Published online May 16, 2015. doi: 10.4253/wjge.v7.i5.555
Peer-review started: November 19, 2014
First decision: December 29, 2014
Revised: January 28, 2015
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: May 16, 2015
Processing time: 180 Days and 21.9 Hours
AIM: To compare the yield of adenomas between narrow band imaging and white light when using high definition/magnification.
METHODS: This prospective, non-randomized comparative study was performed at the endoscopy unit of veteran affairs medical center in Phoenix, Arizona. Consecutive patients undergoing first average risk colorectal cancer screening colonoscopy were selected. Two experienced gastroenterologists performed all the procedures that were blinded to each other’s findings. Demographic details were recorded. Data are presented as mean ± SEM. Proportional data were compared using the χ2 test and means were compared using the Student’s t test. Tandem colonoscopy was performed in a sequential and segmental fashion using one of 3 strategies: white light followed by narrow band imaging [Group A: white light (WL) → narrow band imaging (NBI)]; narrow band imaging followed by white light (Group B: NBI → WL) and, white light followed by white light (Group C: WL → WL). Detection rate of missed polyps and adenomas were evaluated in all three groups.
RESULTS: Three hundred patients were studied (100 in each Group). Although the total time for the colonoscopy was similar in the 3 groups (23.8 ± 0.7, 22.2 ± 0.5 and 24.1 ± 0.7 min for Groups A, B and C, respectively), it reached statistical significance between Groups B and C (P < 0.05). The cecal intubation time in Groups B and C was longer than for Group A (6.5 ± 0.4 min and 6.5 ± 0.4 min vs 4.9 ± 0.3 min; P < 0.05). The withdrawal time for Groups A and C was longer than Group B (18.9 ± 0.7 min and 17.6 ± 0.6 min vs 15.7 ± 0.4 min; P < 0.05). Overall miss rate for polyps and adenomas detected in three groups during the second look was 18% and 17%, respectively (P = NS). Detection rate for polyps and adenomas after first look with white light was similar irrespective of the light used during the second look (WL → WL: 13.7% for polyps, 12.6% for adenomas; WL → NBI: 14.2% for polyps, 11.3% for adenomas). Miss rate of polyps and adenomas however was significantly higher when NBI was used first (29.3% and 30.3%, respectively; P < 0.05). Most missed adenomas were ≤ 5 mm in size. There was only one advanced neoplasia (defined by size only) missed during the first look.
CONCLUSION: Our data suggest that the tandem nature of the procedure rather than the optical techniques was associated with the detection of additional polyps’ and adenomas.
Core tip: The role of narrow band imaging for polyp detection is controversial. We studied 3 groups of 100 patients each, undergoing tandem colonoscopy by (1) white light followed by narrow band imaging; (2) narrow band followed by white light; and (3) white light followed by white light. Detection rate for polyps with white light used first was similar irrespective of the light used afterwards. Miss rate of polyps and adenomas was higher when narrow band imaging was used first (29.3% and 30.3%, respectively; P < 0.05). Our study suggests that the tandem nature of colonoscopy rather than the optical techniques, detects missing pathology.
- Citation: Gilani N, Stipho S, Panetta JD, Petre S, Young MA, Ramirez FC. Polyp detection rates using magnification with narrow band imaging and white light. World J Gastrointest Endosc 2015; 7(5): 555-562
- URL: https://www.wjgnet.com/1948-5190/full/v7/i5/555.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i5.555
Colonoscopy and polypectomy is aimed at prevention or identification of early colorectal cancer[1-3]. Colonoscopy however is not infallible in the detection of polyps and adenomas with reported miss rates in the order of 14% to 32% using tandem colonoscopy[4-10] and 23.3% for lesions (polyps and cancers) in resected colonic specimens[11]. Advances in the optics of endoscopy such as high definition, magnification and narrow band imaging have been introduced in clinical practice, and amongst others, are aimed at improving the yield of polyp and adenoma detection[12-20]. Our hypothesis was that narrow band imaging (NBI) detects more polyps and adenomas than white light (WL) when used in a tandem fashion during screening colonoscopy.
As part of a quality improvement assessment of new technology, we sought to assess the yield of polyp and tubular adenoma detection when using wide angle magnification colonoscopy either with narrow band imaging or white light in average risk patients referred for their first colorectal cancer screening colonoscopy. These procedures were performed using the Olympus 180 H series colonoscopies (Olympus America Inc., Center Valley, PA). Cecal intubation was carried out using WL and without magnification. Once the cecum was reached, the electronic magnification featured at 1.5X was turned on. All these procedures were performed by one of two experienced board certified gastroenterologists (who had performed > 2500 and > 5000 colonoscopies each and > 250 colonoscopies using narrow band imaging) using one of the following strategies: (1) Group A: white light followed by narrow band imaging (WL → NBI); (2) Group B: narrow band imaging followed by white light (NBI → WL) and; (3) Group C: white light followed by white light (WL → WL) in a sequential and segmental fashion of tandem endoscopy every 15-20 cm. Measurements included: cecal intubation, withdrawal and total procedure times; grading of bowel preparation; anatomical location, size and histological diagnosis of polyps detected with white light or NBI, when using either of the strategies. Removed polypoid lesions, were classified based on histology as neoplastic (adenomas, hyperplastic and other tumors) and non-neoplastic (normal mucosa, hyperplastic mucosa, prominent lymphoid aggregates).
Patients underwent bowel cleansing with 4 L of polyethylene glycol solution and 4 bisacodyl tablets (20 mg total dose). Patients with suboptimal preparation (as determined by the colonoscopist during the insertion portion of the examination) were not included in the study. All procedures except four were performed using moderate sedation with incremental doses of midazolam and meperidine or fentanyl. Cecal intubation was confirmed by photo documentation of appendiceal orifice and ileocecal valve. Procedure times (cecal intubation, withdrawal and total procedure times) were documented by the Olympus stopwatch built in the processors. The watch was not stopped for rinsing and cleaning or while performing polypectomy. Polyp’s size was estimated using an open biopsy forceps or the snare used for polypectomy. All polyps were removed during the withdrawal portion of the procedure even if visualized during the insertion phase. Colon was anatomically divided into proximal (proximal to splenic flexure) and distal (splenic flexure or distal to it) portions. Advanced neoplasia was defined as the presence of a tubular adenoma ≥ 10 mm, villous component, or the presence of high grade dysplasia or invasive carcinoma on histology.
The study was approved by the local Institutional Review Board and exemption for informed consent was granted due to non-randomized design and the fact that all patients underwent standard white light colonoscopy. However, informed consent for colonoscopy was obtained from all patients undergoing procedures.
SPSS 16.0 was used for statistical analysis. Data are presented as mean ± SEM. Proportional data were compared using the χ2 test and means were compared using the Student’s t test.
Three-hundred patients, 100 consecutive in each Group were studied. Table 1 shows the demographics, adequacy of bowel preparation and procedure-related times in each group. Although the total time for the colonoscopy was similar in the 3 groups, it reached statistical significance between Groups B and C (P < 0.05). The cecal intubation time in Groups B and C was longer than for Group A. The withdrawal time for Groups A and C was longer than Group B (P < 0.05).
| Group A(WL→NBI) | Group B(NBI→WL) | Group C(WL→WL) | P < 0.05 | |
| Age (mean ± SEM) in years | 62.2 ± 0.7 | 59.3 ± 0.6 | 62.0 ± 0.7 | |
| Gender | ||||
| Men | 99 | 98 | 98 | |
| Women | 1 | 2 | 2 | |
| Cecal intubation time (mean ± SEM) in minute | 4.9 ± 0.3 | 6.5 ± 0.4 | 6.5 ± 0.4 | A vs C A vs B |
| Withdrawal time (mean ± SEM) in minute | 18.9 ± 0.7 | 15.7 ± 0.4 | 17.6 ± 0.6 | A vs B C vs B |
| Total procedure time (mean ± SEM) in minute | 23.8 ± 0.7 | 22.2 ± 0.5 | 24.1 ± 0.7 | B vs C |
| Bowel preparation | ||||
| Excellent | 36 (%) | 18 (%) | 22 (%) | |
| Good | 56 (%) | 74 (%) | 67 (%) | |
| Fair adequate | 8 (%) | 8 (%) | 11 (%) | |
| Patients with polyps | 78 | 67 | 73 | |
| Total polyps detected | 211 | 147 | 219 | |
| Polyps/patient with polyps | 2.7 | 2.2 | 3.0 | |
| Of patients with adenomas | 47 | 47 | 57 | |
| Total adenomas detected | 97 | 76 | 111 | |
| Adenomas/ patient with adenomas | 2.1 | 1.6 | 1.9 | |
In Group A, (WL → NBI) 211 polyps were detected in 78 patients (2.7 polyps/ patient); in Group B (NBI → WL) 147 polyps were detected in 67 patients (2.2 polyps/ patient) whereas in Group C (WL → WL) 219 polyps were detected in 73 patients (3.0 polyps/ patient). Adenomas were detected in 151 patients (50% of all patients) and similar in the 3 groups (47%, 47% and 57% for Groups A, B and C, respectively).
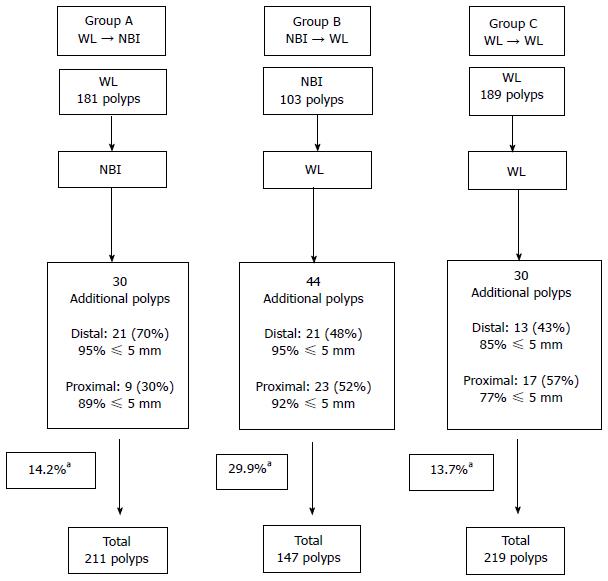
As shown in Figure 1, in Group A (WL → NBI), the withdrawal with WL detected 181 polyps (62.4% distal and 37.6% proximal). Of those detected distally, 89.4% were ≤ 5 mm in size; 7.1%, 6-9 mm in size and, 3.5%, ≥ 1 cm. Of those detected proximally, 72% were ≤ 5 mm in size; 17.7%, 6-9 mm and, 10.3%, ≥ 1 cm in size. Switching to NBI detected 30 additional polyps (14.2% of all polyps detected in Group A) of which 70% were distal and 30% proximal. Ninety-five percent and 89% of the newly detected distal and proximal polyps were ≤ 5 mm in size, respectively.
In Group B (NBI → WL), the first withdrawal with NBI detected 103 polyps (59.2% distal and 40.8% proximal). Of those detected distally, 91.8% were ≤ 5 mm; 6.6%, 6-9 mm and, 1.6% was ≥ 1 cm in size. Of those polyps detected proximally, 83.3% were ≤ 5 mm; 14.3%, 6-9 mm and, 2.4%, ≥ 10 mm in size. Switching to WL detected 44 additional polyps (30.8% of all polyps detected in Group B) of which 48% were distal and 52% proximal. Ninety-five percent and 92% of the newly detected distal and proximal polyps were ≤ 5 mm in size, respectively.
In Group C, (WL → WL) the first withdrawal with white light detected 189 polyps (61.9% distal and 38.1% proximal). Of the polyps detected distally, 76.9% were ≤ 5 mm in size, 17.1% were 6-9 mm and the remaining 6% were ≥ 10 mm in size. Of the polyps detected proximally, 65.3% were ≤ 5 mm in size, 23.6% were 6-9 mm and 11.1% were ≥ 10 mm. When the second look with white light again was used, 30 additional polyps (13.7% of all polyps in Group C) were detected and of which 56.7% were proximal and 43.3% distal. Eighty-five percent and 76.5% of the polyps newly found in the distal and proximal colon were ≤ 5 mm in size, respectively.
The newly diagnosed polyps detected with NBI (Group A, 14.2%) and white light (Group C, 13.7%) during the second look were significantly fewer than the ones detected using the WL after NBI (Group B, 30.8%) (P < 0.05). Overall, the second look of the tandem segmental colonoscopy detected 18% new polyps (104/577 polyps).
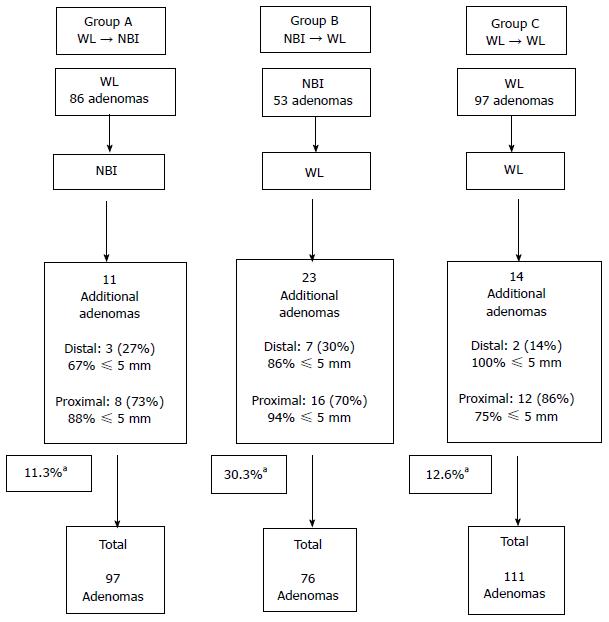
As can be seen in Figure 2, In Group A (WL → NBI), the first withdrawal with WL detected 86 adenomas: 50 (58.1%) proximal (64%: ≤ 5 mm in size, 20%: 6-9 mm and, 14%: ≥ 10 mm in size) and, 36 (41.9%) distal (77.8%: ≤ 5 mm, 13.9%: 6-9 mm and 8.3%: ≥ 10 mm). Switching to NBI detected 11 additional adenomas (11.3% of all adenomas detected in Group A): 8 (73%) proximal (88%: ≤ 5 mm and 12%: 6-9 mm), and 3 (27%) distal (67%: ≤ 5 mm and 37%: 6-9 mm).
In Group B (NBI → WL), the first withdrawal with NBI detected 53 adenomas: 34 (64.2%) proximal (79.4%: ≤ 5 mm, 17.6%: 6-9 mm and 2.9%: ≥ 10 mm) and 19 (35.8%) distal (89.5%: ≤ 5 mm, 5.3%: 6-9 mm and 5.3%: ≥ 10 mm). Switching to WL detected 23 additional adenomas (30.3% of all adenomas detected in Group B): 16 (70%) proximal (94%: ≤ 5 mm, 6%: ≥ 10 mm) and 7 (30%) distal (86%: ≤ 5 mm, 14%: 6-9 mm).
In Group C (WL → WL), there were 97 adenomas detected by white light during the first withdrawal: 61 (62.9%) proximal (70.5%: < 5 mm, 23%: 6-9 mm, 6.5%: > 10 mm) and 36 (37.1%) distal (58.3%: ≤ 5 mm, 25%: 6-9 mm, 16.7%: ≥ 10 mm). During the second withdrawal with white light, 14 additional adenomas were detected (12.6% of all adenomas detected in Group C): 12 (85.7%) proximal (75%: ≤ 5 mm, 25%: 6-9 mm) and 2 (14.3%) distal (100%: ≤ 5 mm).
The newly diagnosed adenomas detected with NBI (Group A, 11.3%) and WL (Group C, 12.6%) during the second look were significantly fewer than those detected using the WL after NBI (Group B, 30.3%) (P < 0.05). The second look of the tandem segmental colonoscopy thus, detected 16.9% new adenomas (48 out of 284 adenomas).
In Group A (WL → NBI), there were 8 patients (10 polyps) with advanced neoplasia (all defined by size ≥ 10 mm only). None of these advanced neoplasias were detected during the second look performed by NBI. In Group B (NBI → WL), there were 3 patients (3 polyps) with advanced neoplasia (all defined by size ≥ 10 mm only), and one of these (10 mm polyp in ascending colon) was detected during the second look using WL. In Group C (WL → WL), there were 9 patients (11 polyps) with advanced neoplasia including 1 villous adenoma in the sigmoid and 1 invasive carcinoma in the rectum (the remaining 9 adenomas were defined as advanced neoplasia by a size ≥ 10 mm). None of the advanced neoplasias were detected during the second look with WL.
When NBI was used as the second look, it diagnosed 2 patients (1 adenoma each) that otherwise would have been diagnosed as having no adenomas at all and representing 4.3% (2 out of 47) of patients with adenomas. When WL was used as the second look, it identified 8 patients that otherwise would have been missed as having any adenomas (6 of these had single adenomas, and the other 2 had 2 adenomas each) and representing a pick up rate of 17% (8 out of 47 patients with adenomas). For Group C, when a second look with white light was performed, 5 patients (1 adenoma each) were detected that otherwise would have been missed as having any adenomas at all and representing 10.6% (5 out of 57) of patients with adenomas. The differences among the groups were not statistically significant. None of these patients in either group had advanced neoplasia that would have been undetected at all.
Non-neoplastic polyps represented 17.8% (103/577) of all polyps and were similarly distributed among the 3 groups.
There was one case of post-polypectomy bleeding requiring admission and endoscopic intervention to secure hemostasis with endoscopic clips. There were no sedation-related complications.
The impact of new optical technologies such as high-definition, magnification and NBI on polyp detection rate is unknown. Tandem colonoscopy studies have yielded an additional detection rate up to 22% for adenomas and 27% for non-adenomas[9]. Our study showed that the detection rate of missed polyps and adenomas after a first look with white light was similar when using narrow band imaging (14.2% for polyps, 11.3% for adenomas) or white light (13.7% for polyps and 12.6% for adenomas) as the second look modality. We also found that when white light was used after narrow band imaging, the detection rate of missed polyps (29.9%) and adenomas (30.3%) was higher in comparison to where white light was used as first modality. The explanation for this unexpected finding is not completely clear. To further address this issue, we studied 100 additional consecutive patients undergoing screening colonoscopy using the following strategy (NBI → NBI → WL). Out of 198 polyps (92 adenomas) detected, the second look with NBI added 24 new polyps (7 adenomas) and the “third look” with WL added 28 additional polyps (15 adenomas) representing 12.1% polyps and 7.6% adenomas with the second NBI look and, 14.1% polyps and 16.3% adenomas with the “third look” using WL. Thus, the combined miss rate after a first look with NBI (26.2% and 23.3% for polyps and adenomas, respectively) was similar to the one reported in the present study when using the NBI → WL strategy. In our study, the bowel cleanliness was not associated with improved polyp detection. Another shortcoming of NBI appears to be relative poor visualization unless endoscope is held closer (more so than the WL) to the inspected area.
The adenoma miss rate in the tandem colonoscopy studies is inversely related to the size and directly related to the number detected during the first look[9]. In a prospective multicenter study[10] of tandem colonoscopy the miss rates for polyps, adenomas, polyps > 5 mm, adenomas > 5 mm and advanced neoplasia was 28%, 20%, 12%, 9% and 11%, respectively. The sessile or flat shape and left colonic location were associated with higher miss rates. Interestingly, in that study, not all recto-sigmoid polyps (thought to be hyperplastic) were removed. The explanation for rather significant and fairly similar miss rates reported by experienced endoscopists remains speculative at best. Operator’s-related factors that may influence the miss rate include: technique, rate of withdrawal, difference in recognition of pathology (only applicable when two different endoscopists with different levels of expertise are involved) and thus related to inter-observer variability, a more careful look performed by the second endoscopist because of the prior knowledge of the goals/objectives of the study (bias). Other factors may be polyp-related: location (i.e., behind folds) that possibly becomes “more exposed” to the second look and, estimated polyp size; and/or, bowel preparation-related: a cleaner colon resultant from the cleaning performed during the first look. Optical enhancements in endoscopy are expected to reduce the miss rate of both polyps/adenomas; better predict histology and, enhance demarcation of neoplastic tissue and thus improve the rate of complete polypectomy. The development of these technologies in part, is in response to the lack of complete protection against interval cancer development[21], polyp detection and clearance such as adequacy of bowel preparation[22,23], operator’s expertise and completeness (cecal intubation) of examination[24-28], adequate withdrawal times[29,30], incomplete polyp resection[31,32] and inherent limitations of the colonoscopy itself[33-35]. NBI was initially reported to increase the yield of detection of polyps and adenomas[12,15,17,19,20]. The studies investigating the role of NBI in the detection of colonic polyps have yielded controversial results. In a study[36], of 40 patients undergoing screening colonoscopy, NBI detected 51 additional polyps (41.5% of total polyps) and 29 adenomas (40.3% of total adenomas). The polyp/adenoma miss rate appeared somewhat higher than what has been reported in the literature (10%-20%), even if a potential gain provided by NBI from 5% to 15% was added. The study included WL → NBI arm but lacked NBI → WL and WL → WL) arms. In another study[15], NBI detected numerically more adenomas (23%) than conventional endoscopy (17%). However, procedures were not performed in a tandem fashion. There also appeared to be a learning effect upon adenoma recognition/detection due to involvement of multiple endoscopists, some with less experience even in conventional endoscopy. In a randomized controlled study[37], again, tandem colonoscopy was not performed, and thus the miss rate with each of the lights remained unknown. Nevertheless, in that study the authors found no difference in the detection rates of overall adenomas or adenomas of any size. To compare, detection rate for adenomas in our group of 300 patients was 50% (range: 47% to 57%) which is similar to the above mentioned study[37]. This may suggest that in the hand of experienced endoscopists with a high detection rate, NBI may not have an added benefit. In another randomized study comparing conventional vs pan-colonic narrow band imaging[38], NBI detected significantly more adenomas, especially diminutive (< 5 mm) in the distal colon without compromising the withdrawal time than conventional colonoscopy. The main limitation of the study again was the lack of tandem colonoscopy. Finally, a randomized tandem colonoscopy study[39] comparing NBI → WL vs WL → WL showed that there were no significant differences either in the miss or detection rates between two modalities (12.6% miss rate in NBI and 12.1% in WL group). Although, the miss rates in the, WL → WL group was similar to ours, the miss rate in the NBI → WL was lower than that found in our study.
The main limitations of our study are a non-randomized nature and being carried out by two experienced endoscopists at a single center, and thus the results may not be generalized.
In summary, the overall miss rate of adenomas by segmental tandem endoscopy was 17%; being highest (30%) after NBI had been used as the first modality. Most missed adenomas were in the proximal colon and were ≤ 5 mm in size. When white light was used first, the detection rate of missed adenomas was similar with white light and NBI. In conclusion, our data suggest that the tandem nature of the procedure rather than the optical technique used was the most important factor for detecting missed pathology. We recommend taking extra time to “take a second look” at each segment during colonoscopy to increase the yield for detection of pathology.
Polyp detection is of paramount importance during colonoscopy. Conventional colonoscopy may miss polyps, some of which could be pre-cancerous. Narrow band imaging (NBI) is one of the several modalities that are being investigated to enhance polyp and adenoma detection rates.
In narrow band imaging, light of specific blue and green wavelengths is used to enhance the details of certain aspects of the mucosa. NBI has been utilized to classify the colon polyps based on their pit patterns, to differentiate normal from dysplastic tissue in Barrett’s esophagus and ulcerative colitis, and in some cases to improve the detection of colonic polyps/lesions.
The impact of new optical techniques such as high-definition, wide angle, magnification and NBI on polyp detection rate is unknown. The authors know, that a second look back-to-back colonoscopy when performed by a second endoscopist (tandem colonoscopy), may yield additional polyps. The study showed, that the additional detection of missed polyps and adenomas after a first look with white light (WL) was similar when either NBI (WL → NBI) or white light (WL → WL) were used as a second look. This suggests that NBI did not increase the rate of detection of polyps/adenomas but that the tandem nature of the procedure did.
This study suggests that white light may be a relatively better modality in comparison to narrow band imaging when routinely used for purposes of polyp detection during colonoscopy.
NBI: Light of specific blue and green wavelengths that can be used in endoscopy to enhance the details of certain aspects of the lining of gastrointestinal tract; Adenoma: A potentially pre-cancerous polyp.
The authors present a well-designed study investigating the use of second look with narrow band vs white light endoscopy and the effect on polyp detection rates.
P- Reviewer: Cologne KG, Shen ZY S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3128] [Article Influence: 97.8] [Reference Citation Analysis (1)] |
| 2. | Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2174] [Article Influence: 67.9] [Reference Citation Analysis (1)] |
| 3. | Winawer S, Fletcher R, Rex D, Bond J, Burt R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology. 2003;124:544-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1501] [Cited by in RCA: 1438] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 4. | Hixson LJ, Fennerty MB, Sampliner RE, Garewal HS. Prospective blinded trial of the colonoscopic miss-rate of large colorectal polyps. Gastrointest Endosc. 1991;37:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 181] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28. [PubMed] |
| 6. | Matsushita M, Hajiro K, Okazaki K, Takakuwa H, Tominaga M. Efficacy of total colonoscopy with a transparent cap in comparison with colonoscopy without the cap. Endoscopy. 1998;30:444-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 118] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Rex DK, Chadalawada V, Helper DJ. Wide angle colonoscopy with a prototype instrument: impact on miss rates and efficiency as determined by back-to-back colonoscopies. Am J Gastroenterol. 2003;98:2000-2005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Ahn SB, Han DS, Bae JH, Byun TJ, Kim JP, Eun CS. The Miss Rate for Colorectal Adenoma Determined by Quality-Adjusted, Back-to-Back Colonoscopies. Gut Liver. 2012;6:64-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 917] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 10. | Heresbach D, Barrioz T, Lapalus MG, Coumaros D, Bauret P, Potier P, Sautereau D, Boustière C, Grimaud JC, Barthélémy C. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 371] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 11. | Postic G, Lewin D, Bickerstaff C, Wallace MB. Colonoscopic miss rates determined by direct comparison of colonoscopy with colon resection specimens. Am J Gastroenterol. 2002;97:3182-3185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Machida H, Sano Y, Hamamoto Y, Muto M, Kozu T, Tajiri H, Yoshida S. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004;36:1094-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 371] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 13. | DaCosta RS, Wilson BC, Marcon NE. Optical techniques for the endoscopic detection of dysplastic colonic lesions. Curr Opin Gastroenterol. 2005;21:70-79. [PubMed] |
| 14. | Gross SA, Wallace MB. Hold on Picasso, narrow band imaging is here. Am J Gastroenterol. 2006;101:2717-2718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Adler A, Pohl H, Papanikolaou IS, Abou-Rebyeh H, Schachschal G, Veltzke-Schlieker W, Khalifa AC, Setka E, Koch M, Wiedenmann B. A prospective randomised study on narrow-band imaging versus conventional colonoscopy for adenoma detection: does narrow-band imaging induce a learning effect? Gut. 2008;57:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 16. | Atkinson RJ, Hurlstone DP. Narrow-band imaging: the next frontier in colonoscopy? Gastrointest Endosc. 2007;66:317-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Hirata M, Tanaka S, Oka S, Kaneko I, Yoshida S, Yoshihara M, Chayama K. Magnifying endoscopy with narrow band imaging for diagnosis of colorectal tumors. Gastrointest Endosc. 2007;65:988-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Soetikno R, Kaltenbach T. The beginning of a new paradigm in colonoscopy? Gastrointest Endosc. 2007;65:996-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Chiu HM, Chang CY, Chen CC, Lee YC, Wu MS, Lin JT, Shun CT, Wang HP. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 20. | East JE, Suzuki N, Stavrinidis M, Guenther T, Thomas HJ, Saunders BP. Narrow band imaging for colonoscopic surveillance in hereditary non-polyposis colorectal cancer. Gut. 2008;57:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, Schnell TG, Chejfec G, Campbell DR, Kidao J. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 22. | Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 560] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 23. | Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 700] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 24. | Atkin W, Rogers P, Cardwell C, Cook C, Cuzick J, Wardle J, Edwards R. Wide variation in adenoma detection rates at screening flexible sigmoidoscopy. Gastroenterology. 2004;126:1247-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 124] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Harewood GC. Relationship of colonoscopy completion rates and endoscopist features. Dig Dis Sci. 2005;50:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102:856-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 306] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 27. | Bernstein C, Thorn M, Monsees K, Spell R, O’Connor JB. A prospective study of factors that determine cecal intubation time at colonoscopy. Gastrointest Endosc. 2005;61:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Chak A, Cooper GS, Blades EW, Canto M, Sivak MV. Prospective assessment of colonoscopic intubation skills in trainees. Gastrointest Endosc. 1996;44:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Simmons DT, Harewood GC, Baron TH, Petersen BT, Wang KK, Boyd-Enders F, Ott BJ. Impact of endoscopist withdrawal speed on polyp yield: implications for optimal colonoscopy withdrawal time. Aliment Pharmacol Ther. 2006;24:965-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 951] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 31. | Pabby A, Schoen RE, Weissfeld JL, Burt R, Kikendall JW, Lance P, Shike M, Lanza E, Schatzkin A. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest Endosc. 2005;61:385-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Farrar WD, Sawhney MS, Nelson DB, Lederle FA, Bond JH. Colorectal cancers found after a complete colonoscopy. Clin Gastroenterol Hepatol. 2006;4:1259-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 242] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 33. | Triadafilopoulos G, Watts HD, Higgins J, Van Dam J. A novel retrograde-viewing auxiliary imaging device (Third Eye Retroscope) improves the detection of simulated polyps in anatomic models of the colon. Gastrointest Endosc. 2007;65:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 309] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 35. | Harrison M, Singh N, Rex DK. Impact of proximal colon retroflexion on adenoma miss rates. Am J Gastroenterol. 2004;99:519-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Rastogi A, Bansal A, Wani S, Callahan P, McGregor DH, Cherian R, Sharma P. Narrow-band imaging colonoscopy--a pilot feasibility study for the detection of polyps and correlation of surface patterns with polyp histologic diagnosis. Gastrointest Endosc. 2008;67:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 37. | Rex DK, Helbig CC. High yields of small and flat adenomas with high-definition colonoscopes using either white light or narrow band imaging. Gastroenterology. 2007;133:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 38. | Inoue T, Murano M, Murano N, Kuramoto T, Kawakami K, Abe Y, Morita E, Toshina K, Hoshiro H, Egashira Y. Comparative study of conventional colonoscopy and pan-colonic narrow-band imaging system in the detection of neoplastic colonic polyps: a randomized, controlled trial. J Gastroenterol. 2008;43:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 123] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 39. | Kaltenbach T, Friedland S, Soetikno R. A randomised tandem colonoscopy trial of narrow band imaging versus white light examination to compare neoplasia miss rates. Gut. 2008;57:1406-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |