Published online May 16, 2015. doi: 10.4253/wjge.v7.i5.573
Peer-review started: October 8, 2014
First decision: November 14, 2014
Revised: January 3, 2015
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: May 16, 2015
Processing time: 222 Days and 6.1 Hours
We report an unexpected, previously unreported complication of Bravo pH capsule dislodgement. During Bravo pH testing of a 44-year-old man with gastroesophageal reflux disease, we were unable to endoscopically visualize the capsule attached to the esophageal wall after deployment. After multiple attempts to detect the capsule, it was visualized in the left pyriform sinus. As there was significant risk for pulmonary dislodgement, ENT and pulmonary physicians were immediately consulted to review options for safe removal. Ultimately, ENT successfully retrieved the capsule with a foreign body removal forceps. The Bravo pH test is generally a well-tolerated diagnostic tool used to confirm the presence of abnormal esophageal acid reflux. While few complications have been reported, technical difficulties can occur, including poor data reception, misplacement, and early dislodgement. Rarely, more serious complications can occur, ranging from esophageal wall trauma to capsule aspiration. Gastroenterologists performing this procedure should be aware of the low, but non-trivial, risk of complications.
Core tip: We report an unexpected, and so far unreported, complication of a Bravo pH capsule dislodgment. While Bravo probe placement is generally a well-tolerated procedure, dislodgment into the pyriform sinus in this case necessitated immediate action by an interdisciplinary team. Complications of Bravo capsule use range from technical difficulties, such as poor data reception and non-deployment, to more serious events such as esophageal wall trauma and capsule aspiration. Gastroenterologists performing this procedure should be aware of the risk of potential complications.
- Citation: Kumar A, Kramer E, Chokhavatia S. Unreported complication of Bravo pH capsule dislodged into the pyriform sinus. World J Gastrointest Endosc 2015; 7(5): 573-574
- URL: https://www.wjgnet.com/1948-5190/full/v7/i5/573.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i5.573
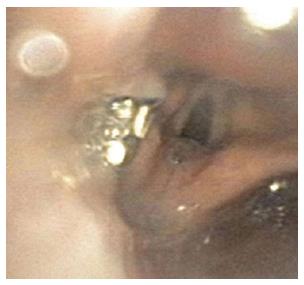
We report an unanticipated, previously undocumented complication of Bravo capsule dislodgement. A forty-five year old patient with gastroesophageal reflux disease (GERD), non-compliant with medical therapy, presented with increasing cough, hoarseness, and other acid reflux symptoms. To verify presence of acid reflux, he underwent upper endoscopy and Bravo pH testing at our hospital. The gastroesophageal junction (Z line) was visualized at a distance of 40.0 cm from dentition. The Bravo device was deployed at 34.0 cm from dentition (6 cm above the Z line). When the capsule was not endoscopically visualized to be adherent to the esophageal wall, the endoscope was advanced beyond 34.0 cm to assess for possible device movement to the distal esophagus or stomach. When the capsule was not visualized at these locations, the endoscope was withdrawn. When the scope was withdrawn from the upper esophageal sphincter, the device was seen in the left pyriform sinus (Figure 1). The nonadherent capsule likely was either pulled up by the endoscope during withdrawal or coughed up by the patient. ENT and pulmonary physicians were immediately consulted for assistance in ensuring safe removal of the capsule from this precarious location, as there was significant risk for pulmonary dislodgement. After the anesthesiologist performed endotracheal intubation, ENT successfully retrieved the capsule with a foreign body removal forceps without further complications.
The Bravo pH test is generally a well-tolerated diagnostic tool that can verify the presence of abnormal esophageal acid reflux and determine if treatment refractory symptoms are due to persistent acid reflux in patients with GERD. As the deployment of the Bravo pH device is typically a innocuous procedure[1], very few complications have been reported. Technical difficulties most commonly include non-deployment, non-attachment, misplacement, premature dislodgement, and insufficient data reception. Infrequently, patients develop significant chest pain after capsule placement[2], necessitating removal. Rarely, more serious complications can occur in less than 2% and include esophageal wall trauma, excessive bleeding, and capsule aspiration[3]. In one reported case, the patient aspirated the capsule into the bronchus immediately after deployment, causing retching, heavy coughing, and desaturation to 74%[4]. After initial pushing into stomach with a transnasal video-endoscope, this capsule was removed with grasping forceps.
Gastroenterologists using the Bravo pH test should be cognizant of the low but non-trivial risk of complications, ranging from technical difficulties to aspiration of a dislodged capsule. Providers can use reports of documented complications to troubleshoot and resolve difficulties that may arise during deployment of Bravo pH capsules.
P- Reviewer: Amornyotin S, Rabago L S- Editor: Tian YL L- Editor: A E- Editor: Wu HL
| 1. | Wenner J, Johnsson F, Johansson J, Oberg S. Wireless oesophageal pH monitoring: feasibility, safety and normal values in healthy subjects. Scand J Gastroenterol. 2005;40:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | de Hoyos A, Esparza EA. Technical problems produced by the Bravo pH test in nonerosive reflux disease patients. World J Gastroenterol. 2010;16:3183-3186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | von Renteln D, Kayser T, Riecken B, Caca K. An unusual case of Bravo capsule aspiration. Endoscopy. 2008;40 Suppl 2:E174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Ho KK, Joyce AM. Complications of capsule endoscopy. Gastrointest Endosc Clin N Am. 2007;17:169-178, viii-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |