Published online May 16, 2015. doi: 10.4253/wjge.v7.i5.518
Peer-review started: October 20, 2014
First decision: November 27, 2014
Revised: December 6, 2014
Accepted: February 10, 2015
Article in press: February 12, 2015
Published online: May 16, 2015
Processing time: 210 Days and 1.6 Hours
With over a third of Americans being considered obese, bariatric procedures have now become the most performed operation be general surgeons in the United States. The most common operations are the Laparoscopic Roux-en-Y Gastric Bypass, the Laparoscopic Sleeve Gastrectomy, and the Laparoscopic Adjustable Gastric Band. With over 340000 bariatric procedures preformed worldwide in 2011, the absolute number of complications related to these operations is also increasing. Complications, although few, can be life threatening. One of the most dreaded acute complication is the anastomotic/staple line leak. If left undiagnosed or untreated they can lead to sepsis, multi organ failure, and death. Smaller or contained leaks can develop into fistulas. Although most patients with an acute anastomotic leak return to the operating room, there has been a trend to manage the stable patient with an endoscopic stent. They offer an advantage by creating a barrier between enteric content and the leak, and will allow the patients to resume enteral feeding much earlier. Fistulas are a complex and chronic complication with high morbidity and mortality. Postoperative bleeding although rare may also be treated locally with endoscopy. Stenosis is a more frequent late complication and is best-managed with endoscopic therapy. Stents may not heal every fistula or stenosis, however they may prevent certain patients the need for additional revisional surgery.
Core tip: The majority of general surgeons and all bariatric surgeons will be faced with complications related to bariatric surgery. Understanding the new anatomy and most frequent complications is paramount to treating these patients appropriately. The use of endoscopic self-expanding stents alone or in combination with an operation can stabilize and occasionally completely heal anastomotic leaks and fistulas. Endoscopy can also be useful in the diagnosis and treatment of bleeding, stenosis, and ulcerations. This review will summarize the current literature on endoscopy for bariatric complications.
- Citation: Walsh C, Karmali S. Endoscopic management of bariatric complications: A review and update. World J Gastrointest Endosc 2015; 7(5): 518-523
- URL: https://www.wjgnet.com/1948-5190/full/v7/i5/518.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i5.518
Obesity is a complex and chronic disease that is attributed to a combination of genetics and environmental factors. In the United States in 2011-2012, 69% of adults aged over 20 were considered overweight, 35.1% were obese, and 6.8% were morbidly obese. Similar trend are also seen in children (2-19 years) with obesity rate of 16.9% during the same period[1]. It is the second leading cause of preventable death in the United States, second only by smoking. The gap between these two has be diminishing and obesity is thought to overtake smoking in the near future[2]. Although lifestyle modifications have good short term results (1 year)[3], longer follow up has demonstrated a significant advantage to patients who have undergone a bariatric procedure[4]. The number of bariatric procedure performed worldwide in 2011 is estimated at 340768. The most commonly performed operations are the [Roux-en-Y gastric bypass RYGB (46.6%)], sleeve gastrectomy [SG (27.8%)], bilio-pancreatic diversion with duodenal-Switch [BPD/DS (2.2%)], and the Adjustable Gastric Band [AGB (17.8%)][5]. Over 90% these procedure are performed laparoscopically. The mean percentage of excess weight loss is 61.6%, 57%, 70.1%, and 47.5% respectively[6,7]. With an increase in the number of procedure being performed worldwide, as will the complications. These can be divided into intraoperative, perioperative, and late. The two latter can be further subdivided into local and systemic (Table 1). The sleeve gastrectomy is noteworthy as is does not create any mesenteric defects, thus the potential for internal hernia is eliminated.
| Local | Systemic | |
| Intraoperative | Iatrogenic splenectomy (0.41%) | |
| Perioperative | Anastomotic leak (1.1%) GI hemorrhage (2.5%) Trocar injury (0.1%) | Deep vein thrombosis (1%) Pulmonary embolism (0.5%) Bowel obstruction (1.7%) Wound infection (3%) Pneumonia (0.2%) Cardiac event Mortality (0.2%-1%) |
| Late | Anastomotic stricture (3%-12%) Marginal ulcer (0.5%-20%) “Candy Cane” syndrome Gastroesophageal reflux | Bowel obstruction (2.5%) Incisional hernia (0.5%-8%) Internal hernia (1%-3%) Dumping syndrome (up to 30%) Cholecystitis (up to 30%) Anemia Vitamin deficiencies |
Perioperative complications, although rare, are life threatening and must be diagnosed and treated promptly. Many of the clinical signs and symptoms are vague and subtle and can easily be overlooked. Late complications, although less life threatening, can be a diagnostic dilemma. Endoscopy is an excellent first line tool and may be simultaneously diagnostic and therapeutic. We will explore the pathophysiology, incidence and management of anastomotic/staple line leak, fistulas, stenosis, ulcers, and bleeding.
Leaks occur when there is discontinuity of tissue apposition at the site where the tissue has been stapled and divided. It is generally felt that leaks within 48 h are caused by a technical failure. This can be a result of stapler misfire, wrong staple size for the tissue, or tissue trauma. Leaks occurring after several days are more likely due to tissue ischemia cause by tension on the anastomosis, distal bowel obstruction, or hematoma. In both situations, the intraluminal pressure exceeds the strength of the staple line[8]. Risk factors for leaks are increased age, male gender, sleep apnea (SA), and reversional surgery[9]. The incidence of leaks after RYGB has been as high as 8.3%, however most recent data would suggest the incidence to be closer to 1.1%[8,10,11]. The most common sites for anastomotic leak in the Roux-en-Y gastric bypass is the gastrojejunal anastomosis (GJA) 42.2%-67.8%, gastric pouch 10.2%, excluded stomach 3.4%, jejunojenual anastomosis 5.5%-7.8%, or in a combination or these sites in 14%[11,12]. As for sleeve gastrectomies, the most common location of staple line leak is the proximal third of the stomach occurring at the level of the cardiac notch in approximately 75%-87.5%[13,14]. Overall leak rate-related mortality is low (0.6%) in RYGB, however leak associated mortality is significantly higher (14.7%-17%)[9,15]. The results are similar in the sleeve gastrectomy population with an incidence of 1%-2.7%[14,16-18], overall leak-related mortality 0.14%, and leak associated mortality 9%[14].
Anastomotic leaks can be classified as acute < 7 d, early 1-6 wk, late 6-12 wk, chronic > 12 wk[17]. Regardless of the time at which the anastomotic leak occurs, a thorough clinical assessment must me performed. Diagnosis of these leaks can be quite difficult with the most commonly found abnormality being sustained tachycardia > 120 bpm[19,20]. Other symptoms that have been reported are abdominal pain, use of more analgesics than expected, no ambulation within 2 h of surgery, and shortness of breath[11]. Laboratory abnormalities may show leukocytosis or an elevated C-reactive protein, although these are not always present. The use of an upper gastrointestinal series with water-soluble contrast or computed tomography may confirm the diagnosis, however these tests should not delay a return to the operating room. Most surgeons (86%) would take the patient to the OR with an unconfined and persistently symptomatic patient[17]. The majority (39%-81%) of patients with acute or early anastomotic leaks will ultimately return the the OR[11,12,19,20]. In the subgroup of patients who have minimal symptoms, are hemodynamically stable, and have a contained leak, conservative management may be warranted. Traditionally this management was NPO status, broad-spectrum antibiotics, percutaneous drains, and parenteral nutrition[11].
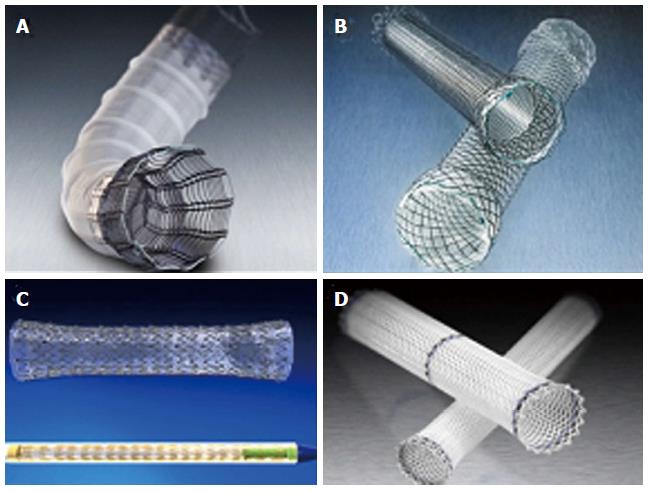
Endoscopic stents were initially designed as a tool of palliation for obstructing esophageal, gastric, and colorectal cancer. Some of the first published data for using stents across an anastomotic leak was in the thoracic population after esophageal resections. Leak rates as well as mortality after re-operation in this population was much higher therefore prompting a more conservative solution[21]. Most endoscopic stents used today are covered self-expanding metal stents (SEMS), partially covered self-expanding metal stents, and covered self-expanding plastic stents (SEPS) (Figure 1). These stents will provide a barrier between endoluminal bacteria and the acidic enteric content and the anastomotic disruption. Having an intraluminal device that will keep the anastomosis patent may also prevent wound contraction and the subsequent development of stenosis. The presence of these stents also confers the advantage of early enteral feeding. Healing success is defined as radiological confirmation of no leak after removal of stent. Stents are successful in 80%-94% of acute anastomotic leaks with stents left in place ranging from a mean of 41 d to 3.2 mo. Most patient may resume an oral liquid diet within 1-3 d. The most common side effects of the stent are early satiety, nausea, epigastric pain, and hypersialosis[22-24]. In a recent international expert panel consensus including 24 centres and over 12000 cases of laparoscopic sleeve gastrectomy (LSG), 93% of responders found the use of a stent for and acute proximal leak is a valid treatment option[17]. The most frequent complication of stent placement is stent migration seen in 16.9%-59%[25]. Most migrations are only a few centimetres, however this is enough to uncover the leak. The stents may also migrate distally with most passing per rectum. Only a few require an elective operation for stent retrieval. An urgent OR for erosion through the gastrointestinal wall and laceration of a blood vessel has also been described. Partially covered SEMS, larger diameter (18-22 mm), and longer length (15 cm) seem to have the least potential to migrate. The procedure of stent placement is most commonly performed in the operating room under general anesthesia with edotracheal intubation. The endoscope is use to identify the location of the leak and mark the location with radio-opaque clips. A guide-wire is also placed through the Roux limb. Under fluoroscopy, the stent deployment system is positioned across the leak and released. The length of the procedure can range from 23-47 min[24,26,27]. Endoscopic extraction is easiest with fully covered SEMS or SEPS. They can be grasped with large toothed graspers and extracted with firm steady pressure. Partially covered SEMS may have tissue ingrowth at either end. Two common techniques from removal are argon plasma coagulation and insertion of SEPS within the SEMS to induce tissue necrosis and easy extraction at a later date.
Early bleeding after surgery can be intraluminal or extraluminal. The most frequent site of bleeding is the site of the anastomosis of staple lines. A risk factor for early bleeding is the presence of diabetes mellitus. The minority of bleeds will require an intervention more involved than a simple blood transfusion, and even fewer will require reoperation (21%)[28]. Bleeding is most often diagnosed with a postoperative decrease in hemoglobin. Uncommon clinical findings are hemoptysis, bright red blood pre rectum, or melena. A patient with hemodynamic instability, a distended or tender abdomen, or falling hemoglobin should be managed with an expeditious return to the operating room.
The gastroscope may be used may be used cautiously in the early post op with minimal air insufflation to avoid undue tension on the fresh anastomosis. The use of endoclips (Figure 2) alone or in combination with epinephrine is preferred to electrocautery[29].
Anastomotic disruption with a more indolent and contained leak may ultimately form a fistula. A theory for the formation of a gastrogastric fistula is an incomplete transection of the gastric pouch and gastric remnant. The most common locations of an enteric fistula after bariatric surgery are gastrogastric, gastrocutaneous, duodenocutaneous, gastroperitoneal, and more rarely gastro-bronchial[8,26,30]. The incidence of gastric fistulas after bariatric surgery has not been well described, it may be in the order of 14.2% after an anastomotic leak[19]. The presence of a fistula will increase mortality with an order of magnitude of 8%-37.5%. It will also increased morbidity associated with a prolonged hospital stay, frequent hospital/clinic visits, and home care[31]. Success after stenting has been much less favourable than in the acute leaks. The success ranges from 19%-81%[19,26,32]. During an international expert panel for LSG, 89% of centres agree that stenting has a limited utility for chronic leaks (> 12 wk)[17]. Bège et al[25] have described a series of interventions starting with endoscopic drainage and debridement (± Amikacin 500 mg into the cavity), placement of a nasocystic tube, and placement of a plastic double-pigtail stent. A stent was inserted if the opening was more than 1 cm in diameter. The stent was secured proximally with endo-clips. If there was no resolution after 6 wk, therapeutic endoscopy was performed with placement of clips and/or injection of synthetic glue (N-butyl-2-cyanoacrylate) within the fistula cavity. Success after the first intervention was 64% of patients with late leaks/fistulas. Eisendrath et al[26] had a 61.9% success after stent alone, and an increased success rate of 80.9% the use after biologic glue, fistula plug, or clips.
Marginal ulceration may be seen in 0.49%-20% after RYGB[33-35]. The most common symptoms include epigastric pain, nausea, vomiting, food intolerance and bleeding. It is one of the most common finding on endoscopy in patients presenting with abdominal pain (52%)[36]. Risk factors include smoking (OR = 30.6), NSAIDs (OR = 11.5), diabetes (OR = 5.6), ischemia, increased stomach acid, bile acid reflux, Helicobacter pylori (H. pylori), steroids, alcohol, and foreign body[35,37-40]. Management is largely directed to the suspected etiology. Cessation of smoking, NSAIDs, and good blood glucose control is paramount. Proton pump inhibitors taken twice daily and tapered for 3-6 mo have had good results. If sampling of gastric fluid reveals normal or alkaline pH, sucralfate four times daily may have better results[41]. Biopsy proven H. pylori should be treated and visible suture should be removed. Non-healing ulcers should raise the possibility of a gastrogastric fistula.
This late complication can present with early satiety, nausea, vomiting, dysphagia, obstruction, retrosternal or abdominal pain[35]. These most commonly occur at the GJA and have an incidence of approximately 3%-12%[42-45]. Less frequently, stenosis can be seen at the enteroenteric anastomosis, the passage of the Roux limb through the mesocolon (Retrocolic approach only), and the Petersens defect. They most commonly present after 4-8 wk post op[46,47]. GJA with a linear stapler has a lower stricture rate of 2% compared to the 21 mm circular EEA stapler with a rate of 14%[19]. Risk factors include small (< 25 mm) circular stapler and marginal ulcers. The majority (90%) of patients will be amenable to endoscopic dilatation[47-49]. Dilatation may be attempted cautiously in as early as 4 wk post operatively. Frequently two, three or more dilatation may be required. With conscious sedation, the endoscope is passed to the level of the GJA. The diameter of the stricture is frequently be smaller than 3 mm and precludes passage of the endoscope. Caution must be applied when passing a guide wire and the balloon dilator through the stenosis blindly. If any resistance is encountered, it should raise the possibility of passage into the blind limb. The balloon dilator is passed through the structured segment until its midpoint is at the maximal level of the stenosis. The smallest balloon is used initially and the size is progressively increased with every successful dilatation. This is felt to reduce the risk of perforation reported to be 3%-5%[46]. Dilatations of up to 15 mm, even in the first procedure, have been shown to be safe. The use of stents for treating strictures that have failed dilatation has not been fruitful. Puig et al[32] have had minimal success with only 2 of 16 patients not requiring and operative revision.
As the number obese patients increases, as will the number bariatric procedures. We will be left with a large number of patients with complications requiring adequate diagnosis and treatment. The surgeon is expected to promptly identify and appropriately manage early and late complications. Only surgeons who have performed the operations truly understand the new anatomy. Diagnostic and therapeutic endoscopy should be considered a first line tool in stable patients with perioperative complications such as anastomotic/staple line leaks, and bleeding. The placement of self-expanding metal or plastic stents in a patient with an anastomotic leak has shown favourable results. Late complication often present with vague complaints such as nausea, vomiting, or abdominal pain. Endoscopy is an excellent instrument for early diagnosis and treatment. SEMS, SEPS alone or in combination with metal clips, biologic glues, and biologic fistula plugs for treatment of fistulas should be considered first line therapy despite modest results. This strategy should greatly decrease the morbidity and mortality by reducing the rate of a revision surgery.
P- Reviewer: De Lusong MAA, Wang B S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6227] [Cited by in RCA: 5921] [Article Influence: 538.3] [Reference Citation Analysis (1)] |
| 2. | Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3857] [Cited by in RCA: 3383] [Article Influence: 161.1] [Reference Citation Analysis (0)] |
| 3. | Martins C, Strømmen M, Stavne OA, Nossum R, Mårvik R, Kulseng B. Bariatric surgery versus lifestyle interventions for morbid obesity--changes in body weight, risk factors and comorbidities at 1 year. Obes Surg. 2011;21:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3301] [Cited by in RCA: 3029] [Article Influence: 144.2] [Reference Citation Analysis (0)] |
| 5. | Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1020] [Cited by in RCA: 1004] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 6. | Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5073] [Cited by in RCA: 4706] [Article Influence: 224.1] [Reference Citation Analysis (1)] |
| 7. | Wölnerhanssen B, Peterli R. State of the art: sleeve gastrectomy. Dig Surg. 2014;31:40-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Baker RS, Foote J, Kemmeter P, Brady R, Vroegop T, Serveld M. The science of stapling and leaks. Obes Surg. 2004;14:1290-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 274] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 9. | Fernandez AZ, DeMaria EJ, Tichansky DS, Kellum JM, Wolfe LG, Meador J, Sugerman HJ. Experience with over 3,000 open and laparoscopic bariatric procedures: multivariate analysis of factors related to leak and resultant mortality. Surg Endosc. 2004;18:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 242] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 10. | Podnos YD, Jimenez JC, Wilson SE, Stevens CM, Nguyen NT. Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg. 2003;138:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 447] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 11. | Jacobsen HJ, Nergard BJ, Leifsson BG, Frederiksen SG, Agajahni E, Ekelund M, Hedenbro J, Gislason H. Management of suspected anastomotic leak after bariatric laparoscopic Roux-en-y gastric bypass. Br J Surg. 2014;101:417-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Ballesta C, Berindoague R, Cabrera M, Palau M, Gonzales M. Management of anastomotic leaks after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2008;18:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Burgos AM, Braghetto I, Csendes A, Maluenda F, Korn O, Yarmuch J, Gutierrez L. Gastric leak after laparoscopic-sleeve gastrectomy for obesity. Obes Surg. 2009;19:1672-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 14. | Sakran N, Goitein D, Raziel A, Keidar A, Beglaibter N, Grinbaum R, Matter I, Alfici R, Mahajna A, Waksman I. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 285] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 15. | Lee S, Carmody B, Wolfe L, Demaria E, Kellum JM, Sugerman H, Maher JW. Effect of location and speed of diagnosis on anastomotic leak outcomes in 3828 gastric bypass cases. J Gastrointest Surg. 2007;11:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Clinical Issues Committee of the American Society for Metabolic and Bariatric Surgery. Updated position statement on sleeve gastrectomy as a bariatric procedure. Surg Obes Relat Dis. 2010;6:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Rosenthal RJ, Diaz AA, Arvidsson D, Baker RS, Basso N, Bellanger D, Boza C, El Mourad H, France M, Gagner M. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of & gt; 12,000 cases. Surg Obes Relat Dis. 2012;8:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 712] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 18. | Vix M, Diana M, Marx L, Callari C, Wu HS, Perretta S, Mutter D, Marescaux J. Management of staple line leaks after sleeve gastrectomy in a consecutive series of 378 patients. Surg Laparosc Endosc Percutan Tech. 2015;25:89-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Gonzalez R, Sarr MG, Smith CD, Baghai M, Kendrick M, Szomstein S, Rosenthal R, Murr MM. Diagnosis and contemporary management of anastomotic leaks after gastric bypass for obesity. J Am Coll Surg. 2007;204:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 20. | Carucci LR, Conklin RC, Turner MA. Roux-en-Y gastric bypass surgery for morbid obesity: evaluation of leak into excluded stomach with upper gastrointestinal examination. Radiology. 2008;248:504-510. [PubMed] |
| 21. | Hünerbein M, Stroszczynski C, Moesta KT, Schlag PM. Treatment of thoracic anastomotic leaks after esophagectomy with self-expanding plastic stents. Ann Surg. 2004;240:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | Salinas A, Baptista A, Santiago E, Antor M, Salinas H. Self-expandable metal stents to treat gastric leaks. Surg Obes Relat Dis. 2006;2:570-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Edwards CA, Bui TP, Astudillo JA, de la Torre RA, Miedema BW, Ramaswamy A, Fearing NM, Ramshaw BJ, Thaler K, Scott JS. Management of anastomotic leaks after Roux-en-Y bypass using self-expanding polyester stents. Surg Obes Relat Dis. 2008;4:594-559; discussion 599-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Puli SR, Spofford IS, Thompson CC. Use of self-expandable stents in the treatment of bariatric surgery leaks: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Bège T, Emungania O, Vitton V, Ah-Soune P, Nocca D, Noël P, Bradjanian S, Berdah SV, Brunet C, Grimaud JC. An endoscopic strategy for management of anastomotic complications from bariatric surgery: a prospective study. Gastrointest Endosc. 2011;73:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 26. | Eisendrath P, Cremer M, Himpens J, Cadière GB, Le Moine O, Devière J. Endotherapy including temporary stenting of fistulas of the upper gastrointestinal tract after laparoscopic bariatric surgery. Endoscopy. 2007;39:625-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 27. | Eubanks S, Edwards CA, Fearing NM, Ramaswamy A, de la Torre RA, Thaler KJ, Miedema BW, Scott JS. Use of endoscopic stents to treat anastomotic complications after bariatric surgery. J Am Coll Surg. 2008;206:935-938; discussion 938-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Rabl C, Peeva S, Prado K, James AW, Rogers SJ, Posselt A, Campos GM. Early and late abdominal bleeding after Roux-en-Y gastric bypass: sources and tailored therapeutic strategies. Obes Surg. 2011;21:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Kumar N, Thompson CC. Endoscopic management of complications after gastrointestinal weight loss surgery. Clin Gastroenterol Hepatol. 2013;11:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Campos JM, Pereira EF, Evangelista LF, Siqueira L, Neto MG, Dib V, Falcão M, Arantes V, Awruch D, Albuquerque W. Gastrobronchial fistula after sleeve gastrectomy and gastric bypass: endoscopic management and prevention. Obes Surg. 2011;21:1520-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 31. | Csendes A, Burdiles P, Burgos AM, Maluenda F, Diaz JC. Conservative management of anastomotic leaks after 557 open gastric bypasses. Obes Surg. 2005;15:1252-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | Puig CA, Waked TM, Baron TH, Wong Kee Song LM, Gutierrez J, Sarr MG. The role of endoscopic stents in the management of chronic anastomotic and staple line leaks and chronic strictures after bariatric surgery. Surg Obes Relat Dis. 2014;10:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Higa KD, Ho T, Boone KB. Laparoscopic Roux-en-Y gastric bypass: technique and 3-year follow-up. J Laparoendosc Adv Surg Tech A. 2001;11:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Nguyen NT, Goldman C, Rosenquist CJ, Arango A, Cole CJ, Lee SJ, Wolfe BM. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234:279-289; discussion 289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 630] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 35. | Keith JN. Endoscopic management of common bariatric surgical complications. Gastrointest Endosc Clin N Am. 2011;21:275-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Marano BJ. Endoscopy after Roux-en-Y gastric bypass: a community hospital experience. Obes Surg. 2005;15:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Ramaswamy A, Lin E, Ramshaw BJ, Smith CD. Early effects of Helicobacter pylori infection in patients undergoing bariatric surgery. Arch Surg. 2004;139:1094-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Hedberg J, Hedenström H, Nilsson S, Sundbom M, Gustavsson S. Role of gastric acid in stomal ulcer after gastric bypass. Obes Surg. 2005;15:1375-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 39. | Wilson JA, Romagnuolo J, Byrne TK, Morgan K, Wilson FA. Predictors of endoscopic findings after Roux-en-Y gastric bypass. Am J Gastroenterol. 2006;101:2194-2199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Azagury DE, Abu Dayyeh BK, Greenwalt IT, Thompson CC. Marginal ulceration after Roux-en-Y gastric bypass surgery: characteristics, risk factors, treatment, and outcomes. Endoscopy. 2011;43:950-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 41. | Rasmussen JJ, Fuller W, Ali MR. Marginal ulceration after laparoscopic gastric bypass: an analysis of predisposing factors in 260 patients. Surg Endosc. 2007;21:1090-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 42. | Smith SC, Edwards CB, Goodman GN, Halversen RC, Simper SC. Open vs laparoscopic Roux-en-Y gastric bypass: comparison of operative morbidity and mortality. Obes Surg. 2004;14:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 43. | Carrodeguas L, Szomstein S, Zundel N, Lo Menzo E, Rosenthal R. Gastrojejunal anastomotic strictures following laparoscopic Roux-en-Y gastric bypass surgery: analysis of 1291 patients. Surg Obes Relat Dis. 2006;2:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | Mathew A, Veliuona MA, DePalma FJ, Cooney RN. Gastrojejunal stricture after gastric bypass and efficacy of endoscopic intervention. Dig Dis Sci. 2009;54:1971-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Higa K, Ho T, Tercero F, Yunus T, Boone KB. Laparoscopic Roux-en-Y gastric bypass: 10-year follow-up. Surg Obes Relat Dis. 2011;7:516-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 285] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 46. | Go MR, Muscarella P, Needleman BJ, Cook CH, Melvin WS. Endoscopic management of stomal stenosis after Roux-en-Y gastric bypass. Surg Endosc. 2004;18:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Goitein D, Papasavas PK, Gagné D, Ahmad S, Caushaj PF. Gastrojejunal strictures following laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 2005;19:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Peifer KJ, Shiels AJ, Azar R, Rivera RE, Eagon JC, Jonnalagadda S. Successful endoscopic management of gastrojejunal anastomotic strictures after Roux-en-Y gastric bypass. Gastrointest Endosc. 2007;66:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Espinel J, De-la-Cruz JL, Pinedo E, Canga J, De-la-Cruz F. Stenosis in laparoscopic gastric bypass: management by endoscopic dilation without fluoroscopic guidance. Rev Esp Enferm Dig. 2011;103:508-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |