised study[51]. Prospective reports validating this approach are awaited.
Forward viewing echoendoscope: A multicentre randomised trial reported use of a forward viewing echoendoscope for drainage of PFCs. The technical success rates, mean procedure times, ease of access and complication rates were similar to the oblique-viewing echoendoscope indicating lack of any benefit with use of forward viewing echoendoscope for drainage of PFCs[52].
Access to the cavity may be difficult in patients with thick wall between the gastric/duodenal lumen and the cavity and therefore the tract may be difficult to dilate. To overcome this use of wire guided bent needle knife to obtain a wide access has been used[53]. A double guidewire technique utilising a double lumen catheter has been advocated to avoid the hassle of repeated need for cannulation of pseudocyst for placing multiple endoprosthesis[54]. A modification of the dual-lumen biliary brush catheter has also been used to place multiple guidewires into the cyst cavity and thereby allowing placement of multiple stents[55]. A novel exchange free access device has also been used for EUS guided drainage of PFCs and has an inner trocar for puncture and an outer dual balloon for dilatation of the tract reducing the need for multiple exchanges[56,57]. Numerous other innovations like use of hydrogen peroxide and streptokinase have been used but comparative data vis-à-vis control group is not yet available[58,59].
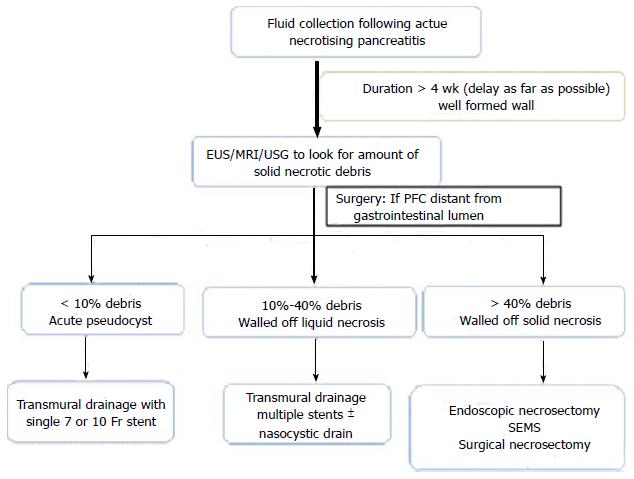
Drainage of PFCs is an important therapeutic application of EUS with excellent technical and clinical outcomes. We believe that merely dividing walled off PFCs into pseudocysts and WON may be too simplistic and it would be better to have three subgroups including acute postnecrotic pseudocyst (< 10% solid debris), walled off liquid necrosis (10%-40% solid content) and walled off solid necrosis (> 40% solid debris) as this has implications on management and success of endoscopic drainage[60]. We have previously shown that the amount of necrosis predicts the therapy needed in PFCs. Whilst those with < 10% debris need only one session of drainage, those with 10%-40% solid debris needed ≥ 2 sessions and the group with even higher (> 40%) debris needed direct endoscopic debridement or surgical necrosectomy[8]. Based on this, we follow an algorithmic approach (Figure 1) for management of PFCs at our institution.
Endoscopic retrograde cholangio-pancreaticography (ERCP) is the standard approach to drain an obstructed biliary tract but may fail due to a number of factors like inaccessible papilla or a failure to cannulate it. In these situations, radiological or surgical drainage is needed. EUS guided biliary drainage is emerging as an alternative to a failed ERCP[16]. EUS guided approaches include transmural drainage (hepaticogastrostomy or choledochoduodenostomy), a rendezvous procedure or an antegrade approach[61]. EUS guided transluminal drainage (EUS-TLD) is achieved by bile duct puncture from the stomach or the duodenum using EUS-FNA needle. Occasionally choledochoantrostomy or hepaticoesophagostomy has also been described for achieving biliary drainage[62-64]. After obtaining a cholangiogram a guidewire is placed into the biliary system and the tract dilated followed by insertion of stent to achieve drainage of biliary system into the stomach or the duodenum. While duodenal station is used to achieve access into the common bile duct, gastric station allows access to the left lobe intrahepatic biliary radicals[61]. Access to right sided biliary system has also been described[65]. Table 4 depicts the major reports of EUS guided transluminal access to biliary system. EUS guided rendezvous is achieved by creation of a temporary access to the biliary tree using EUS guided approach in patients with failed cannulation but with accessible papilla. The guidewire is then negotiated across the obstruction into the duodenum through the papilla and is then retrieved using snare and thereby providing a conduit for further ERCP[61]. This approach is, therefore, useful in failed ERCP but accessible papilla. The approach from the stomach and first part of duodenum is considered to be stable but the ampullary direction of guidewire is achieved best from the stomach and second part of duodenum[61]. Table 5 depicts the major reports of EUS guided rendezvous procedures and their outcomes. EUS guided antegrade approach is the use of temporary EUS guided access created from the duodenum or stomach for placement of stents or balloon dilatation without the scope reaching the papilla. The reported success rate for this procedure is 77% and the complication rate is 5%, however large studies are lacking[61].
| Ref. | Number | Etiology | Technical success | Clinical success | Complication rates |
| Takada et al[66] | 26 17 CCD, 6 HG, 2 CCA, 1 HJ | Malignant | 90.6% | 100% | 20.7% |
| Kawakubo et al[67] | 64 CCD: 44 HG: 20 | Malignant | 95% | 100% | 19% |
| Prachayakul et al[68] | 21 CCD: 6 HG: 15 | Malignant | 95.2% | 90.2% | 9.5% |
| Hara et al[69] | 18 CCD | Malignant | 94% | 94% | 11% |
| Song et al[70] | 15 CCD | Malignant | 86.7% | 100% | 23.1% |
| Kim et al[71] | 13 CCD: 9 HG: 4 | Malignant | 92.3% | 91.7% | 30.7% |
| Park do et al[72] | 57 CCD: 26 HG: 31 | Both benign and malignant | 96.5% | 89% | 20% |
| Komaki et al[73] | 15 CCD | Malignant | 93% | 100% | 26.7% |
| Hara et al[74] | 18 CCD | Malignant | 94% | 100% | 17% |
| Khashab et al[64] | 20 HG: 3 CCD: 15 HE: 2 | Malignant | 95% | 86.3% | 10% |
| Vila et al[75] | 60 HG: 34 CCD: 26 | Both benign and malignant | 64.7% and 86.3% | 63.2% | 15.1% |
| Attasaranya et al[76] | 25 HG: 16 CCD: 9 | Both benign and malignant | 77% | 96% | 35% |
| Ref. | Number | Technical success | Clinical success | Complications |
| Khashab et al[64] | 13 (EH: 11, IH: 2) | 100% | 100% | 15% |
| Tarantino et al[77] | 4 (EH: 4) | 50% | 100% | 13% |
| Dhir et al[78] | 20 | 100% | 100% | 15% |
| Dhir et al[79] | 17 TH, 18 EH | 100% for EH and 94.1% for TH | 100% | Higher for TH vs EH |
| Park do et al[80] | 20 (14 IH and 6 EH) | 80% | 10% | |
| Kawakubo et al[81] | 14 (9 EH and 5 IH) | 100% | 100% | 14% |
| Dhir et al[82] | 58 (all EH) | - | 98% | 3.4% |
| Iwashita et al[83] | 40 (31 EH and 9 IH) | 73% | 13% |
EUS-TLD is associated with significant complications including perforation, bile leak, bleeding, and stent dysfunction or migration. The use of EUS-TLD has also been reported to be as efficacious as transpapillary drainage in patients with previous duodenal stents with a higher stent patency rate with EUS-TLD[84]. SEMS are preferred over plastic stents as they provide a larger diameter and therefore are likely to remain patent for longer periods and the risk of bile leaks is likely to be less with SEMS. SEMS also make a reinsertion of stent easier as stent can be placed into the previous SEMS[85]. Both EUS-TLD and placement of duodenal SEMS in patients with obstructive jaundice and duodenal obstruction due to unresectable periampullary lesions has been reported as a single step procedure with use of linear echo-endoscope[86].
EUS guided approaches have also been compared with percutaneous approach for biliary drainage. In a randomised study comparing percutaneous and EUS guided approaches in 25 patients with unresectable biliary obstruction, the technical success, clinical success, cost and complications were similar amongst both the groups suggesting that either could be used as an alternative for biliary drainage[87]. However a recent report comparing 51 patients who underwent percutaneous transhepatic biliary drainage (PTBD) with 22 patients who underwent EUS-TLD indicated that the technical success was higher for PTBD. The authors however recommended EUS-TLD as the initial procedure of choice as it needed lesser re-interventions reducing costs of therapy as also a lower adverse event rate[88]. In a similar report where 50 patients were retrospectively evaluated success of internal stenting as well as complication rates were more favourable in the EUS-TLD group. While internal stenting could be achieved in 92% patients in EUS-TLD group, it could be achieved only in 46% of PTBD group[89]. Amongst EUS guided approaches, transhepatic access seems to increase the risk of complications vis-à-vis transduodenal access of the biliary tree[78]. An approach has been suggested for the use of various EUS guided methods for achieving biliary drainage in different clinical settings. If ampulla is inaccessible, EUS-TLD is the initial choice. If papilla is accessible rendezvous should be attempted but if it is not possible to cross the lesion/stricture then EUS-TLD can be undertaken. Antegrade approach may be better suited for surgically altered anatomy where the procedure is needed for benign lesions[61].
The emergent gall bladder drainage is usually done radiologically but availability of EUS has made it possible to drain the gall bladder endoscopically. This may be indicated in situations like acute cholecystitis in patients who are unsuitable for surgery and have not improved with antibiotics[90]. In a systematic review of endoscopic drainage of gallbladder using nasogallbladder drainage in 194 patients and gallbladder stenting in 127 patients the technical success rates were 81% and 96%, clinical success rates were 75% and 88% and complication rates were 3.6% and 6.3%, respectively[90,91]. In a randomised study of patients with acute cholecystitis who were assigned to undergo either an EUS guided drainage or a percutaneous drainage of gall bladder the technical success rates were similar as were the complication rates suggesting that EUS guided approach is feasible for gall bladder drainage with outcomes comparable to the percutaneous approach[92]. Major reports (> 10 patients) on EUS guided drainage of gall bladder are shown in Table 6.
Gall Bladder drainage can be achieved by use of either plastic or metallic stents or use of naso-gallbladder drains[94,95]. The complications may include bile leak, perforation and pneumo-peritoneum. In a report evaluating long term outcomes in 56 patients with acute cholecystitis who had underwent EUS guided gallbladder drainage the stent patency was 86% over 3 years. Four patients had late adverse events including distal stent migration in 2 patients and acute cholecystitis due to stent occlusion in 2 patients. The stent occlusions were treated endoscopically[97]. A single step procedure for insertion for lumen opposing metallic stent using AXIOS system has also been reported[98]. EUS guided gallbladder drainage has also been used as an approach for drainage in unresectable pancreatic cancer with use of anti-migratory fully covered metallic stents[99]. EUS guided gall bladder drainage may be of value in situation where a percutaneous procedure is dificult or more risky (presence of ascites and coagulopathy) but comes at an increased risk associated with sedation in patients with various comorbidities and the risk of bile leak.
EUS guided pancreatic ductal (PD) drainage may be indicated for patients with failed transpapillary drainage like in failed cannulation of non-negotiable strictures in chronic pancreatitis or pancreatic fistulae or pancreaticogastric or pancreaticojejunal stenosis after pancreatic surgery[100]. Both trans-enteric stenting and rendezvous procedures can be accomplished after EUS guided access to the pancreatic duct has been obtained. Once an access has been achieved using EUS-FNA needle and a guidewire is placed into the PD, and dilatation of the tract is done. SEMS are not used to drain the pancreatic duct for the associated risk of obstructive pancreatitis due to blockage of the side branches of the pancreatic duct. Complications associated with EUS guided PD drainage include leakage of pancreatic juice, pancreatitis, perforation or bleeding[101,102]. In a systematic review of 9 studies including 205 patients the pancreatic duct drainage was successful in 58%-100% with clinical success in 74% and a complication rate of 20%[102]. Success rates were lesser in a nationwide retrospective study form Spain[75]. Both rendezvous and transenteric drainage has been reported to have similar efficacy although it may be difficult to do a rendezvous in tight strictures[103,104]. The EUS guided PD access can be utilised for taking brushings to confirm malignancy in pancreatic stricture[105]. Access may be easier to obtain in dilated duct[104]. Some data is available about long term clinical success which indicates that at a median follow-up of 37 mo pain relief was present in 72% patients[106]. Another report indicated complete pain relief in 83% of patients[107]. It is important to suspect underlying malignancy in those with lack of pain relief[108]. Anterograde pancreatic drainage including stricture dilatation and removal of stone has also been reported[109,110]. To summarise EUS guided pancreatic duct drainage can be of use in rescue management of failed ERCP or in patients with surgically altered anatomy but the technique is still evolving and better accessories are needed.
Recently EUS guided brachytherapy has also been evaluated with radioactive seeds being placed into the tumour of interest under EUS guidance with the help of linear echoendoscope[111,112]. The most popular radioactive seeds are Iodine 125, palladium 103 and iridium 192. In pancreatic cancers where the cells divide quite rapidly, iodine is the radioactive material of choice as it has got a long half life of 60 d. The radioactive spill over the region of interest is definitely an issue of concern but in human tissue the penetration distance of the radiation into surrounding tissue is very small. The seeds of EUS guided brachytherapy were sowed by Sun et al[111] with their study in pigs. Sun et al[111] published the use of iodine 125 in unresectable pancreatic cancer in 15 patients. The result revealed a median survival of 10.6 mo with 27% patients having partial tumour response[111]. In another study in 22 patients with advanced pancreatic cancer where combination of gemcitabine and Iodine 125 brachytherapy was used, the overall survival rate didn’t improve[112].
For external beam radiation to the cranium, bony landmarks are used for guiding the therapy while in intraabdominal malignancy fiducial markers are placed inside the tumour for guiding therapy. These markers are radioactive spheres, coils or seeds. Earlier fiducials were placed under surgical or radiological guidance but with advent of interventional EUS, these fiducials can be placed under EUS guidance also. Pishvaian et al[113] reported EUS guided fiducail placement in 13 patients with technical success achieved in 11/13 patients. An average of 3-4 fiducials were placed in each patient. There have been multiple studies where EUS guided fiducials have been placed successfully in pancreatic cancers, esophageal cancers and neuroendocrine tumours[114-116]. To compare the 2 types of fiducials a study was conducted in 39 patients with advanced pancreatic cancer. Traditional fiducials of 5 mm length and viscoil fiducials of 10 mm length were compared. It was observed that traditional fiducials had better visibility scores as compared to viscoil fiducials and the migration rate between the two types of fiducials was similar[117].
Ethanol causes cell death by membrane lysis, vascular occlusion and protein denaturation and has been used for ablation of solid and cystic lesions of thyroid, liver, adrenals, etc. EUS guided ethanol ablation has been used recently for ablation of pancreatic lesions, neuroendocrine tumors (NETs) and metastatic abdominal lesions. EUS guided fine needle injection therapy using alcohol is safe and better than percutaneous approach as it is delivers alcohol to target tissue with more accuracy, identify surrounding structures and perform injection therapy in real time monitoring.
In a study by Gan et al[118] including 25 patients with pancreatic cysts who underwent ablation with variable concentrations of alcohol (5%-80%), the results revealed complete resolution in 8 patients and epithelial ablation in 5 patients who underwent surgery. In another study ethanol injection was compared with saline injection alone. In this study 25/42 patients were initially treated with alcohol and rest 17 with saline. After 3 mo, patients in both the groups were treated with ethanol injection. The results showed that 80% ethanol injection resulted in a greater decrease in size as compared to saline injection. Nine patients who were followed up for 2 years had no recurrence of cyst[119]. In another study of 42 patients with cystic tumours of the pancreas who were initially injected with 99% ethanol followed by paclitaxel. Complete resolution was achieved in 29 patients. No complications were observed[120]. EUS guided ethanol ablation of pancreatic neuroendocrine tumours has been reported in patients who are not good candidates for surgery either because of age or comorbidities. There have been published reports where even multiple NETs have been injected with alcohol and ablation has been achieved with patient remaining symptom free post injection. But there is a risk of recurrence and metastasis. So long term follow up studies are required to adequately define the role of ethanol ablation in NETs[121-125].
Multiple metastatic lesions have also been ablated with EUS guided ethanol injection but its role in these situations need to assessed in larger studies. These include hepatic metastases from carcinoma colon, pelvic lymph nodal metastases from rectal cancer, left adrenal metastases from non-small cell carcinoma lung, hepatic metastases from pancreatic carcinoma and ablation of a gastrointestinal stromal tumour in a patient whose comorbidities precluded surgery[126-129].
Pancreatic carcinoma has got a poor response to chemotherapeutic agents and radiation. In presence of locally advance disease and borderline resectability, neoadjuvant chemotherapy has been tried, but it carries a poor response rate as the tumour is hypovascular and produces a desmoplastic reaction around it leading to poor delivery of drugs. So various local antitumour agents have been tried in patients with advanced pancreatic carcinoma for palliation and in locally advanced lesions for downstaging before surgery (Table 7).
| Name of the agent | Drug | Ref. | Reported use |
| CYTOIMPLANT | Allogenic mixed lymphocyte culture | Chang et al[130] | Advanced pancreatic cancer |
| TNFerade | cDNA expressing TNF-α (adenovector) | Hecht et al[131], Chang et al[132] and Citrin et al[133] | Pancreatic, esophageal and rectal cancer |
| ONY X-015 | Adenovirus | Mulvihill et al[134] | Advanced pancreatic cancer |
| Oncogel | Paclitaxel and ReGel | Linghu et al[135], Matthes et al[136] and Vukelja et al[137] | Pancreatic, esophageal cancer |
| Gemcitabine | Gemcitabine | Levy et al[138] | Advanced pancreatic cancer |
| DC’s | Dendritic cells | Irisawa[139], Hirooka et al[140] | Advanced pancreatic cancer |
The problem with all these studies is that they were small and all these agents in this role are still in experimental stage. So we need much more large prospective studies before these techniques can be put into clinical practice.
Thermal injury leading to coagulation necrosis has been the principle of radiofrequency ablation (RFA). This principle has been exploited for treatment of solid tumours like hepatocellular carcinoma (HCC) and liver metastases. Percutaneous, open or laparoscopic approach have been associated with morbidity and mortality. Recently EUS guided RFA has been performed under real time guidance in porcine models. Studies of EUS RFA done in porcine models have used the technique for ablation of lymph nodes and pancreatic lesions[141,142]. Majority of pigs tolerated the procedure well except for few complications.
Photodynamic therapy is another modality for tumour ablation. Here a photosensitizer drug is injected and application of light is done to the area of interest. The tumour cells are killed by direct cytotoxic effects, vascular changes and inflammatory reaction[143,144]. A study in porcine models where EUS guided photodynamic therapy has been done to liver, pancreas and kidney showed that 100% necrosis was seen in pancreas only[145].
It is an evolving technique and recently a case was reported where EUS guided laser ablation of a left lobe HCC was performed using 22 G needle and patient was followed up for 2 mo with no recurrence of the lesion[146].
EUS guided internal drainage of abdominal and pelvic abscesses has emerged as an alternative to traditional percutaneous drainage. Abscesses in areas close to the gastrointestinal lumen including mediastinum, lesser sac, perihepatic and subphrenic space, and pelvis can be drained using EUS guidance. The procedure involves the usual steps described earlier for PFC drainage: access using 19 G EUS-FNA needle, use of guidewire, dilatation of tract and placement of drainage catheter or pigtail stents. The suggested dilatation diameters for esophagus is 6 mm, for colon and jejunum is 6-8 mm, for duodenum 8-10 mm and in stomach 8-15 mm[147]. Table 8 shows various reports of EUS guided drainage of pelvic abscesses.
| Ref. | Number | Site | Technical success | Clinical success | Complications |
| Hadithi et al[148] | 8 | Abdominal (pelvic) | 100% | 100% | 0 |
| Puri et al[149] | 30 | Pelvic (4 prostatic) | 93.3% | 83.5% | 0 |
| Ramesh et al[150] | 38 | 11 transcolonic, 27 transrectal | 100% | 87% | 10.5% |
| Puri et al[151] | 14 | Pelvic | 100% | 93% | 0 |
| Varadarajulu et al[152] | 25 | Pelvic | 100% | 96% | 0 |
| Giovannini et al[153] | 12 | Pelvic | 100% | 75% | 25% |
Mediastinal abscesses have also been drained under EUS guidance including placement of lumen opposing stents[154,155]. A few reports have also involved aspiration of splenic abscess in setting of pancreatitis[156,157]. Liver abscess have also been drained using EUS guidance and placement of lumen apposing stent has also been done[158,159].
Percutaneous celiac plexus neurolysis (CPN) has been used for management of pain in pancreatic cancer and sometimes for chronic pancreatitis. EUS guided CPN has emerged as a more effective technique in recent times[160]. Using the linear echoendoscope at the level of gastroesophageal junction the aorta is located and celiac artery traced. While alcohol is used to obtain CPN, bupivacaine is used for celiac plexus block (CPB). Although triamcinolone is often added to bupivacaine but a randomised study found no benefit with addition of triamcinolone[161]. The average efficacy of CPB for pain relief is around 3 mo. Transient hypotension and diarrhea may occur as side effects of the procedure. The EUS guided technique avoids passage through the vertebrae and muscles at the back as required for a CT guided celiac block and therefore unlikely to have related adverse events like paraparesis[162]. Interestingly, ganglion cells have now been visualised on EUS and it may be better to target ganglions directly[163,164]. In a randomised trial comparing CPN with celiac ganglia neurolysis (CGN), the positive response was higher in the CGN group (73.5% vs 45.5%). Half of the patients in CGN group obtained complete relief vis-à-vis 18% in CPN group[165].
Multiple comparative reports have emerged which have compared radiologic vs EUS guided CPN. EUS guided CPN provided a more long lasting pain relief (30% up to 24 wk) while with CT guided CPN only 12% had some relief at 12 wk[166]. In a trial comparing one vs two injections for pain relief during CPN, no incremental benefit in pain relief was observed with two injections[167]. EUS guided CPB was more efficacious for pain relief in patients with chronic pancreatitis in a randomised comparison with percutaneous CPB[168]. Only a subset of patients with EUS guided CPN obtain complete pain relief and the duration of pain relief is variable. The predictors of pain relief are not established. Also the benefit of performing CPN rather than CPB in chronic pancreatitis is not clear[169].
It is apparent that the availability of interventional EUS has allowed gastroenterologists to make forays into areas which traditionally remained the domain of surgeons and interventional radiologists. With further improvements in accessories and development of EUS-natural orifice transluminal endoscopic surgery, the endosonologist will have to do multiple roles[170].
P- Reviewer: Merrett ND S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | Brun A, Agarwal N, Pitchumoni CS. Fluid collections in and around the pancreas in acute pancreatitis. J Clin Gastroenterol. 2011;45:614-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Rana SS, Bhasin DK, Reddy YR, Sharma V, Rao C, Sharma RK, Gupta R. Morphological features of fluid collections on endoscopic ultrasound in acute necrotizing pancreatitis: do they change over time? Ann Gastroenterol. 2014;27:258-261. [PubMed] |
| 3. | Bakker OJ, van Santvoort HC, van Brunschot S, Geskus RB, Besselink MG, Bollen TL, van Eijck CH, Fockens P, Hazebroek EJ, Nijmeijer RM. Endoscopic transgastric vs surgical necrosectomy for infected necrotizing pancreatitis: a randomized trial. JAMA. 2012;307:1053-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 4. | Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 331] [Article Influence: 27.6] [Reference Citation Analysis (1)] |
| 5. | Fisher JM, Gardner TB. Endoscopic therapy of necrotizing pancreatitis and pseudocysts. Gastrointest Endosc Clin N Am. 2013;23:787-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Rana SS, Bhasin DK, Rao C, Gupta R, Singh K. Non-fluoroscopic endoscopic ultrasound-guided transmural drainage of symptomatic non-bulging walled-off pancreatic necrosis. Dig Endosc. 2013;25:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Bang JY, Varadarajulu S. Endoscopic ultrasound-guided management of pancreatic pseudocysts and walled-off necrosis. Clin Endosc. 2014;47:429-431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Rana SS, Bhasin DK, Sharma RK, Kathiresan J, Gupta R. Do the morphological features of walled off pancreatic necrosis on endoscopic ultrasound determine the outcome of endoscopic transmural drainage? Endosc Ultrasound. 2014;3:118-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Park DH, Lee SS, Moon SH, Choi SY, Jung SW, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009;41:842-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. 2008;68:1102-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 277] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 12. | Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Barthet M, Lamblin G, Gasmi M, Vitton V, Desjeux A, Grimaud JC. Clinical usefulness of a treatment algorithm for pancreatic pseudocysts. Gastrointest Endosc. 2008;67:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Panamonta N, Ngamruengphong S, Kijsirichareanchai K, Nugent K, Rakvit A. Endoscopic ultrasound-guided versus conventional transmural techniques have comparable treatment outcomes in draining pancreatic pseudocysts. Eur J Gastroenterol Hepatol. 2012;24:1355-1362. [PubMed] |
| 15. | Rana SS, Sharma V, Sharma R, Gupta R, Bhasin DK. Endoscopic ultrasound-guided transmural drainage of calcified pseudocyst in a patient with chronic calcific pancreatitis. Available from: http://www.annalsgastro.gr/files/journals/1/earlyview/2014/ev-10-2014-13-AG2106.pdf. |
| 16. | Widmer JL, Michel K. Endoscopic Ultrasound-Guided Treatment beyond Drainage: Hemostasis, Anastomosis, and Others. Clin Endosc. 2014;47:432-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Talreja JP, Shami VM, Ku J, Morris TD, Ellen K, Kahaleh M. Transenteric drainage of pancreatic-fluid collections with fully covered self-expanding metallic stents (with video). Gastrointest Endosc. 2008;68:1199-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 18. | Arvanitakis M, Delhaye M, Bali MA, Matos C, De Maertelaer V, Le Moine O, Devière J. Pancreatic-fluid collections: a randomized controlled trial regarding stent removal after endoscopic transmural drainage. Gastrointest Endosc. 2007;65:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Rana SS, Bhasin DK, Rao C, Sharma R, Gupta R. Consequences of long term indwelling transmural stents in patients with walled off pancreatic necrosis & amp; disconnected pancreatic duct syndrome. Pancreatology. 2013;13:486-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Giovannini M, Pesenti C, Rolland AL, Moutardier V, Delpero JR. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Devière J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Krüger M, Schneider AS, Manns MP, Meier PN. Endoscopic management of pancreatic pseudocysts or abscesses after an EUS-guided 1-step procedure for initial access. Gastrointest Endosc. 2006;63:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Antillon MR, Shah RJ, Stiegmann G, Chen YK. Single-step EUS-guided transmural drainage of simple and complicated pancreatic pseudocysts. Gastrointest Endosc. 2006;63:797-803. [PubMed] |
| 24. | Lopes CV, Pesenti C, Bories E, Caillol F, Giovannini M. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Ardengh JC, Coelho DE, Coelho JF, de Lima LF, dos Santos JS, Módena JL. Single-step EUS-guided endoscopic treatment for sterile pancreatic collections: a single-center experience. Dig Dis. 2008;26:370-376. [PubMed] |
| 26. | Varadarajulu S, Tamhane A, Blakely J. Graded dilation technique for EUS-guided drainage of peripancreatic fluid collections: an assessment of outcomes and complications and technical proficiency (with video). Gastrointest Endosc. 2008;68:656-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Ahn JY, Seo DW, Eum J, Song TJ, Moon SH, Park do H, Lee SS, Lee SK, Kim MH. Single-Step EUS-Guided Transmural Drainage of Pancreatic Pseudocysts: Analysis of Technical Feasibility, Efficacy, and Safety. Gut Liver. 2010;4:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Will U, Wanzar C, Gerlach R, Meyer F. Interventional ultrasound-guided procedures in pancreatic pseudocysts, abscesses and infected necroses - treatment algorithm in a large single-center study. Ultraschall Med. 2011;32:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Seewald S, Ang TL, Richter H, Teng KY, Zhong Y, Groth S, Omar S, Soehendra N. Long-term results after endoscopic drainage and necrosectomy of symptomatic pancreatic fluid collections. Dig Endosc. 2012;24:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Puri R, Mishra SR, Thandassery RB, Sud R, Eloubeidi MA. Outcome and complications of endoscopic ultrasound guided pancreatic pseudocyst drainage using combined endoprosthesis and naso-cystic drain. J Gastroenterol Hepatol. 2012;27:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Kato S, Katanuma A, Maguchi H, Takahashi K, Osanai M, Yane K, Kim T, Kaneko M, Takaki R, Matsumoto K. Efficacy, Safety, and Long-Term Follow-Up Results of EUS-Guided Transmural Drainage for Pancreatic Pseudocyst. Diagn Ther Endosc. 2013;2013:924291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Künzli HT, Timmer R, Schwartz MP, Witteman BJ, Weusten BL, van Oijen MG, Siersema PD, Vleggaar FP. Endoscopic ultrasonography-guided drainage is an effective and relatively safe treatment for peripancreatic fluid collections in a cohort of 108 symptomatic patients. Eur J Gastroenterol Hepatol. 2013;25:958-963. [PubMed] |
| 33. | Siddiqui AA, Dewitt JM, Strongin A, Singh H, Jordan S, Loren DE, Kowalski T, Eloubeidi MA. Outcomes of EUS-guided drainage of debris-containing pancreatic pseudocysts by using combined endoprosthesis and a nasocystic drain. Gastrointest Endosc. 2013;78:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 34. | Hocke M, Will U, Gottschalk P, Settmacher U, Stallmach A. Transgastral retroperitoneal endoscopy in septic patients with pancreatic necrosis or infected pancreatic pseudocysts. Z Gastroenterol. 2008;46:1363-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Jürgensen C, Neser F, Boese-Landgraf J, Schuppan D, Stölzel U, Fritscher-Ravens A. Endoscopic ultrasound-guided endoscopic necrosectomy of the pancreas: is irrigation necessary? Surg Endosc. 2012;26:1359-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Yasuda I, Nakashima M, Iwai T, Isayama H, Itoi T, Hisai H, Inoue H, Kato H, Kanno A, Kubota K. Japanese multicenter experience of endoscopic necrosectomy for infected walled-off pancreatic necrosis: The JENIPaN study. Endoscopy. 2013;45:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 37. | Yamamoto N, Isayama H, Kawakami H, Sasahira N, Hamada T, Ito Y, Takahara N, Uchino R, Miyabayashi K, Mizuno S. Preliminary report on a new, fully covered, metal stent designed for the treatment of pancreatic fluid collections. Gastrointest Endosc. 2013;77:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Desilets DJ, Banerjee S, Barth BA, Bhat YM, Gottlieb KT, Maple JT, Pfau PR, Pleskow DK, Siddiqui UD, Tokar JL. New devices and techniques for management of pancreatic fluid collections. Gastrointest Endosc. 2013;77:835-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Penn DE, Draganov PV, Wagh MS, Forsmark CE, Gupte AR, Chauhan SS. Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 2012;76:679-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 40. | Belle S, Collet P, Post S, Kaehler G. Temporary cystogastrostomy with self-expanding metallic stents for pancreatic necrosis. Endoscopy. 2010;42:493-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Fabbri C, Luigiano C, Cennamo V, Polifemo AM, Barresi L, Jovine E, Traina M, D’Imperio N, Tarantino I. Endoscopic ultrasound-guided transmural drainage of infected pancreatic fluid collections with placement of covered self-expanding metal stents: a case series. Endoscopy. 2012;44:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Weilert F, Binmoeller KF, Shah JN, Bhat YM, Kane S. Endoscopic ultrasound-guided drainage of pancreatic fluid collections with indeterminate adherence using temporary covered metal stents. Endoscopy. 2012;44:780-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 43. | Shah RJ, Shah JN, Waxman I, Kowalski TE, Sanchez-Yague A, Nieto J, Brauer BC, Gaidhane M, Kahaleh M. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin Gastroenterol Hepatol. 2015;13:747-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 44. | Walter D, Will U, Sanchez-Yague A, Brenke D, Hampe J, Wollny H, López-Jamar JM, Jechart G, Vilmann P, Gornals JB. A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: a prospective cohort study. Endoscopy. 2015;47:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 45. | Gornals JB, De la Serna-Higuera C, Sánchez-Yague A, Loras C, Sánchez-Cantos AM, Pérez-Miranda M. Endosonography-guided drainage of pancreatic fluid collections with a novel lumen-apposing stent. Surg Endosc. 2013;27:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 46. | Itoi T, Binmoeller KF, Shah J, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos). Gastrointest Endosc. 2012;75:870-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 47. | Sarkaria S, Sethi A, Rondon C, Lieberman M, Srinivasan I, Weaver K, Turner BG, Sundararajan S, Berlin D, Gaidhane M. Pancreatic necrosectomy using covered esophageal stents: a novel approach. J Clin Gastroenterol. 2014;48:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Seicean A, Stan-Iuga R, Badea R, Tantau M, Mocan T, Seicean R, Iancu C, Pascu O. The safety of endoscopic ultrasonography-guided drainage of pancreatic fluid collections without fluoroscopic control: a single tertiary center experience. J Gastrointestin Liver Dis. 2011;20:39-45. [PubMed] |
| 49. | Rasmussen DN, Hassan H, Vilmann P. Only few severe complications after endoscopic ultrasound guided drainage of pancreatic pseudocysts. Dan Med J. 2012;59:A4406. [PubMed] |
| 50. | Ross AS, Irani S, Gan SI, Rocha F, Siegal J, Fotoohi M, Hauptmann E, Robinson D, Crane R, Kozarek R. Dual-modality drainage of infected and symptomatic walled-off pancreatic necrosis: long-term clinical outcomes. Gastrointest Endosc. 2014;79:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 51. | Varadarajulu S, Phadnis MA, Christein JD, Wilcox CM. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc. 2011;74:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 189] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 52. | Voermans RP, Ponchon T, Schumacher B, Fumex F, Bergman JJ, Larghi A, Neuhaus H, Costamagna G, Fockens P. Forward-viewing versus oblique-viewing echoendoscopes in transluminal drainage of pancreatic fluid collections: a multicenter, randomized, controlled trial. Gastrointest Endosc. 2011;74:1285-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Azar RR, Oh YS, Janec EM, Early DS, Jonnalagadda SS, Edmundowicz SA. Wire-guided pancreatic pseudocyst drainage by using a modified needle knife and therapeutic echoendoscope. Gastrointest Endosc. 2006;63:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 54. | Itoi T, Itokawa F, Tsuchiya T, Kawai T, Moriyasu F. EUS-guided pancreatic pseudocyst drainage: simultaneous placement of stents and nasocystic catheter using double-guidewire technique. Dig Endosc. 2009;21 Suppl 1:S53-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 55. | Khashab MA, Lennon AM, Singh VK, Kalloo AN, Giday SA. Endoscopic ultrasound (EUS)-guided pseudocyst drainage as a one-step procedure using a novel multiple-wire insertion technique (with video). Surg Endosc. 2012;26:3320-3323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Binmoeller KF, Smith I, Gaidhane M, Kahaleh M. A kit for eus-guided access and drainage of pancreatic pseudocysts: efficacy in a porcine model. Endosc Ultrasound. 2012;1:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Binmoeller KF, Weilert F, Shah JN, Bhat YM, Kane S. Endosonography-guided transmural drainage of pancreatic pseudocysts using an exchange-free access device: initial clinical experience. Surg Endosc. 2013;27:1835-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Siddiqui AA, Easler J, Strongin A, Slivka A, Kowalski TE, Muddana V, Chennat J, Baron TH, Loren DE, Papachristou GI. Hydrogen peroxide-assisted endoscopic necrosectomy for walled-off pancreatic necrosis: a dual center pilot experience. Dig Dis Sci. 2014;59:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Gupta R, Shenvi SD, Nada R, Rana SS, Khullar M, Kang M, Singh R, Bhasin DK. Streptokinase may play role in expanding non-operative management of infected walled off pancreatic necrosis: preliminary results. Pancreatology. 2014;14:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 60. | Rana SS, Bhasin DK. Should all fluid collections in delayed phase of acute necrotizing pancreatitis labeled as walled-off pancreatic necrosis? Dig Dis Sci. 2014;59:1338-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Iwashita T, Doi S, Yasuda I. Endoscopic ultrasound-guided biliary drainage: a review. Clin J Gastroenterol. 2014;7:94-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 62. | Itoi T, Itokawa F, Tsuchiya T, Tsuji S, Tonozuka R. Endoscopic ultrasound-guided choledochoantrostomy as an alternative extrahepatic bile duct drainage method in pancreatic cancer with duodenal invasion. Dig Endosc. 2013;25 Suppl 2:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Will U, Thieme A, Fueldner F, Gerlach R, Wanzar I, Meyer F. Treatment of biliary obstruction in selected patients by endoscopic ultrasonography (EUS)-guided transluminal biliary drainage. Endoscopy. 2007;39:292-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 64. | Khashab MA, Valeshabad AK, Modayil R, Widmer J, Saxena P, Idrees M, Iqbal S, Kalloo AN, Stavropoulos SN. EUS-guided biliary drainage by using a standardized approach for malignant biliary obstruction: rendezvous versus direct transluminal techniques (with videos). Gastrointest Endosc. 2013;78:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 65. | Ogura T, Sano T, Onda S, Imoto A, Masuda D, Yamamoto K, Kitano M, Takeuchi T, Inoue T, Higuchi K. Endoscopic ultrasound-guided biliary drainage for right hepatic bile duct obstruction: novel technical tips. Endoscopy. 2015;47:72-75. [PubMed] |
| 66. | Takada J, Carmo AM, Artifon EL. EUS-guided biliary drainage for malignant biliary obstruction in patients with failed ERCP. J Interv Gastroenterol. 2013;3:76-81. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Kawakubo K, Isayama H, Kato H, Itoi T, Kawakami H, Hanada K, Ishiwatari H, Yasuda I, Kawamoto H, Itokawa F. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci. 2014;21:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 68. | Prachayakul V, Aswakul P. A novel technique for endoscopic ultrasound-guided biliary drainage. World J Gastroenterol. 2013;19:4758-4763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Hara K, Yamao K, Hijioka S, Mizuno N, Imaoka H, Tajika M, Kondo S, Tanaka T, Haba S, Takeshi O. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy. 2013;45:392-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 70. | Song TJ, Hyun YS, Lee SS, Park do H, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided choledochoduodenostomies with fully covered self-expandable metallic stents. World J Gastroenterol. 2012;18:4435-4440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Kim TH, Kim SH, Oh HJ, Sohn YW, Lee SO. Endoscopic ultrasound-guided biliary drainage with placement of a fully covered metal stent for malignant biliary obstruction. World J Gastroenterol. 2012;18:2526-2532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Park do H, Jang JW, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 243] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 73. | Komaki T, Kitano M, Sakamoto H, Kudo M. Endoscopic ultrasonography-guided biliary drainage: evaluation of a choledochoduodenostomy technique. Pancreatology. 2011;11 Suppl 2:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 74. | Hara K, Yamao K, Niwa Y, Sawaki A, Mizuno N, Hijioka S, Tajika M, Kawai H, Kondo S, Kobayashi Y. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol. 2011;106:1239-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 75. | Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, Abadia MA, Pérez-Millán A, González-Huix F, Gornals J, Iglesias-Garcia J, De la Serna C, Aparicio JR. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: a Spanish national survey. Gastrointest Endosc. 2012;76:1133-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 76. | Attasaranya S, Netinasunton N, Jongboonyanuparp T, Sottisuporn J, Witeerungrot T, Pirathvisuth T, Ovartlarnporn B. The Spectrum of Endoscopic Ultrasound Intervention in Biliary Diseases: A Single Center’s Experience in 31 Cases. Gastroenterol Res Pract. 2012;2012:680753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Tarantino I, Barresi L, Repici A, Traina M. EUS-guided biliary drainage: a case series. Endoscopy. 2008;40:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Dhir V, Artifon EL, Gupta K, Vila JJ, Maselli R, Frazao M, Maydeo A. Multicenter study on endoscopic ultrasound-guided expandable biliary metal stent placement: choice of access route, direction of stent insertion, and drainage route. Dig Endosc. 2014;26:430-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 79. | Dhir V, Bhandari S, Bapat M, Joshi N, Vivekanandarajah S, Maydeo A. Comparison of transhepatic and extrahepatic routes for EUS-guided rendezvous procedure for distal CBD obstruction. United European Gastroenterol J. 2013;1:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 80. | Park do H, Jeong SU, Lee BU, Lee SS, Seo DW, Lee SK, Kim MH. Prospective evaluation of a treatment algorithm with enhanced guidewire manipulation protocol for EUS-guided biliary drainage after failed ERCP (with video). Gastrointest Endosc. 2013;78:91-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 81. | Kawakubo K, Isayama H, Sasahira N, Nakai Y, Kogure H, Hamada T, Miyabayashi K, Mizuno S, Sasaki T, Ito Y. Clinical utility of an endoscopic ultrasound-guided rendezvous technique via various approach routes. Surg Endosc. 2013;27:3437-3443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 82. | Dhir V, Bhandari S, Bapat M, Maydeo A. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos). Gastrointest Endosc. 2012;75:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 83. | Iwashita T, Lee JG, Shinoura S, Nakai Y, Park DH, Muthusamy VR, Chang KJ. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy. 2012;44:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 84. | Hamada T, Isayama H, Nakai Y, Kogure H, Yamamoto N, Kawakubo K, Takahara N, Uchino R, Mizuno S, Sasaki T. Transmural biliary drainage can be an alternative to transpapillary drainage in patients with an indwelling duodenal stent. Dig Dis Sci. 2014;59:1931-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 85. | Sarkaria S, Sundararajan S, Kahaleh M. Endoscopic ultrasonographic access and drainage of the common bile duct. Gastrointest Endosc Clin N Am. 2013;23:435-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 86. | Artifon EL, Frazão MS, Wodak S, Carneiro FO, Takada J, Rabello C, Aparício D, de Moura EG, Sakai P, Otoch JP. Endoscopic ultrasound-guided choledochoduodenostomy and duodenal stenting in patients with unresectable periampullary cancer: one-step procedure by using linear echoendoscope. Scand J Gastroenterol. 2013;48:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768-774. [PubMed] |
| 88. | Khashab MA, Valeshabad AK, Afghani E, Singh VK, Kumbhari V, Messallam A, Saxena P, El Zein M, Lennon AM, Canto MI. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 89. | Bapaye A, Dubale N, Aher A. Comparison of endosonography-guided vs. percutaneous biliary stenting when papilla is inaccessible for ERCP. United European Gastroenterol J. 2013;1:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 90. | Súbtil JC, Betes M, Muñoz-Navas M. Gallbladder drainage guided by endoscopic ultrasound. World J Gastrointest Endosc. 2010;2:203-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 91. | Itoi T, Coelho-Prabhu N, Baron TH. Endoscopic gallbladder drainage for management of acute cholecystitis. Gastrointest Endosc. 2010;71:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 92. | Jang JW, Lee SS, Song TJ, Hyun YS, Park do H, Seo DW, Lee SK, Kim MH, Yun SC. Endoscopic ultrasound-guided transmural and percutaneous transhepatic gallbladder drainage are comparable for acute cholecystitis. Gastroenterology. 2012;142:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 93. | Lee SS, Park do H, Hwang CY, Ahn CS, Lee TY, Seo DW, Lee SK, Kim MW. EUS-guided transmural cholecystostomy as rescue management for acute cholecystitis in elderly or high-risk patients: a prospective feasibility study. Gastrointest Endosc. 2007;66:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Song TJ, Park do H, Eum JB, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided cholecystoenterostomy with single-step placement of a 7F double-pigtail plastic stent in patients who are unsuitable for cholecystectomy: a pilot study (with video). Gastrointest Endosc. 2010;71:634-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 95. | Jang JW, Lee SS, Park do H, Seo DW, Lee SK, Kim MH. Feasibility and safety of EUS-guided transgastric/transduodenal gallbladder drainage with single-step placement of a modified covered self-expandable metal stent in patients unsuitable for cholecystectomy. Gastrointest Endosc. 2011;74:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 96. | de la Serna-Higuera C, Pérez-Miranda M, Gil-Simón P, Ruiz-Zorrilla R, Diez-Redondo P, Alcaide N, Sancho-del Val L, Nuñez-Rodriguez H. EUS-guided transenteric gallbladder drainage with a new fistula-forming, lumen-apposing metal stent. Gastrointest Endosc. 2013;77:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 97. | Choi JH, Lee SS, Choi JH, Park do H, Seo DW, Lee SK, Kim MH. Long-term outcomes after endoscopic ultrasonography-guided gallbladder drainage for acute cholecystitis. Endoscopy. 2014;46:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 98. | Teoh AY, Binmoeller KF, Lau JY. Single-step EUS-guided puncture and delivery of a lumen-apposing stent for gallbladder drainage using a novel cautery-tipped stent delivery system. Gastrointest Endosc. 2014;80:1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 99. | Widmer J, Alvarez P, Gaidhane M, Paddu N, Umrania H, Sharaiha R, Kahaleh M. Endoscopic ultrasonography-guided cholecystogastrostomy in patients with unresectable pancreatic cancer using anti-migratory metal stents: a new approach. Dig Endosc. 2014;26:599-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Giovannini M. EUS-guided pancreatic duct drainage: ready for prime time? Gastrointest Endosc. 2013;78:865-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Itoi T, Yasuda I, Kurihara T, Itokawa F, Kasuya K. Technique of endoscopic ultrasonography-guided pancreatic duct intervention (with videos). J Hepatobiliary Pancreat Sci. 2014;21:E4-E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Fabbri C, Luigiano C, Lisotti A, Cennamo V, Virgilio C, Caletti G, Fusaroli P. Endoscopic ultrasound-guided treatments: are we getting evidence based--a systematic review. World J Gastroenterol. 2014;20:8424-8448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 103. | Will U, Fueldner F, Thieme AK, Goldmann B, Gerlach R, Wanzar I, Meyer F. Transgastric pancreatography and EUS-guided drainage of the pancreatic duct. J Hepatobiliary Pancreat Surg. 2007;14:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 104. | Barkay O, Sherman S, McHenry L, Yoo BM, Fogel EL, Watkins JL, DeWitt J, Al-Haddad MA, Lehman GA. Therapeutic EUS-assisted endoscopic retrograde pancreatography after failed pancreatic duct cannulation at ERCP. Gastrointest Endosc. 2010;71:1166-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 105. | Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. EUS-guided pancreaticogastrostomy: analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2007;65:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 106. | Ergun M, Aouattah T, Gillain C, Gigot JF, Hubert C, Deprez PH. Endoscopic ultrasound-guided transluminal drainage of pancreatic duct obstruction: long-term outcome. Endoscopy. 2011;43:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 107. | Fujii LL, Topazian MD, Abu Dayyeh BK, Baron TH, Chari ST, Farnell MB, Gleeson FC, Gostout CJ, Kendrick ML, Pearson RK. EUS-guided pancreatic duct intervention: outcomes of a single tertiary-care referral center experience. Gastrointest Endosc. 2013;78:854-864.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 108. | Tessier G, Bories E, Arvanitakis M, Hittelet A, Pesenti C, Le Moine O, Giovannini M, Devière J. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 109. | Hisa T, Momoi T, Shimizu T, Furutake M, Takamatsu M, Ohkubo H. Endoscopic ultrasound-guided antegrade stone removal in a patient with pancreatic stones and anastomotic stricture after end-to-side pancreaticojejunostomy. Pancreatology. 2013;13:452-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 110. | Shah JN, Marson F, Weilert F, Bhat YM, Nguyen-Tang T, Shaw RE, Binmoeller KF. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc. 2012;75:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 111. | Sun S, Qingjie L, Qiyong G, Mengchun W, Bo Q, Hong X. EUS-guided interstitial brachytherapy of the pancreas: a feasibility study. Gastrointest Endosc. 2005;62:775-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 112. | Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 113. | Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 114. | Choi JH, Seo DW, Park do H, Lee SK, Kim MH. Fiducial placement for stereotactic body radiation therapy under only endoscopic ultrasonography guidance in pancreatic and hepatic malignancy: practical feasibility and safety. Gut Liver. 2014;8:88-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 115. | Law JK, Singh VK, Khashab MA, Hruban RH, Canto MI, Shin EJ, Saxena P, Weiss MJ, Pawlik TM, Wolfgang CL. Endoscopic ultrasound (EUS)-guided fiducial placement allows localization of small neuroendocrine tumors during parenchymal-sparing pancreatic surgery. Surg Endosc. 2013;27:3921-3926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 116. | Fernandez DC, Hoffe SE, Barthel JS, Vignesh S, Klapman JB, Harris C, Almhanna K, Biagioli MC, Meredith KL, Feygelman V. Stability of endoscopic ultrasound-guided fiducial marker placement for esophageal cancer target delineation and image-guided radiation therapy. Pract Radiat Oncol. 2013;3:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 117. | Khashab MA, Kim KJ, Tryggestad EJ, Wild AT, Roland T, Singh VK, Lennon AM, Shin EJ, Ziegler MA, Sharaiha RZ. Comparative analysis of traditional and coiled fiducials implanted during EUS for pancreatic cancer patients receiving stereotactic body radiation therapy. Gastrointest Endosc. 2012;76:962-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 118. | Gan SI, Thompson CC, Lauwers GY, Bounds BC, Brugge WR. Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest Endosc. 2005;61:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 119. | DeWitt J, DiMaio CJ, Brugge WR. Long-term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest Endosc. 2010;72:862-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 120. | Oh HC, Seo DW, Song TJ, Moon SH, Park do H, Soo Lee S, Lee SK, Kim MH, Kim J. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology. 2011;140:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 121. | Jürgensen C, Schuppan D, Neser F, Ernstberger J, Junghans U, Stölzel U. EUS-guided alcohol ablation of an insulinoma. Gastrointest Endosc. 2006;63:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 122. | Muscatiello N, Nacchiero M, Della Valle N, Di Terlizzi F, Verderosa G, Salcuni A, Macarini L, Cignarelli M, Castriota M, D’Agnessa V. Treatment of a pancreatic endocrine tumor by ethanol injection (PEI) guided by endoscopic ultrasound. Endoscopy. 2008;40 Suppl 2:E83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 123. | Deprez PH, Claessens A, Borbath I, Gigot JF, Maiter D. Successful endoscopic ultrasound-guided ethanol ablation of a sporadic insulinoma. Acta Gastroenterol Belg. 2008;71:333-337. [PubMed] |
| 124. | Vleggaar FP, Bij de Vaate EA, Valk GD, Leguit RJ, Siersema PD. Endoscopic ultrasound-guided ethanol ablation of a symptomatic sporadic insulinoma. Endoscopy. 2011;43 Suppl 2 UCTN:E328-E329. [PubMed] |
| 125. | Levy MJ, Thompson GB, Topazian MD, Callstrom MR, Grant CS, Vella A. US-guided ethanol ablation of insulinomas: a new treatment option. Gastrointest Endosc. 2012;75:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 126. | Barclay RL, Perez-Miranda M, Giovannini M. EUS-guided treatment of a solid hepatic metastasis. Gastrointest Endosc. 2002;55:266-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 127. | Hu YH, Tuo XP, Jin ZD, Liu Y, Guo Y, Luo L. Endoscopic ultrasound (EUS)-guided ethanol injection in hepatic metastatic carcinoma: a case report. Endoscopy. 2010;42 Suppl 2:E256-E257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 128. | Artifon EL, Lucon AM, Sakai P, Gerhardt R, Srougi M, Takagaki T, Ishioka S, Bhutani MS. EUS-guided alcohol ablation of left adrenal metastasis from non-small-cell lung carcinoma. Gastrointest Endosc. 2007;66:1201-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 129. | DeWitt J, Mohamadnejad M. EUS-guided alcohol ablation of metastatic pelvic lymph nodes after endoscopic resection of polypoid rectal cancer: the need for long-term surveillance. Gastrointest Endosc. 2011;74:446-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 130. | Chang KJ, Nguyen PT, Thompson JA, Kurosaki TT, Casey LR, Leung EC, Granger GA. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer. 2000;88:1325-1335. [PubMed] |
| 131. | Hecht JR, Farrell JJ, Senzer N, Nemunaitis J, Rosemurgy A, Chung T, Hanna N, Chang KJ, Javle M, Posner M. EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: a phase I/II study. Gastrointest Endosc. 2012;75:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 132. | Chang KJ, Reid T, Senzer N, Swisher S, Pinto H, Hanna N, Chak A, Soetikno R. Phase I evaluation of TNFerade biologic plus chemoradiotherapy before esophagectomy for locally advanced resectable esophageal cancer. Gastrointest Endosc. 2012;75:1139-46.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 133. | Citrin D, Camphausen K, Wood BJ, Quezado M, Denobile J, Pingpank JF, Royal RE, Alexander HR, Seidel G, Steinberg SM. A pilot feasibility study of TNFerade™ biologic with capecitabine and radiation therapy followed by surgical resection for the treatment of rectal cancer. Oncology. 2010;79:382-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 134. | Mulvihill S, Warren R, Venook A, Adler A, Randlev B, Heise C, Kirn D. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther. 2001;8:308-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 199] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 135. | Linghu E, Matthes K, Mino-Kenudson M, Brugge WR. Feasibility of endoscopic ultrasound-guided OncoGel (ReGel/paclitaxel) injection into the pancreas in pigs. Endoscopy. 2005;37:1140-1142. [PubMed] |
| 136. | Matthes K, Mino-Kenudson M, Sahani DV, Holalkere N, Fowers KD, Rathi R, Brugge WR. EUS-guided injection of paclitaxel (OncoGel) provides therapeutic drug concentrations in the porcine pancreas (with video). Gastrointest Endosc. 2007;65:448-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 137. | Vukelja SJ, Anthony SP, Arseneau JC, Berman BS, Cunningham CC, Nemunaitis JJ, Samlowski WE, Fowers KD. Phase 1 study of escalating-dose OncoGel (ReGel/paclitaxel) depot injection, a controlled-release formulation of paclitaxel, for local management of superficial solid tumor lesions. Anticancer Drugs. 2007;18:283-289. [PubMed] |
| 138. | Levy MJ, Alberts SR, Chari ST, Farnell MB, Haddock MG, Kendrick ML, Mukhopadhyay D, Petersen GM, Takahashi N. 716 EUS guided intra-tumoral gemcitabine therapy for locally advanced and metastatic pancreatic cancer. Gastrointestinal Endoscopy. 2011;73:AB144-AB145. [DOI] [Full Text] |
| 139. | Irisawa A, Takagi T, Kanazawa M, Ogata T, Sato Y, Takenoshita S, Ohto H, Ohira H. Endoscopic ultrasound-guided fine-needle injection of immature dendritic cells into advanced pancreatic cancer refractory to gemcitabine: a pilot study. Pancreas. 2007;35:189-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 140. | Hirooka Y, Itoh A, Kawashima H, Hara K, Nonogaki K, Kasugai T, Ohno E, Ishikawa T, Matsubara H, Ishigami M. A combination therapy of gemcitabine with immunotherapy for patients with inoperable locally advanced pancreatic cancer. Pancreas. 2009;38:e69-e74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 141. | Sethi A, Ellrichmann M, Dhar S, Hadeler KG, Kahle E, Seehusen F, Klapper W, Habib N, Fritscher-Ravens A. Endoscopic ultrasound-guided lymph node ablation with a novel radiofrequency ablation probe: feasibility study in an acute porcine model. Endoscopy. 2014;46:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 142. | Kim HJ, Seo DW, Hassanuddin A, Kim SH, Chae HJ, Jang JW, Park do H, Lee SS, Lee SK, Kim MH. EUS-guided radiofrequency ablation of the porcine pancreas. Gastrointest Endosc. 2012;76:1039-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 143. | Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4038] [Cited by in RCA: 3766] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 144. | Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4122] [Cited by in RCA: 3554] [Article Influence: 253.9] [Reference Citation Analysis (0)] |
| 145. | Chan HH, Nishioka NS, Mino M, Lauwers GY, Puricelli WP, Collier KN, Brugge WR. EUS-guided photodynamic therapy of the pancreas: a pilot study. Gastrointest Endosc. 2004;59:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 146. | Di Matteo F, Grasso R, Pacella CM, Martino M, Pandolfi M, Rea R, Luppi G, Silvestri S, Zardi E, Costamagna G. EUS-guided Nd: YAG laser ablation of a hepatocellular carcinoma in the caudate lobe. Gastrointest Endosc. 2011;73:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 147. | Prasad GA, Varadarajulu S. Endoscopic ultrasound-guided abscess drainage. Gastrointest Endosc Clin N Am. 2012;22:281-290, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 148. | Hadithi M, Bruno MJ. Endoscopic ultrasound-guided drainage of pelvic abscess: A case series of 8 patients. World J Gastrointest Endosc. 2014;6:373-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 149. | Puri R, Choudhary NS, Kotecha H, Shah SP, Paliwal M, Misra SR, Bhagat S, Madan K, Saraf N, Sud R. Endoscopic ultrasound-guided pelvic and prostatic abscess drainage: experience in 30 patients. Indian J Gastroenterol. 2014;33:410-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 150. | Ramesh J, Bang JY, Trevino J, Varadarajulu S. Comparison of outcomes between endoscopic ultrasound-guided transcolonic and transrectal drainage of abdominopelvic abscesses. J Gastroenterol Hepatol. 2013;28:620-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 151. | Puri R, Eloubeidi MA, Sud R, Kumar M, Jain P. Endoscopic ultrasound-guided drainage of pelvic abscess without fluoroscopy guidance. J Gastroenterol Hepatol. 2010;25:1416-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 152. | Varadarajulu S, Drelichman ER. Effectiveness of EUS in drainage of pelvic abscesses in 25 consecutive patients (with video). Gastrointest Endosc. 2009;70:1121-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 153. | Giovannini M, Bories E, Moutardier V, Pesenti C, Guillemin A, Lelong B, Delpéro JR. Drainage of deep pelvic abscesses using therapeutic echo endoscopy. Endoscopy. 2003;35:511-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 154. | Consiglieri CF, Escobar I, Gornals JB. EUS-guided transesophageal drainage of a mediastinal abscess using a diabolo-shaped lumen-apposing metal stent. Gastrointest Endosc. 2015;81:221-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 155. | Saxena P, Kumbhari V, Khashab MA. EUS-guided drainage of a mediastinal abscess. Gastrointest Endosc. 2014;79:998-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 156. | Lee DH, Cash BD, Womeldorph CM, Horwhat JD. Endoscopic therapy of a splenic abscess: definitive treatment via EUS-guided transgastric drainage. Gastrointest Endosc. 2006;64:631-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 157. | Rana SS, Chaudhary V, Sharma V, Sharma R, Dutta U, Bhasin DK. Infected pancreatic pseudocyst of spleen successfully treated by combined endoscopic transpapillary stent placement and transmural aspiration. Gastrointest Endosc. 2014;79:360-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 158. | Alcaide N, Vargas-Garcia AL, de la Serna-Higuera C, Sancho del Val L, Ruiz-Zorrilla R, Perez-Miranda M. EUS-guided drainage of liver abscess by using a lumen-apposing metal stent (with video). Gastrointest Endosc. 2013;78:941-942; discussion 942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 159. | Noh SH, Park do H, Kim YR, Chun Y, Lee HC, Lee SO, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided drainage of hepatic abscesses not accessible to percutaneous drainage (with videos). Gastrointest Endosc. 2010;71:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 160. | Levy MJ, Chari ST, Wiersema MJ. Endoscopic ultrasound-guided celiac neurolysis. Gastrointest Endosc Clin N Am. 2012;22:231-47, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 161. | Stevens T, Costanzo A, Lopez R, Kapural L, Parsi MA, Vargo JJ. Adding triamcinolone to endoscopic ultrasound-guided celiac plexus blockade does not reduce pain in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2012;10:186-191, 191.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 162. | Gress F. Endoscopic ultrasound-guided celiac plexus neurolysis. Gastroenterol Hepatol (N Y). 2007;3:279-281. [PubMed] |
| 163. | Levy M, Rajan E, Keeney G, Fletcher JG, Topazian M. Neural ganglia visualized by endoscopic ultrasound. Am J Gastroenterol. 2006;101:1787-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 164. | Levy MJ, Topazian MD, Wiersema MJ, Clain JE, Rajan E, Wang KK, de la Mora JG, Gleeson FC, Pearson RK, Pelaez MC. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct Ganglia neurolysis and block. Am J Gastroenterol. 2008;103:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 165. | Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, Irisawa A, Mukai T, Katanuma A, Kubota K, Ohnishi T. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy. 2013;45:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 166. | Gress F, Schmitt C, Sherman S, Ikenberry S, Lehman G. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol. 1999;94:900-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 209] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 167. | LeBlanc JK, Al-Haddad M, McHenry L, Sherman S, Juan M, McGreevy K, Johnson C, Howard TJ, Lillemoe KD, DeWitt J. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: one injection or two? Gastrointest Endosc. 2011;74:1300-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 168. | Santosh D, Lakhtakia S, Gupta R, Reddy DN, Rao GV, Tandan M, Ramchandani M, Guda NM. Clinical trial: a randomized trial comparing fluoroscopy guided percutaneous technique vs. endoscopic ultrasound guided technique of coeliac plexus block for treatment of pain in chronic pancreatitis. Aliment Pharmacol Ther. 2009;29:979-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 169. | Wilcox CM. Tinkering with a tarnished technique: isn’t it time to abandon celiac plexus blockade for the treatment of abdominal pain in chronic pancreatitis? Clin Gastroenterol Hepatol. 2012;10:106-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 170. | Sun S. Endoscopic ultrasound’s vision: Probing our way to NOTES. Endosc Ultrasound. 2014;3:141-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |